Understanding immune perspectives and options for the use of checkpoint immunotherapy in HCC post liver transplant
Chimaobi M. Anugwom, Thomas M. Leventhal, Jose D. Debes,3
1HealthPartners Digestive Care, Saint Paul, Minnesota, MN 55130, USA.
2Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition and Division of Infectious Disease and International Medicine, University of Minnesota, Minneapolis, MN 55455, USA.
3Department of Gastroenterology and Hepatology, Erasmus Medical Center, Postbus 2040, The Netherlands.
Abstract Treatment modalities for hepatocellular carcinoma (HCC) vary from surgical techniques and interventional radiologic strategies to systemic therapy. For the latter, the use of immune checkpoint inhibitors (ICIs) has gained popularity due to successful trials showing increased survival. In patients who have undergone liver transplantation, recurrence of HCC poses a significant challenge. There is indeed considerable debate on the efficacy and safety of ICI use in liver transplant recipients due to competing immune interests in maintaining a healthy graft and combating the tumor. Recent reports and case series have highlighted a role for the type of immune therapy, timing of therapy, tissue expression of PD-1 and modulation of immunosuppression, in the understanding of the efficacy and risks of ICIs for HCC in liver transplant. In this article, we appraise the available literature on the usage of ICIs for HCC in liver transplant recipients and provide perspectives on immune concerns as well as potential recommendations to consider during the management of such complex cases.
Keywords: Hepatocellular carcinoma, liver transplant, checkpoint inhibitors
INTRODUCTION
Hepatocellular carcinoma (HCC) is the predominant primary liver malignancy - representing about 75% of all primary liver cancers[1]. Consequently, HCC causes a significant global public health care burden, as it is the seventh most common malignancy and the second most common cause of cancer-related mortality worldwide[2].
In the majority of individuals, HCC occurs as a complication of underlying chronic liver disease. Globally,hepatitis B is the most important risk factor for developing HCC, while hepatitis C, alcohol-related liver injury and non-alcoholic fatty liver disease represent prominent etiological risk factors in resource-rich countries[3-5].
Treatment modalities for HCC include surgical resection, ablation therapies including radiofrequency ablation, microwave ablation and electroporation; as well as, in select candidates, liver transplantation(LT)[6-9]. Resection or ablation of HCC, in those deemed appropriate candidates, can lead to long-term disease-free survival and LT can provide the additional benefit of not only removing the malignancy but eliminating underlying chronic liver disease as well. In patients with HCC, despite the use of strict selection criteria for candidates for LT, there remains a risk of HCC recurrence in the transplant recipient[6,10]. The mean rate of HCC recurrence after LT is about 16%, and can be as high as 20%, with 75% of cases occurring within the first two years of the post-transplant period[10-12]. Even more concerning is the dramatic course of tumor recurrence. It is considered a systemic event, as the transplanted liver alone is involved in only 30% of cases while approximately 50% of cases of HCC recurrence post-LT involves multiple organs: the lungs,skeletal system, and adrenal glands being the most common sites of recurrence[13,14].
The great strides in cancer therapy in recent years include the emergence of immunotherapeutic agents,which have become commonplace in the management of most cancers, including HCC[15]. Indeed, over the last few years, checkpoint immunotherapy for HCC has advanced at an explosive pace and despite the cost of treatment (including copays, office visits, and laboratory tests), the cost of management of adverse events and its contribution to the overall cost of cancer care; it is now considered first-line therapy for advanced HCC in those individuals that can tolerate it[16]. There is, however, still ongoing debate about the safety and efficacy of these medications in patients who have undergone LT, given the contrasting mechanism of action of these immunotherapeutic agents compared to immunosuppression for LT [Figure 1]. Moreover,there is an incomplete understanding of the effects caused by the inter-relation between the non-cancerrelated activation of immune exhaustion triggered by immune-therapy and immune-modulation related to anti-rejection medications in these patients. In this review, we discuss critical aspects of checkpoint immunotherapy for HCC following LT based on existing data and as well as providing insight into controversial issues in the field.
CHECKPOINT IMMUNOTHERAPY IN THE TREATMENT OF HEPATOCELLULAR CARCINOMA
Many drug classes are employed in the systemic treatment of hepatocellular carcinoma. Sorafenib was the first agent to demonstrate survival benefit as a first-line therapy for unresectable HCC based on the SHARP and Asian-Pacific trials and remained the sole resource for advanced HCC for over ten years[17,18]. Sorafenib is a multi-kinase inhibitor that acts by inhibiting a variety of tyrosine-kinase receptors, including vascular endothelial growth factor receptor and platelet-derived growth factor receptor and has mainly been shown to be effective in selected patients such as those with hepatitis C and favorable neutrocyte-lymphocyte ratio[19-21]. Additional therapies such as lenvatinib, regorafenib, and cabozantinib, all targeting a combination of tyrosine kinase receptors, as well as ramicirumab with specific targeting of VEGF, have been approved for the treatment of HCC[22-25].

Figure 1. Interplay between immune checkpoint inhibitors and liver transplant immunosuppression in a recipient with hepatocellular carcinoma, emphasizing the effect of these medications on components of the immune system. NK: Natural killer; IL: interleukin.
Although lymphocyte infiltration of HCC is variable, its presence, as reported by Yoonget al.[26], may allude to the apparent immunogenicity of this tumor, thus making immunotherapy an exciting prospect for HCC therapy. This can be accomplished by either removing barriers to the body’s natural immune response to tumor antigens - in the case of checkpoint inhibitors - or by stimulating a novel response by targeting specific HCC antigenic molecules.
During T-lymphocyte activation, binding of the programmed death ligand-1 (PD-L1) to the PD-1 receptor or the cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) to B7-1/B7-2, produces a co-inhibitory signal,which prevents lymphocytes from attacking specific host cells[27,28]. Tumor cells may hijack these checkpoint mechanisms, thus escaping immunologic surveillance. Immune checkpoint inhibitors (ICIs), as the name indicates, are immunotherapeutic agents that are effective in targeting and inhibiting these checkpoints,thereby activating T-lymphocytes, and potentially those with anti-tumoral activity[28]. By removing this coinhibitory signal, ICIs augment the immune response toward the tumor. However, removal of these checkpoints, in addition to the subsequent activation of other non-specific T-cells, lead to an increased risk of immune-related adverse effects (IRAEs) in the host[29]. The various ICIs approved or being studied for the systemic treatment of HCC are shown in Table 1.
Single-agent treatment of HCC with the PD-L1/PD-1 inhibitors comprises most of the initial data on this topic. Nivolumab and pembrolizumab (both PD-1 inhibitors) have shown significant promise in the treatment of HCC and were initially approved for use as second-line therapy in patients who have been exposed to sorafenib. The CheckMate-040 study was a single-arm, non-comparative, dose escalation andexpansion trial showing median overall survival of 7.6 months with Nivolumab in patients exposed to Sorafenib. This led to the approval of nivolumab for the indication of treatment of HCC by the FDA in 2017[30]. In the KEYNOTE-240 trial, pembrolizumab showed a median survival of 13.9 months compared to 10.6 months with placebo [Hazard ratio (HR) = 0.78][31]. Nivolumab was studied as a first-line agent compared to sorafenib in the CheckMate-459 study. Although there was some evidence of increased survival in the Nivolumab group, this study did not meet its primary endpoint of statistically significant improvement in overall survival[32].
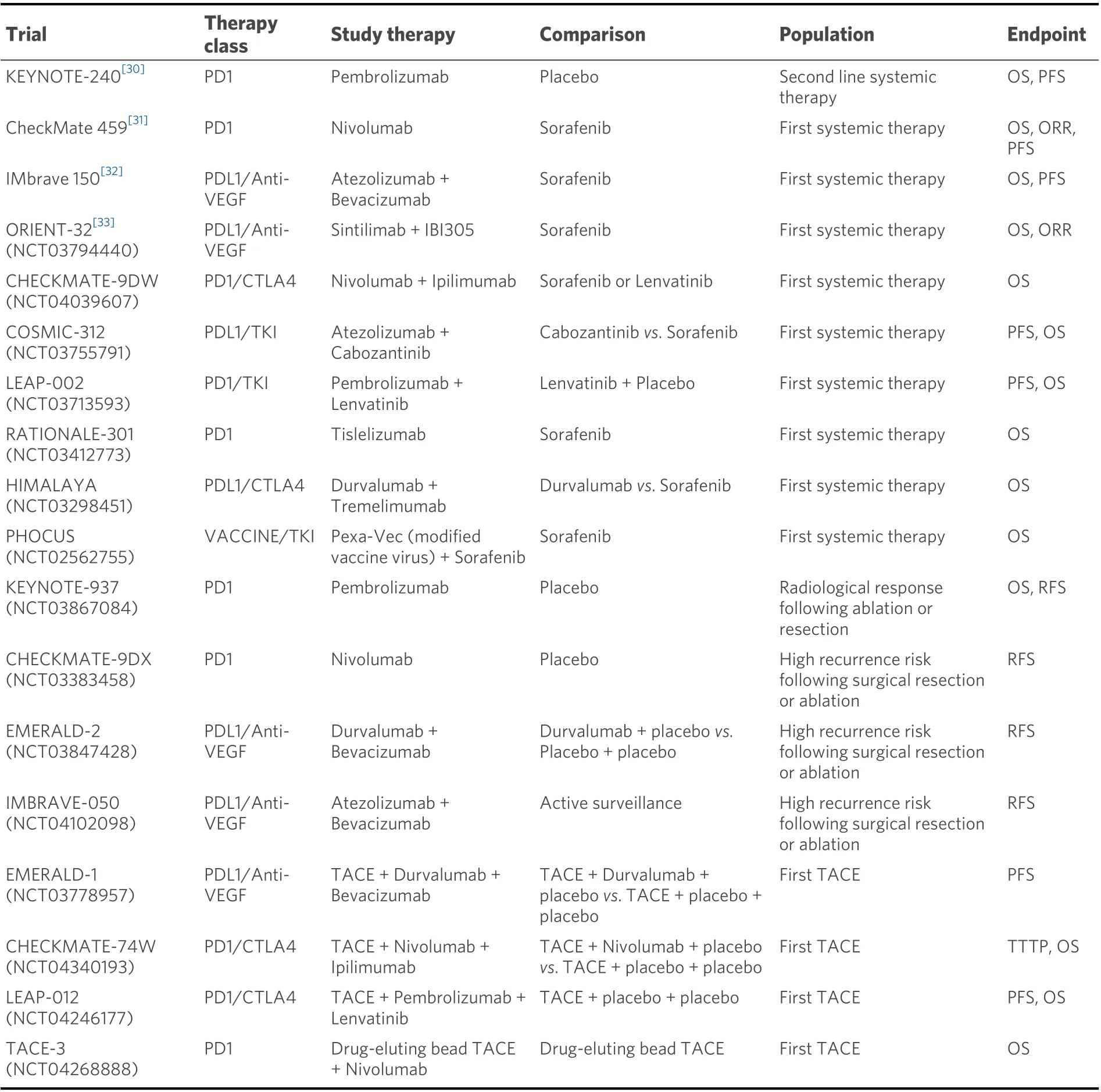
Table 1. Immune checkpoint inhibitors in the systemic therapy of hepatocellular carcinoma
The combination therapy with atezolizumab (PD-L1 inhibitor) and bevacizumab (Anti-VEGF) was compared to sorafenib in the IMbrave study[33]. This was a multicenter, randomized, phase III open-label trial, that showed a median progression-free survival of 6.8 months with atezolizumab/bevacizumab compared to 4.3 months with sorafenib (HR = 0.59), as well as increased overall survival (HR = 0.58) in the atezolizumab/bevacizumab group[33]. This led to the approval of this combination by the FDA as first-line therapy for advanced HCC in 2020. Sintilimab (PD-L1 inhibitor) combined with a bevacizumab biosimilar(a biologic medical product highly similar to the already approved biological) has been compared to sorafenib in the ORIENT-32 trial. The overall survival and progression-free survival were both higher in the sintilimab/bevacizumab-biosimilar group (HR = 0.57 for both outcomes)[34]. Other combinations including ICIs in ongoing trials include: atezolizumab/cabozantinib (COSMIC-312, NCT03755791),lenvatinib/pembrolizumab (LEAP-002, NCT03713593) nivolumab/ipilimumab (CHECKMATE-9DW,NCT04039607) and durvalumab/bevacizumab (EMERALD-2, NCT03847428)[35]. It is expected that these additional ICIs will expand the immunologic treatment options for patients with HCC in the near future.
THE IMMUNOLOGICAL MILLEU OF THE TRANSPLANT RECIPIENT
Immunosuppression is essential to long-term patient and graft survival after LT. Compared to transplantation for other solid organs, the liver is quite immune tolerant, and this is related to the unique immunologic microenvironment created by the liver-derived dendritic cells, liver sinusoidal endothelial cells, liver-derived natural killer cells, and Kupffer cells[36]. This unique environment is crucial in maintaining organ homeostasis and keeping a balance between immune tolerance and inflammation when exposed to infectious and tumorigenic triggers[37,38]. In the LT recipient, this unique immune-environment may explain the need for less overall systemic immunosuppression and potential for immunosuppression withdrawal after LT[38,39].
There has been significant advancement in the strategies aimed at successfully preventing rejection of the allograft since the first successful liver transplantation by Starzlet al.[40,41]in the 1960’s. In the early days of LTs, corticosteroids and azathioprine were used as the primary immunosuppression strategy and this has evolved to more recent immunosuppression modalities such as calcineurin inhibitors (CNIs), antimetabolites, mammalian targets of Rapamycin inhibitors (mTORs), T-cell depleting and T-cell inhibiting antibodies[42,43].
The consequent effect of transplantation on the native immune system is the reduction of T-cell stimulation, proliferation and differentiation, impairment of natural killer cell proliferation, and significant downregulated production of co-stimulatory molecules by antigen-presenting cells with a decrease in the production of pro-inflammatory cytokines[44-46]. These changes, though necessary for long-term allograft survival, have a deleterious effect on the ability of the immune system to actively detect and attack cancer cells, so it is no surprise that the risk of some malignancies increases in the post-LT period. As previously alluded to, in those transplanted for HCC, tumor recurrence can be as high as 20%, and this risk is affected by immunosuppression, obesity, donor age, etiology of liver disease[47-49]. Thede-novocancer risk in patients post-LT, based on over 108,000 recipients between 1987 and 2015, was obtained from the United States Scientific Registry of Transplant Recipients database, and this estimated the cumulative incidence ofde novoextrahepatic cancer to be about 1.3% (95%CI: 1.3-1.4) in the first year after LT; and up to 18.8% (18.4-19.3)at 20 years[47]. The most commonde-novomalignancies in the LT population include Non-Hodgkin’s lymphoma, keratinocyte skin cancer (basal cell cancer and squamous cell cancer), cervical cancer and head/neck cancers; and so, strategies such as judicious use of immunosuppression with reduction when possible, cancer screening (dermatologic visits, regular pap smears) and avoidance of excessive sun exposure may promote early detection[50,51].
THE IMPACT OF CHECKPOINT IMMUNOTHERAPY FOLLOWING LIVER TRANSPLANTATION
The treatment of recurrent HCC in the LT recipient is a complex endeavor. Given the rates of multiorgan involvement with tumor recurrence in this population, there is a limit to the treatment modalities available[13]. Furthermore, after HCC recurrence, the overall survival at 5 years is about 50%, even with treatment[52]. Historically, treatment of HCC in the post-transplant patient has focused on the use of targeted therapies such as sorafenib with a demonstrated mortality benefit. Additionally, stereotactic body radiation therapy in localized bone disease and localized ablation and/or resection of solitary, small recurrent tumors are adjunctive treatments that can be employed[14,53,54].
Consideration for the use of immunologic therapy in LT recipients is wrought with a complex interplay between the provision of adequate immunosuppression to protect the graft and augmentation of the immune response to detect and kill cancer cells. In addition to the typically reported immune-related adverse effects from ICI therapy such as hypophysitis, diarrhea/colitis and dermatitis, there is an additional risk of acute immune-mediated hepatitis in the liver allograft and an increased risk of acute cellular rejection (ACR)[55].
Registry trials that led to the ultimate approval of ICI use for the treatment of HCC did not include liver(and other solid organ) transplant recipients as study participants. Hence, most of the data on the efficacy and safety of ICIs in these patients are drawn from case reports, case series and single center experiences[56-60]. A summary of some studies evaluating the efficacy and adverse events of these medications in the transplant population is summarized in Table 2. Relative safety, especially with close monitoring, has been described in a few case reports, but severe and sometimes fatal outcomes have also been published[56,57,59]. Some of the main adverse effects to be considered, especially in an LT recipient, are that of venous (sub-distribution HR up to 1.36 depending on the agent) and arterial thrombosis[61,62]. Poor wound healing is also a concern given the overlapping cellular and molecular processes between wound healing and cancer; but this increased risk has not been apparent in studies, and ICIs have been suggested to be safe in the peri-operative period[63-65]. The severity of checkpoint inhibitor-induced injury in the allograft can vary,and it is unclear if the altered immunologic milieu associated with solid organ transplant (SOT) and need for chronic immunosuppression play a role in the incidence and severity of this phenomenon. The most commonly reported liver injury is hepatocellular injury, and this pattern of injury is predominant in those who have undergone SOT[66]. Moreover, complications beyond hepatocellular injury have been exposed.Our group reported a case of severe cholestatic disease in the allograft after the treatment of recurrent HCC with nivolumab during the post-transplant period. This patient had no evidence of ACR on liver biopsy, but died from complications related to the confluent hepatic necrosis, consequent synthetic dysfunction and concurrent esophagitis and gastrointestinal hemorrhage[58].
The gravest concern regarding the use of ICIs in the post-transplant setting is related to severe graft rejection or even allograft failure. Initial reports documented rates of rejection in transplant recipients treated with ICIs, anywhere from 36% in LT to 54% in kidney transplant recipients[67]. Systematic reviews have evaluated the risk of rejection in SOT recipients treated with ICIs[60,68]. These reviews are quite heterogenous: including a mix of SOT recipients with a variety of solid tumor malignancies. One singlecenter analysis of 17 SOT (including 8 LT) recipients treated with ICIs found that 18% of patients had acute allograft rejection, a cumulative incidence of cancer progression of 50% at 6 months, and 65% mortality over the median follow up period of 4.6 months[60]. Another pooled analysis of 64 SOT recipients documented the rate of allograft rejection at 41% following checkpoint immunotherapy for malignancies in the posttransplant period. Of note, the highest risk of ACR was seen in those treated with PD-1 inhibitors, and thelowest risk was in those on CTLA-4 inhibitor therapy[68.]This finding supports the previously proposed theory that the PD-1 pathway could play a critical role in determining graft tolerance[69.]In an LT recipient cohort, Munkeret al.[70]carried out a systemic review of 14 cases of LT recipients treated with ICIs, with ACR reported in 29% of patients - and lethal outcomes in 75% of those with ACR.

Table 2. Efficacy and adverse events noted with immune checkpoint inhibitors in the liver transplant recipient
It is important to note that ICIs have been investigated for use in the pre-transplant setting, with mixed outcomes. One study reported the use of a pretransplant toripalimab (Anti-PD-1) with resultant post-transplant fatal acute hepatic necrosis[71.]Another case series of nivolumab use for pre-transplant tumor treatment reported the absence of allograft loss, tumor recurrence and death[72.]Though this is worth mentioning, the safety and efficacy of ICIs in the pretransplant setting are quite broad and beyond the scope of this review[73.]
A variety of factors may affect the development of rejection of liver allograft during ICI therapy. The timing of ICI use has been implicated by a few studies. In the LT population, the use of ICIs in those with a median interval of ~2 to 8 years post-LT has been associated with little to no reports of ACR, but rejection seems much higher when used in the early post-transplant period, up to a year following transplantation[74-76]. This phenomenon may be explained by the development of transplant immunological tolerance, which refers to the decreased immune activity against the allograft, and thus reduced immunosuppression needs[77]. Being an immunologically privileged organ, liver allograft immune tolerance can occur many years post-LT and is evidenced by maturation and depletion of self-reactive T-cells, progressive upregulation of CD4+ regulatory T-cells (which can suppress the injurious activity of Th cells) and regulatory dendritic cells, as well as the ongoing interaction of these alloreactive cells with hepatocytes and cholangiocytes in the allograft[78,79]. This reduced immune activity against the allograft is responsible for the decline in basal immune activity in those with more remote transplantation as opposed to those in the early post-transplant period, hence the importance of considering the time interval from LT in the use of ICIs.
Modification of immunosuppression in LT recipients undergoing treatment with ICI is somewhat unclear.Data suggests that immunosuppression could be reduced in LT patients prior to starting ICIs; as a robust Tcell response is required for successful activity of ICIs and less stringent immunosuppression could facilitate this[28,80]. Different approaches to achieving this goal have been documented. The use of prophylactic corticosteroids in a patient maintained on Tacrolimus and Everolimus, with close monitoring was reported in a case by Biondaniet al.[56]. This patient was managed successfully with no evidence of ACR or IRAEs.Systemic corticosteroids serve as a potent treatment for IRAEs or ACR, and this may explain the utility of their use[67]. De Toniet al.[57]reported a patient who was managed by progressive tapering of immunosuppression while being closely monitored on ICIs with no apparent adverse effects. A few studies showed improved survival in patients managed with ICIs and mTOR inhibitors. Compared to the CNIs, the mTOR inhibitors have been postulated as having significant anti-neoplastic, anti-angiogenetic and antiproliferative properties, related to the selective inhibition of protein synthesis required for cancer cell growth and proliferation, with the induction of G1 cell cycle arrest, promoting cancer cell apoptosis,decreased translation of DNA damage, as well as restoration of radiosensitivity in some radioresistant tumors[81,82]. It has been suggested that these properties may be responsible for the improved survival shown in these studies[83,84]. However, all these observations are based on case reports and small series, and there is no consensus recommendation for an immunosuppression strategy prior to initiating ICIs.
Interestingly, in a study reviewing liver biopsies of persons who were post-LT and on ICIs, Munkeret al.[70]demonstrated that liver biopsies with ACR had increased levels of PD-1 expression, whereas those without ACR did not have increased PD-1 expression. This suggested a relationship between PD-1 expression and risk of acute cellular rejection following treatment with ICIs that could be further studied to better implement treatment in these patients (as this study evaluated only seven samples)[70].
IMPORTANT CONSIDERATIONS FOR THE USE OF CHECKPOINT INHIBITORS AFTER LIVER TRANSPLANTATION
Although there are no societal recommendations on the strategies for the use of ICIs in the treatment of HCC in LT recipients, some guidance can be drawn from published studies. The timing of ICI use should be considered. Based on the limited data available, initiating ICIs should be approached with caution in the early years post-LT for HCC or other tumors[74,75,85]. However, the choice of agent or agent combinations should be guided primarily by the tumor type, as well as available data behind its safety, efficacy and response. Moreover, in the case of HCC, choosing ICIs should be considered after other systemic therapies have failed. Where possible, a liver biopsy should be performed prior to initiation of ICIs in post-transplant patients. Appropriate staining for, and measurement of, PD-1 expression can be performed. In this regard,overexpression of PD-1 may suggest an increased risk of rejection with PD-1 inhibitor use, and may therefore prompt consideration of anti-CTLA-4 therapy[70]. Limited data suggests that combination therapy with PD1/CTLA-4 inhibition can have lower rejection than monotherapy[86]. Moreover, anti-PD-1/PD-L1 monotherapy has been suggested to confer a higher risk of rejection than anti CTLA-4 monotherapy. This finding was suggested by an analysis of 12 post-LT recipients on ICI therapy with ACR occurring in 50% of the subjects on anti-PD1 therapy compared to none of those on anti-CTLA-4 therapy[87]. Also, a review of 34 published reports of ICI therapy in SOT recipients showed that 85% of the documented cases of ACR occurred in those on anti-PD1 therapy[88]. The putative explanation for this is the role of the PD1 pathway in the development of transplant immune tolerance based on its ability to alter the balance between pathogenic and regulatory T-cells[89]. It is important to note that another study of 28 LT recipients reported similar rates of ACR in both groups of subjects[90]. Nonetheless, most reports of post-transplant ICI use in the treatment of HCC are based on cases where anti PD-1 therapy was used (as it is preferred for HCC), making it difficult to assess if anti-PD-1 therapy (compared to anti-CTLA-4) has a higher risk of rejection, or if the findings are biased towards its higher use[67,86]. When starting therapy, the highest risk for graft rejection has been reported over the first 3 weeks of therapy, and close follow up should be implemented in this period[86]. The choice of immunosuppression in the LT recipient and the need for changes before commencing ICI are still debatable. At this time, there is no clear data to provide recommendations on if, and when to make changes,as data on corticosteroid pre-treatment or progressive tapering of immunosuppressive medications are limited[56,57]. Lastly, patient preferences should be considered during the selection of these therapeutic agents.Prior to initiating therapy with ICIs, all patients should be properly counseled and provided informed consent on the efficacy and risks of immune-related adverse effects, as well as the risk of acute cellular rejection and even potential graft failure. These discussions should ideally be carried out by both the treating oncologist as well as the transplant expert providing liver-related care.
FUTURE DIRECTIONS
Treatment of HCC with ICI in LT recipients is an area of oncology, hepatology and transplant medicine that is actively advancing. Immunotherapy continues to develop beyond the use of checkpoint inhibitors to include adoptive cell therapy - especially engineered T-cell receptor (TCR) and chimeric antigen receptor(CAR) T-cell therapy. CAR-T-cells have been extensively used in hematological malignancies mainly due to the lack of antigen heterogeneity in heme-derived cells. However, CAR-T therapy is being evaluated in solid tumors, and CAR-T cells directed towards glypican-3 could possibly eliminate glypican-3 positive HCC cells - this is a promising future intervention[91,92]. Another encouraging modality of adoptive cell therapy involves the use of HBV-specific TCR therapy. In a study by Tanet al.[93], it was shown that by utilizing the integrated short segments of HBV DNA in HCC cells, T-cells can be specifically engineered to recognize specific HBV epitopes, thus personalizing therapy. Following administration to two patients with metastatic HCC, one of the patients showed decreased size of most of his pulmonary metastasis[93]. The use of HBVspecific TCR therapy has therefore shown promise in the management of HBV-related HCC recurrence in the LT recipient[94,95]. The results of these adoptive cell therapies, though promising, need further research given the fine balance between optimal efficacy via robust T-cell activity and immunosuppression after LT[94]. The use of other potential approaches includes therapeutic vaccines against HCC tumor-associated antigens (such as glypican-3, alpha-feto protein), as well as the use of oncolytic viruses such as the orthoreovirus to modulate innate immune response[96-98]. More research into these novel methods is needed to determine the efficacy and safety of the LT recipient.
With the use of checkpoint inhibitors in the post-transplant population occurring more frequently, more randomized controlled trials evaluating its efficacy and safety in this specific population are necessary and essential. In this incredible era of precision medicine, further studies should lead to an understanding of ICIs in those on immunosuppressive medications, thus providing a framework for individually optimized therapy in the treatment of HCC in this population.
DECLARATIONS
Authors’ contributions
Conceptualization: Anugwom CM, Debes JD
Literature search: Anugwom CM, Leventhal TM, Debes JD
Writing - original draft preparation: Anugwom CM
Writing - review and editing: Anugwom CM, Leventhal TM, Debes JD
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
Robert Wood Johnson Foundation; AFMDP; University of Minnesota AIRP; EU Horizon2020 program,project number 825510; all to JDD.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.

