Experience of living donor liver transplantation for hepatocellular carcinoma in the University of Hong Kong Hospital
Janice Hoi Man Mok, Ka Wing Ma, Kenneth Siu Ho Chok
1Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
2Department of Surgery, The University of Hong Kong, Hong Kong, China.
Abstract
Keywords: Living donor liver transplantation, hepatocellular carcinoma, high volume centre, LDLT, HCC
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common fatal cancer in Hong Kong with a crude death rate of 21.9 per 100,000 population. Liver transplantation is often regarded as the best curative treatment for HCC with a 5-year overall survival rate of 89.9%[1]. The scarcity of cadaveric liver donation in Asia due to cultural and religious beliefs has undermined the possibility of deceased donor liver transplantation(DDLT)[2]. The deceased graft liver donation rate in Hong Kong is as low as 3.07 per 1,000,000 population,compared to 25.61 in the United States[3]. Since the first report of successful adult-to-adult living donor liver transplantation (LDLT) using extended right lobe graft from our centre in 1997[4], LDLT gained popularity in Hong Kong as well as in other Asian countries. It serves as the last hope of cure for cirrhotic HCC patients amid the shortage of deceased donors. By the end of 2018, our centre completed over 1400 liver transplants with overall 1-year, 3-year, and 5-year survival rates of 93.0%, 88.1%, and 85.7%, respectively[5].Among all the transplant cases, 231 LDLT for HCC patients were performed.
Studies demonstrated improved mortality and other long-term post-transplant outcomes from high volume centres[6,7]. In 2019, South Korea topped the world with 22.87 LDLT per 1,000,000 population, followed by Taiwan, the People’s Republic of China, and Japan[3]. Hong Kong ranked 6th in the world in terms of LDLT rates, with 2.67 LDLT per 1,000,000 population. As experience accumulated, there were continuous modifications of patient management. All these were translated into better treatment outcomes. The Liver Transplant Centre of Queen Mary Hospital, Hong Kong is one of the high-volume centres for LDLT in Asia that adopted the University of California, San Francisco (UCSF) criteria.
In this article, we will discuss the current practise of LDLT, including patient selection criteria, surgical techniques, management of small-for-size syndrome (SFSS) and postoperative complications, oncological outcomes in HCC patients at the high-volume centres, and results of our units.
METHODS
Surgical techniques
Extended right lobe graft and venous outflow regulation
Our centre established the use of the extended right lobe graft in LDLT since 1997[4]. The graft contains the whole right lobe with segments V, VI, VII, and VIII, the middle hepatic vein, and right inferior hepatic veins of size > 5 mm[8]. The procedure of extended right lobectomy is performed through bilateral subcostal incisions with an upward median extension to the xiphoid. Intraoperative ultrasonography is taken to determine configuration of the right, middle, and left hepatic veins. The correct transaction plane displays the longitudinal section of the middle hepatic vein together with the inferior vena cava. Hilar dissection is then performed to free the hepatic artery and right portal vein[9-18].
Hepatic venoplasty
The maintenance of venous outflow is important in healthy functioning of the liver. In contrast to whole liver graft from a deceased donor, hemi-graft used in LDLT carries a shorter hepatic vein caliber, that implies more difficult reconstruction, higher risk of angulation and poorer venous outflow[9]. While inclusion of the middle hepatic vein in extended right lobe graft improves transplant outcome, the reconstruction process of the inferior vena cava anastomosis is technically demanding. Precise adjustment of the length, orientation, and diameter of the anastomosis is required.
In 2000, our centre pioneered a hepatic venoplasty technique that minimizes the above technical difficulties[10]. Following donor hepatectomy, the inflow and outflow vessels of the graft are trimmed at the back table. A 5-mm transverse incision is made to create a depression between the middle and right hepatic veins. Adjacent walls of the vessels are then sutured using two 6-0 prolene stitches placed 1 cm apart. The combined outflow lumen is enlarged to form a single triangular cuff, with the right hepatic vein as the base,and middle hepatic vein as the apex of the triangle. The recipient undergoes total hepatectomy with the inferior vena cava preserved. The modified outflow vessels are then sutured onto a triangular incision on the recipient’s inferior vena cava using 5-0 prolene[11].
The novel technique using extended right lobe graft in LDLT is now widely applied in many centres. It eliminates the need for a sufficient length of the hepatic vein calibre for anastomosis, prevents hepatic venous blockage, and reduces outflow resistance[10].
Microvascular anastomosis
Nowadays, microvascular anastomosis has been adopted as a standard procedure in hepatic artery reconstruction[19]. At our center, this part of the transplantation is performed by the team of Plastic and Reconstructive Surgery. The operating microscope, microinstruments and microsutures using 9-0 prolene are applied in the procedure[11].
Hepatic artery segments from the donor and the recipient are clamped, with distal end blood clots removed by heparin saline irrigation. Two stay sutures are then placed at 0° and 160° with the subsequent stitches made from the lateral to the central part. Any discrepancy in the whole vessel circumference is carefully adjusted. Upon completion of the anastomosis, the vascular clamps are removed with an instant restoration of arterial blood flow. Doppler ultrasonography is applied to assess the efficacy of the anastomosis and the overall perfusion of the liver graft. Extra stitches may be added in instances of bleeding or separation of the vessel wall[11].
Statistical analysis
Graft and patient overall and disease-free survival at 1 year, 3 years, and 5 years after transplantation were the primary endpoints, and the secondary endpoints were complication rate. Data were collected prospectively and presented as the mean values and ranges, or the number of patients in proportion of total patient population.
RESULTS
By May 2020, 231 HCC patients received LDLT at our centre [Table 1]. There were more male than female recipients, at a ratio of 3.62:1. The recipients were predominantly Asian and had a mean age of 56 years(range: 3-73). Of the patients, 76.6% were tested positive for hepatitis B virus (HBV).
Of our patients, 74.9% met the UCSF criteria, and 64.5% met the Milan criteria [Table 2]. The majority of our LDLT recipients had a relatively low AFP level of 22 ug/L. However, there was a wide range (range: 2-117850 ug/L).

Table 1. Demographic data of recipients and donors undergoing living donor liver transplantation for hepatocellular carcinoma in Hong Kong
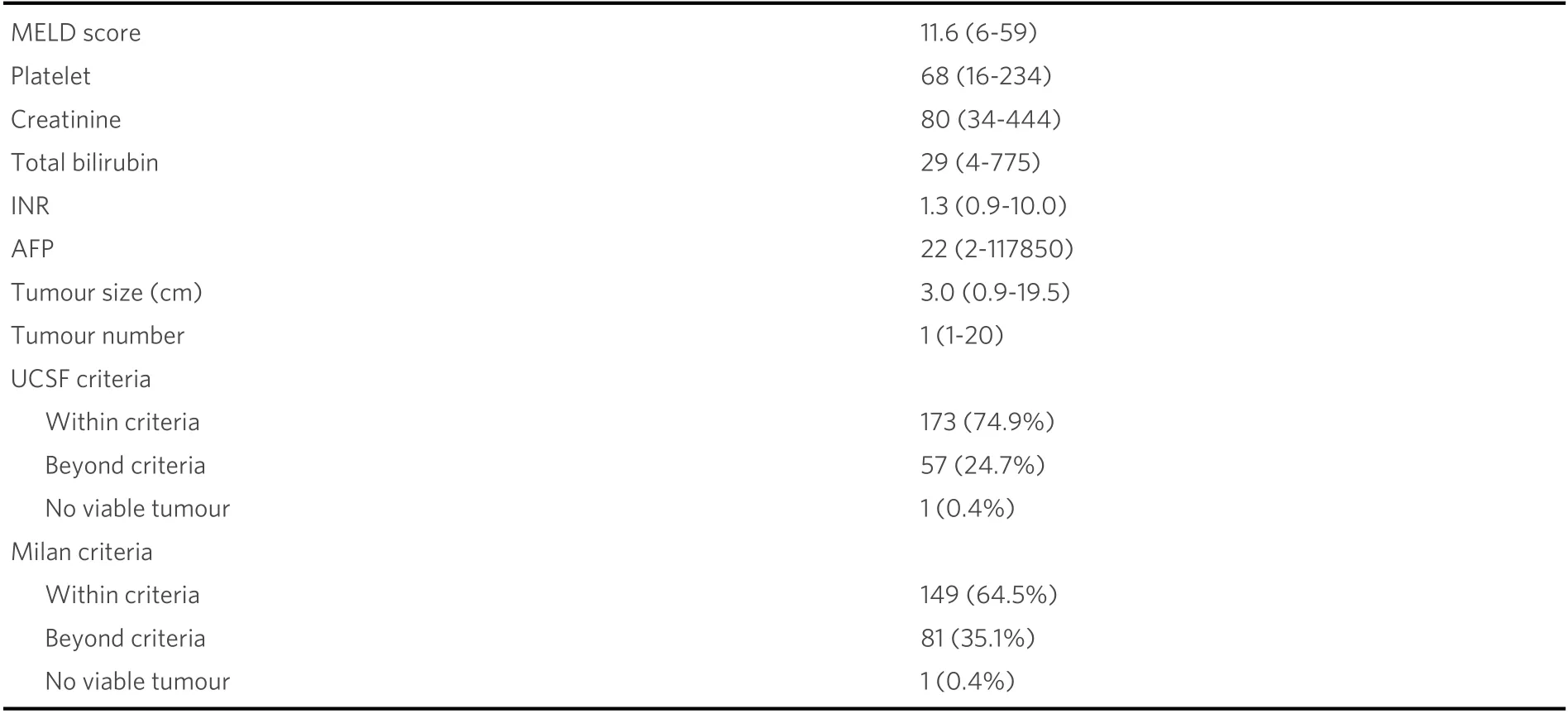
Table 2. Biological and explant pathological profile in recipients undergoing living donor liver transplantation
The standard procedure accounted for 91.8% of the operations and nearly all of the adult-to-adult transplants [Table 3]. Transplants for pediatric patients or recipients with smaller body size sometimesinvolve the use of a left lobe graft, which constituted 18 out of 231 cases. The median graft volume to standard liver volume was 42.8%, including 13 cases of small-for-size graft, which were placed under intensive post-transplant surveillance to reduce SFSS-related complications.

Table 3. Perioperative details of recipients undergoing living donor liver transplantation
Our centre attained a 1-year, 3-year, and 5-year overall survival rate of 96.0%, 84.7%, 78.9%, respectively.The 1-year, 3-year, and 5-year disease-free survival rates were 88.9%, 79.8%, and 76.3%, respectively[Table 4]. Significant disease-free and overall survival were demonstrated among transplant recipients within Milan criteria and the UCSF criteria [Figures 1-4]. Of the patients, 18.6% suffered HCC recurrence.The long-term graft survival rate at 1-year, 3-year, and 5-year follow-up were 93.4%, 83.0%, and 77.3%,while 9.1% of patients suffered from graft rejection and were subjected to conservative treatment and reoperations. Of the patients, 26.4% suffered from Grade III or above, and 5.2% suffered from Grade IV or above early postoperative complications according to the Clavien-Dindo Classification[20]. The most common ones include pleural effusion, intra-abdominal abscess, chest infection, and hepatic artery thrombosis (HAT). Late complications, including biliary stricture, portal vein thrombosis, and stenosis,were recorded. There had been two cases of hospital mortality, relating to acute myocardial infarction and to multiorgan failure from bronchopneumonia and necrotic liver graft.
DISCUSSION
The experience of LDLT for HCC in Hong Kong
Hong Kong is one of the endemic regions for HBV infection. Chronic HBV infection is the primary etiology of developing cirrhosis and HCC[21]. A large proportion of our patients tested positive for the virus. In such cases, LDLT is considered the best therapeutic option, given the shorter waiting time, better graft quality,and essentially no cold ischemic time.
Recipient and donor selection
Patients with HCC were chosen based on the pretransplant radiological tumour staging. Computed tomography (CT) of the abdomen was performed to delineate the tumour size and number. Morphological assessment of HCC based on the Milan criteria (one lesion ≤ 5 cm or two to three lesions ≤ 3 cm) has been the gold standard for patient selection[22]. However, the criteria are often considered too restrictive as tumour biology is only partially represented by tumour size and number. Our centre adopted the UCSFcriteria as the main selection criteria (solitary HCC ≤ 6.5 cm, or ≤ 3 nodules with the largest tumour ≤4.5 cm, and a total diameter ≤ 8 cm)[23,24]. It predicts better post-transplant survival and fewer complications[8]. The UCSF criteria act as a modest expansion on the Milan criteria that allow transplantation in 10% more HCC patients without compromising survival[22]. A multicentre study reported the 5-year survival rates of LDLT within the UCSF criteria to range between 66%-90%, with variations attributed to the transplant team experience and resource availability[25]. Selected patients with more advanced HCC underwent radionucleotide bone scan and positron emitting tomography using a C-11 acetate tracer. Patients with evidence of diffuse HCC, major vascular invasion or extrahepatic metastasis were then excluded.

Table 4. Outcome of living donor liver transplantation for hepatocellular carcinoma patients in Hong Kong

Figure 1. Kaplan-Meier patient disease-free survival after living donor liver transplantation within and beyond Milan criteria (P = 0.008).

Figure 2. Kaplan-Meier patient disease-free survival after living donor liver transplantation within and beyond University of California,San Francisco (UCSF) criteria (P < 0.001).
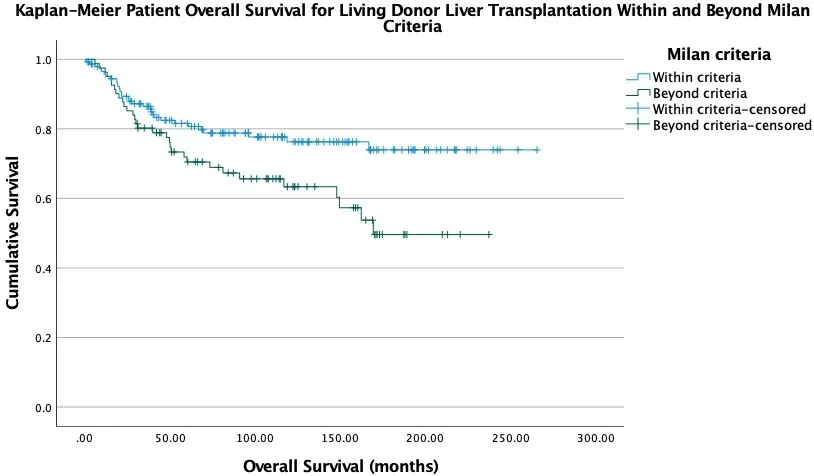
Figure 3. Kaplan-Meier patient overall survival after living donor liver transplantation within and beyond Milan criteria (P = 0.018).

Figure 4. Kaplan-Meier patient overall survival after living donor liver transplantation within and beyond University of California, San Francisco (UCSF) criteria (P < 0.001).
Donor voluntarism provided flexibility in recipient selection. Patients slightly over the UCSF criteria were still accepted, given a favourable biological tumour profile. AFP serves as an indicator of the patients’ liver function. Its role in predicting post-transplant HCC recurrence has been widely supported[26-28]. With available donors, the patients experienced shorter waiting time on the list and were prevented from further deterioration of liver function and disease progression[25].
In terms of donor selection, donor voluntarism serves as the prerequisite before undertaking further assessments for the suitability of donors. Clinical psychologists assess donors in the absence of the recipients and other parties for their voluntarism in liver graft donation. Subsequently, donors are evaluated for ABO blood group compatibility, negative HBV and hepatitis C virus (HCV) serology, and any acute or chronic illness. Donors undergo CT to assess for adequate CT volumetry of the liver graft for the recipient and liver remnant for the donor.
The process of the recipient and donor surgery has been discussed. The use of the extended right lobe graft with the inclusion of the middle hepatic vein provided a larger liver volume and minimized the risk of developing SFSS[15].
Factors associated with HCC recurrence
Tumour recurrence remained one of the significant factors that compromise recipients’ survival[25].
Various factors related to tumour recurrence have been identified. Todoet al.[29]pinpointed elevated preoperative AFP level, tumour size, vascular invasion, and bilobar distribution as independent risk factors for recurrence after LDLT. Our centre’s experience echoed the findings above and demonstrated significantly worse overall and disease-free survival when the Milan criteria and the UCSF criteria were not met[30].
There are ongoing discussions about the survival benefits of primary and salvage liver transplantation (LT).Bhanguiet al.[31]reported a higher incidence of non-transplantable recurrence and lower survival rates in the group receiving salvage LT than primary LT. Hepatic resection followed by salvage LT is performed under the local context of substantial organ shortage. While primary LT may not always be possible, this approach gives patients a possible chance of cure.
Close surveillance is essential for the timely detection of recurrence at a transplantable stage. Patients are follow-up every three months with clinical examination, measurement of AFP level, and CT scan of the abdomen and thorax. Liver biopsy is carried out in cases of suspected recurrence.
Small for size graft and portal venous flow modulation
With donor safety being one of the most important considerations in LDLT, many centres embrace the use of left lobe graft, which reduced the risk to the donors by five times (0.1% with left lobe graft compared to 0.5% with right lobe graft)[12]. Despite that, left lobe grafts are frequently small-for-size graft (graft-torecipient weight ratio < 0.8, or graft volume to standard liver volume < 30%)[13,14]which are associated with the development of small-for-size syndrome (SFSS) in transplant recipients. It may further aggravate into early graft failure due to insufficient liver volume for meeting the metabolic demand of a larger recipient[8].Compared to the left lobe graft, the extended right lobe graft constitutes a higher liver volume[15]and reduces the chance of SFSS.
The development of SFSS is subject to interrelating factors of the graft size, graft quality, portal inflow, and venous outflow[8]. A previous study suggested compromised recipient outcome with graft volume to standard liver volume < 35%[16], modulation of portal venous flow becomes an important technique to minimize the chance of SFSS.
The regulation of flow and portal venous pressure should always be treated with caution. Hyperperfusion,common in the use of small-for-size graft or left lobe graft in LDLT, increases sinusoidal pressure and damages liver endothelium[17]. It is demonstrated that raised portal pressure in the early postoperative phase is linked to poorer recipient survival with small-for-size grafts[18]. At our centre, portal vein flow rate is routinely measured with a flowmeter. We proposed splenic artery ligation for portal inflow >250 mL/min/100 g of graft weight. The subsequent cut in flow from the splenic vein lowers portal vein pressure back to normal[8]. Portocaval shunt and mesocaval shunt are also valid in reducing portal pressure.However, potential complications associated with porto-systemic shunting remains a concern. It also adds difficulty to the second laparotomy, if needed[8]. In case of portal vein hypoperfusion after implantation,which is commonly seen in very cirrhotic patients, pre-existing porto-systemic shunts are identified and ligated to increase the portal venous flow. Effect and magnitude of portal vein flow modulation is reflected by the change in portal venous manometric assessment. With the above techniques, an overall graft and patient survival rate of 84% and 96%, respectively, were achieved[15].
Post-transplant complication and surveillance
HAT is a serious post-transplant complication associated with early graft loss and increased recipient mortality. It remained the most common arterial complication before introduction of the microvascular techniques. Compared to DDLT, the use of a hemi-graft that involves only the left or right hepatic artery in LDLT is also associated with a higher risk of HAT[11]. The application of microsurgery successfully reduced its incidence from 25%[32]to 1.6%-3.8%[33-37].
The highest standard of postoperative care is offered to the recipients of LDLT. The donors are administered to the intensive care unit with strict monitoring of liver function. Fluid restriction is crucial in preserving the post-LT liver function. It helps regulate central venous pressure that ensures a sufficient volume of venous return to the liver remnant. The central venous catheter and urinary catheter are removed as soon as possible to reduce the risk of infection. Breathing exercises are recommended that ensure a speedy recovery[11]. Infusion of insulin and albumin solution allows control of blood glucose level and compensates for the impairment in coagulative function. Infection is one of the common post-LT complications. Prophylactic antibiotics will be continued postoperatively. Frequent monitoring of hepatic artery blood flow using bedside doppler ultrasonography helps detect and salvage early case of HAT[36].Haemodialysis may be required in cases that present with transient renal function impairment. Early resumption of enteral feeding yields better patient outcomes[11].
Postoperative anti-viral and immunosuppressive treatment
Adequate immunosuppressive and antimicrobial treatment after LDLT reduces the chance of graft loss and HCC recurrence.
Our centre has adopted a quadruple immunosuppression regime for all transplant recipients since 2001. It involves induction with basiliximab, an interlukin-2 receptor antibody, and two perioperative injections of steroids. And for postoperative maintenance, mycophenolate mofetil and tacrolimus are given.
In the past, cyclosporin A was used as the first-line immunosuppressive therapy for post-LT recipients. The use of the more potent calcineurin inhibitor, tacrolimus, is associated with superior survival and rejection rates[38]. However, its application is limited by the concerns on the drug’s nephrotoxicity and neurotoxicity[39]. More importantly, studies revealed that tacrolimus is potentially oncogenic, predisposing patients to HCC recurrence[40,41].
In recent years, the mammalian target of rapamycin (mTOR) inhibitors is used as a calcineurin inhibitorsparing agent with anti-tumour properties[42]. Compared with tacrolimus, the application of sirolimus and everolimus minimize the risk of renal impairment[43]. A meta-analysis study demonstrated prolonged overall recipient survival and attenuated tumour recurrence with the use of mTOR inhibitors[44]. Despite concerns on the potential association of sirolimus usage and the increased risk of HAT, there is no proof of the prothrombotic effect by the drug. Two separate studies revealed a reduced incidence of HAT in the treatment group, compared to the control group using corticosteroids[45,46]. In our centre, we adopted an early use of mTOR inhibitor (i.e., 3 months after liver transplantation) together with low maintenance dose of calcineurin inhibitor in patients with high risk of HCC recurrence.
In Hong Kong, HBV-related cirrhosis is the primary cause of HCC, which accounts for a large proportion of all transplant cases. Antiviral agents are commonly applied in post-LT treatment, which reduces the chance of graft loss, viral hepatitis, and HCC recurrence[47,48]. In recent years, entecavir monotherapy has replaced lamivudine and hepatitis B immunoglobulin as the standard antiviral therapy[49]. The antiviral agent is now used at our centre, which gives an excellent long-term overall survival rate of 85%. Its high degree of viral suppression also reduces HBV-related complications[50].
Future directions for LDLT
Laparoscopic and robotic surgery
Our centre practised laparoscopic and robotic hepatectomy surgeries for selected cases. The technique is one of the areas for future advancement with expected extensive application in the practise of LDLT.
Minimally invasive surgery has been reported as a safe and effective approach in the management of liver diseases. Its application expanded since the first laparoscopic cholecystectomy published in 1992[51]. Since then, numerous reviews and meta-analyses validated the benefits of laparoscopic procedures, such as reduced blood loss, shorter hospital stay, fewer postoperative complications, and a similar oncological outcome as in open surgery[51-58]. Many experienced centres now adopt the adult-to-child laparoscopic living donor left lateral sectionectomy[59]. Application of the living donor right hepatectomy remains restricted due to safety concerns in minimally invasive donor hepatectomy[60].
Kimet al.[61]’s report emphasized strict selection criteria based on vascular and biliary structure. Donors with single and more extended right hepatic artery, portal vein, hepatic duct, and favourable hepatic vein anatomy were selected, excluding graft of over 700 g. Laparoscopic hepatectomy still follows surgical techniques[62]in open surgery, including use of the Cavitron ultrasonic surgical aspirator, hanging manoeuvre, and Pringle’s manoeuvre[63,64]. Complete liver mobilization before resection allows better manipulation of the transaction plane. Intraoperative indocyanine green cholangiography guides precise bile duct division and improves patient safety[65].
Robotic-assisted (RA) approaches in liver resection are documented as safe and feasible. Better visualization of the surgical field and improved range of motion are now possible under the robotic system[66]. With the rubber band retraction technique and the Da Vinci Fluorescence imaging vision system, RA provides a clear segmental boundary of the liver parenchyma. It allows a more careful dissection of the hepatic hilum and inferior vena cava[67-69]. In donor hepatectomy, the robotic system closes the hepatic duct stump with a running suture, reducing the risk of donor biliary strictures, especially when shorter bile ducts are used[70].
Series reports by Chenet al.[2]quoted comparable short-term outcomes, vascular complications, and biliary complications in RA, compared to open surgery. Studies indicated a similar intraoperative blood loss and warm ischaemic time with reduction in analgesia, and shorter return to work[71]. However, one should note the protracted learning curve in laparoscopic and robotic hepatectomy. Accumulation of experience is crucial in the success in RA liver transplantation[72,73].
Tumour markers
Tumour markers provide a supplementary evaluation of the disease outcome and recurrence. Specific markers, such as AFP, have been added to the patient selection criteria for LDLT at many centres[21,74]. Its effectiveness in the prediction of tumour aggressiveness and post-transplant tumour recurrence has been validated. Protein induced by vitamin K absence-II (PIVKA-II) is a biomarker developed for the diagnosis of HCC. The marker itself promotes cellular proliferation and migration, as well as induces the expression of angiogenetic factors. It is, therefore, predictive of the tumour aggressiveness and post-LT outcome[75,76].Studies claimed that PIVKA-II has the potential to detect HCC early with improved sensitivity and specificity[77,78]. Some centres support the combination of AFP and PIVKA-II in predicting tumour recurrence[79].
AFP mRNA aims to predict post-LT recurrence by detecting residual cancer cells in the circulating blood[80]. AFP-L3% is a fucosylated form of AFP, predicting HCC recurrence and prognosis following local ablation and hepatectomy[81]. The process of tumour necrosis and angiogenesis release systemic inflammatory markers. Neutrophil-to-lymphocyte ratio is applied in patient selection for DDLT and LDLT in adjunct to the model for end-stage liver disease (MELD) score and the Milan criteria[82]. An increased level of neutrophil-to-lymphocyte ratio (> 5) predicts lower recurrence-free survival and overall survival in individual HCC patients[83].
Many centres now propose a combined use of morphological criteria and tumour markers[27,74,84]. With evolving evidence on the predictive power of individual biomarkers, it seems rational to combine the prognostic ability of different markers for a more accurate prediction of the transplant outcome. For example, the BALAD staging score, developed by Toyodaet al.[85], uses a combination of AFP (>400 ng/mL), AFP-L3 (> 15%), and DCP (> 100 mAU/mL), which showed reduced survival per level of increment in the markers (P< 0.0001).
LDLT is an ideal treatment for HCC in Hong Kong with regard to the critical organ shortage and high demand for transplantation. Our centre practises careful selection for HCC patients using the UCSF criteria,supplemented by AFP level and the MELD score. Slight flexibility is offered to enthusiastic donors and recipients in LDLT while balancing the risks and benefits. We pioneered in using the extended right lobe graft and the novel hepatic venoplasty technique, which lessen the risk of hyperperfusion and SFSS with improved overall recipient survival. A 5-year overall and disease-free survival rate of 78.9% and 76.3%,respectively, were achieved.
DECLARATIONS
Authors’ contributions
Contributed to study design, manuscript writing, and data analysis: Mok JHM, Ma KW Supervised the research project: Chok KSH
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.

