Acetyl-11-keto-beta-boswellic acid promotes sciatic nerve repair after injury:molecular mechanism
Yao Wang,Zong-Liang Xiong,Xiang-Lin Ma,Chong Zhou,Mo-Han Huo,Xiao-Wen Jiang,,Wen-Hui Yu,
Abstract Previous studies showed that acetyl-11-keto-beta-boswellic acid (AKBA),the active ingredient in the natural Chinese medicine Boswellia,can stimulate sciatic nerve injury repair via promoting Schwann cell proliferation.However,the underlying molecular mechanism remains poorly understood.In this study,we performed genomic sequencing in a rat model of sciatic nerve crush injury after gastric AKBA administration for 30 days.We found that the phagosome pathway was related to AKBA treatment,and brain-derived neurotrophic factor expression in the neurotrophic factor signaling pathway was also highly up-regulated.We further investigated gene and protein expression changes in the phagosome pathway and neurotrophic factor signaling pathway.Myeloperoxidase expression in the phagosome pathway was markedly decreased,and brain-derived neurotrophic factor,nerve growth factor,and nerve growth factor receptor expression levels in the neurotrophic factor signaling pathway were greatly increased.Additionally,expression levels of the inflammatory factors CD68,interleukin-1β,prointerleukin-1β,and tumor necrosis factor-α were also decreased.Myelin basic protein-and β3-tubulin-positive expression as well as the axon diameter-to-total nerve diameter ratio in the injured sciatic nerve were also increased.These findings suggest that,at the molecular level,AKBA can increase neurotrophic factor expression through inhibiting myeloperoxidase expression and reducing inflammatory reactions,which could promote myelin sheath and axon regeneration in the injured sciatic nerve.
Key Words:AKBA;axon;genomics;inflammatory;injury and repair;myelin sheath;myeloperoxidase;neurotrophic factor;peripheral nerve;phagosome pathway;regeneration;Sprague-Dawley rat

Graphical Abstract Acetyl-11-keto-beta-boswellic acid (AKBA) promotes sciatic nerve repair after injury
Introduction
Peripheral nerve injury (PNI) is caused by mechanical,physical,chemical,or biological factors,and it results in partial or complete peripheral nerve loss,motor function impairment,and neuropathic pain (Vijayavenkataraman,2020).A series of pathological changes occurs after PNI.Proximal neurons and ganglia may change in addition to the damaged part and its distal area.The inflammatory response to phagocyte recruitment occurs first after PNI.A phagosome is formed when the specific receptors on the phagocyte surface recognize ligands on the particle surface.The main participant in this process is myeloperoxidase (MPO).Changes in MPO levels and activity represent the function and activity status of neutrophil polymorphonuclear leukocytes(Volkman et al.,2019).MPO also indicates the level of inflammation(Wendland et al.,2010).Additionally,MPO reduction was shown to effectively alleviate peripheral nerve diseases (Eastman et al.,2020),protect peripheral nerve cells,and reduce oxidative stress (Wang et al.,2020).Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) play an important role in PNI by promoting and maintaining nerve cell growth,survival,differentiation,and executive function (Liu et al.,2022a).The NGF receptor (NGFR) can bind to NGF,BDNF,neurotrophin-3,and neurotrophin-4 using low-affinity receptors,which can mediate the survival of nerve cells (Ryu et al.,2018) and the survival,differentiation,growth,and apoptosis of Schwann cells that play a key role in nerve repair (Jung et al.,2016).Myelin basic protein (MBP) is an indicator that reflects the amount of myelin (Liu et al.,2019a),and neuronal class III β-tubulin (β3-tubulin) can reflect the presence of immature neuron cell bodies,dendrites,axons,and axon ends,which thereby indicates the drug’s capability to regenerate axons (Li et al.,2020).
Acetyl-11-keto-beta-boswellic acid (AKBA) is a natural small-molecule compound that is extracted from the natural Chinese medicine Boswellia.AKBA is widely used to inhibit inflammation (Abdel-Tawab et al.,2011;Meka et al.,2017;Khan et al.,2019) and the nuclear factor-kappa B signaling pathway (Takada et al.,2006;Ranzato et al.,2017).Its therapeutic effect on nerves can promote nerve damage repair by reducing oxidative stress(Sadeghnia et al.,2017) and protecting ischemic neurons (Ding et al.,2014).Our team members’ previous results showed that Boswellia serrata extract and AKBA can promote the repair of rat sciatic nerve injury by regulating Schwann cells and the extracellular regulated protein kinases signaling pathway (Jiang et al.,2016,2018,2020).However,peripheral nerve regeneration is a complex pathophysiological process that involves numerous changes at different levels,from molecules and cells to biological organisms,and it is affected by many factors.Therefore,we used high-throughput genomics and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to explore the mechanisms associated with the repair of sciatic nerve damage and to elucidate the mechanisms related to AKBA that promote peripheral nerve damage and repair.In this article,we show that AKBA acts on the repair of sciatic nerve injury mainly through the phagosome pathway and that the phagosome is closely related to anti-inflammation,thus further demonstrating the anti-inflammatory effect of AKBA;and we also discuss the ability of AKBA to promote myelin and axons by increasing the neurotrophic factor expression level.This study could represent a new area or a new type of investigation using AKBA and traditional Chinese medicine.
Materials and Methods
Animals
Because estrogen in female rats may affect test results (Acosta et al.,2017),we uniformly used male rats to avoid affecting the test result accuracy.Fifty-five male Sprague-Dawley rats (provided by Harbin Medical University,China;license No.SCXK (Hei) 2017-0001) weighing between 150 and 180 g(approximately 2 months old) were allowed to acclimate to our animal facility for 7 days.Five rats were placed into each cage,and they were housed in temperature-controlled (24 ± 3°C) and light-controlled (12-hour light/dark cycle;lights on at 7:00 a.m.) rooms with standard rodent chow and water availablead libitum.The experiment was conducted in compliance with the requirements of the National Research Council Guide (1996) and Ethical and Animal Welfare Committee of Heilongjiang Province,China (revised 2016).All experiments were designed and reported in accordance with the Animal Research:Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).The study was approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (approval No.NEAU-[2018]-009,approval date:January 5,2018).
Sciatic nerve injury model
Ten rats were randomly selected as the blank (control) group (without any surgical treatment).The remaining 45 rats were randomly and equally divided into the following three groups:Sham group (exposed nerves only);Model group (exposed nerves and a crush injury);and AKBA group (exposed nerves,crush injury,and AKBA).
The rats were anesthetized via an intraperitoneal injection of Zoletil 50(zolazepam+tiletamine) (30 mg/kg;Virbac,Nice,France).Each rat’s right sciatic nerve was exposed surgically and in the Model and AKBA groups,the nerve was squeezed with vascular forceps (Shanghai Medical Devices (Group)Co.,Ltd.,Shanghai,China) to the second button to provide the same force and held for 10 seconds to create the sciatic nerve injury model (Jiang et al.,2018;Remacle et al.,2018).Each nerve was marked with sutures near the distal end of the clamp,and the wound was closed layer by layer using sutures.In the Sham group,only nerve exposure was performed without inflicting nerve damage.
Water and food were withheld for 24 hours after the model was created.Rats in the AKBA group received AKBA intragastrically (6 mg/kg;Shanghai Yuanye Biological Co.,Ltd.,Shanghai,China;dissolved in 1 mL normal saline) every 2 days for 30 days beginning on day 1 after surgery (Jiang et al.,2018).Each AKBA administration time was the same,and the Sham and Model group rats received 1 mL normal saline.The study timeline is shown inFigure 1.
Genomics detection in sciatic nerve
On day 30,the rats were anesthetized via intraperitoneal injection of Zoletil 50 (30 mg/kg) and sacrificed by cervical dislocation.To investigate the mechanism of AKBA in sciatic nerve injury repair,rats in the AKBA and Model groups underwent genomic analysis.Rats in the Sham group also underwent genomic analysis to exclude the effect of surgery.Sciatic nerves(n=5/group) in the three groups were obtained surgically.After the samples were quick-frozen in liquid nitrogen,the genome was sequenced by Tianhao Biotechnology Co.,Ltd.(Shanghai,China).Briefly,Trizol (Thermo Fisher,Waltham,MA,USA) was used for total RNA extraction.Invitrogen Qubit 3.0 Spectrophotometer (Thermo Fisher) was used to assess the accuracy of RNA detection.A Nanodrop 2000 (Thermo Fisher) was used to detect the RNA concentration,and an Agilent 2100 Bioanalyzer (Agilent Technologies,Santa Clara,CA,USA) was used to detect the degree of RNA degradation.The total RNA library was prepared in accordance with the TruSeq Stranded Total RNA Library Prep Kit instructions (Illumina,San Diego,CA,USA).The library was purified using the Agencourt SPRI select Nucleic Acid Fragment Screening kit(Beckman Coulter,Carlsbad,CA,USA).The library was sequenced using the high-throughput sequencing platform (Illumina) with a 2× 150 bp paired-end sequencing strategy.
The quality of raw sequencing data was evaluated using Fast QC software(Babraham Institute,Cambridge,UK) and R software (Free Software Foundation,Boston,MA,USA).The original sequence contained some lowquality reads with connectors.The raw reads were filtered using the Trim Galore method (Liu et al.,2017) (https://ccb.jhu.edu/software/hisat2/index.shtml) to obtain clean reads for subsequent analysis and to ensure the quality of information analysis.The clean reads obtained after filtering were compared with the reference database annotations (the Rno6 version of the rat genome was selected) using HISAT2 software (https://ccb.jhu.edu/software/hisat2/index.shtml).Differentially expressed genes were screened using Cuffdiff software (University of Washington,Washington,WA,USA),and two criteria were used for screening differentially expressed genes.Specifically,a gene was considered to be differentially expressed whenP<0.05 and |log2(fold change)| >1,where log2(fold change) >1 was marked as an upregulated gene and log2(fold change) <-1 was marked as a downregulated gene.
Real-time polymerase chain reaction
Total RNA from the sciatic nerve in four groups (Blank,Sham,Model,and AKBA groups;n=3/group) on day 30 after injury was extracted as previously described.Briefly,the extracted RNA can be used for reverse transcription at the ratio ofA260/A280between 1.8 and 2.0 as detected by NanoDrop 2000(Thermo Fisher).Moreover,cDNA was synthesized using the HiScript III RT SuperMix for real-time polymerase chain reaction (RT-qPCR) (+gDNA wiper)kit (Novizan Biotechnology Co.,Ltd.,Nanjing,China).The target mRNA primers are shown inTable 1.The test was conducted in accordance with the PCR strategy that was provided in the 2× SYBR Green RT-qPCR Master Mix (Bimake,Houston,TX,USA) dye manual.RT-qPCR was performed using a Light Cycler@480 System (Roche,Basel,Switzerland).PCR reaction conditions are shown inTable 2.The relative mRNA level was calculated using the 2-∆∆Ctmethod (Qianru et al.,2021).Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous control for standardization.
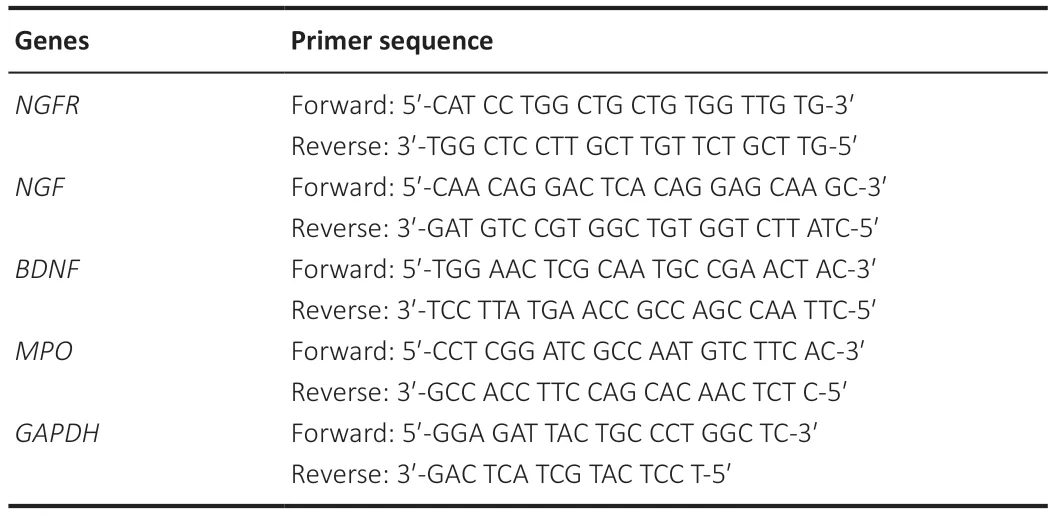
Table 1|Primer sequence list

Table 2|Polymerase chain reaction conditions
Western blot assay
To verify the genomics results,we performed western blot tests on MPO,BDNF,NGFR,and other relative proteins (NGF,interleukin-1β,pro-interleukin-1β,and tumor necrosis factor-α).The injured nerves in the four groups (n=3/group) after 30 days were subjected to total protein extraction in accordance with Hu et al.’s method (Hu et al.,2019).Subsequently,10% sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed,and the sample was then transferred to a polyvinylidene difluoride membrane.The polyvinylidene difluoride membrane was blocked with 5% skimmed milk(Biosharp,Hefei,China) at room temperature for 2 hours and then placed into a solution with diluted primary antibody.The primary antibody information is shown inTable 3.The sample was incubated overnight in a refrigerator at 4°C.Then,the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:5000,Bioss,Beijing,China,Cat# bs-0295G-HRP,RRID:AB_10923693) at 37°C for 2 hours.Image acquisition was performed using the Tanon 5200 gel imaging system (Tanon Bio.,Shanghai,China).Image analysis was performed using ImageJ software v1.53c (Schneider et al.,2012),and relative protein expression was presented as the optical density (OD) ratio of the target protein to glyceraldehyde-3-phosphate dehydrogenase/β-actin.
Table 3|Primary antibodies
Immunohistochemical staining
Nerve crush injuries are accompanied by damage to the myelin sheaths and neurons (Gordon,2020),so myelin basic protein as well as β3-tubulin was detected.Nerve samples from the four groups (n=3/group) after 30 days were fixed in 4% polymethanol and then sectioned in paraffin.The paraffin sections were deparaffinized and washed with distilled water (Jia et al.,2021).Then,the tissue sections were placed in a repair box filled with citric acid antigen retrieval buffer antigen retrieval solution (pH 6.0;G1202,Servicebio,Wuhan,China) in a microwave oven to retrieve the antigens followed by incubation with goat serum for 30 minutes.Then,the samples were then incubated with primary antibody (rabbit anti-MBP antibody,1:100,Servicebio,Cat# GB11226,RRID:AB 2 895014;rabbit anti-β3-tubulin antibody,1:100,Servicebio,Cat# GB11139,RRID:AB_2895013) in a humidified incubator overnight at 4°C.After washing,the slices were dried slightly and incubated with secondary antibody (1:200,horseradish peroxidase-conjugated goat anti-rabbit,Servicebio,Cat# GB23303,RRID:AB2811189) for 50 minutes at roomtemperature.The nucleus was counterstained using 3,3′-diaminobenzidine tertrahydrochloride (Servicebio,G1211) and rinsed with running water.After dehydration and mounting,all images were quantified using Image-pro plus 6.0 (Schneider et al.,2012).Each specimen was randomly selected from five 200-fold visual fields for observation,and the average number of positive cells in the specimen was calculated (Liu et al.,2019b).
Silver-plated staining
The nerve samples from the four groups (n=3/group) after 30 days were fixed in 4% polymethanol and then sectioned in paraffin.The paraffin sections were first dewaxed and washed with tap water,and then stained with acid formaldehyde (Okada et al.,2010).Reducing solutions I and II were then added,and the reduction effect was constantly observed.The slices were quickly removed after a few seconds,washed with distilled water,quickly treated with 5% sodium thiosulfate,and then washed with distilled water.Finally,the sections were dehydrated in gradient absolute ethanol,made transparent with xylene,mounted on slides using neutral gum,and then observed under a microscope (Beijing Ou Bo Tong Optical Technology Co.,Ltd.,Beijing,China) followed by image acquisition.
Ultrastructure observation
After 30 days,the nerve tissue 1 cm distal to the injury site in the Blank,Sham,Model,and AKBA groups was collected,cut on ice into 1 cm cubes using a blade,and fixed in 2.5% glutaraldehyde.The tissue was placed into a refrigerator at 4°C and fixed for 7 days (Deng et al.,2021;Mu et al.,2021).The fixed samples were rinsed with 0.1 M phosphate buffer,fixed with 1%osmium acid,and rinsed again with 0.1 M phosphate buffer.After dehydration and soaking,the samples were embedded,polymerized,trimmed,sliced with a thin microtome (50-60 nm),and double-stained using uranyl acetate-lead citrate (Servicebio).The slices were then observed under a projection electron microscope (HT7700,Hitachi,Tokyo,Japan).The G-ratio was calculated as follows:axon diameter/total diameter,where the diameter=(area/π)1/2.The myelin thickness was calculated as follows:total diameter − axon diameter.
Immunofluorescence
Nerve samples from the four groups (n=3/group) after 30 days were fixed in 4% polymethanol and then sectioned in paraffin.The paraffin sections were deparaffinized and washed with water.Then,the tissue sections were placed into a repair box filled with ethylene diamine tetraacetic acid antigen retrieval solution (pH8.0,G1206,Servicebio) in a microwave oven for antigen retrieval.After cooling at room temperature,the slides were placed in PBS (pH 7.4)and washed three times while shaking on a decolorizing shaker for 5 minutes each time.After the slices were dried,the tissue was incubated with bovine albumin (Servicebio) for 30 minutes.Then,the samples were treated with rabbit anti-CD68 (a marker of inflammatory cells,1:200,Bioss,Cat# bs-0649R,RRID:AB_10857979) in a humidified incubator overnight at 4°C.After the slices were washed and dried,they were incubated with Cy3-conjugated with goat anti-rabbit IgG (1:300,Servicebio,Cat# GB21303,RRID:AB_2861435) at room temperature in the dark for 50 minutes.The nuclei were counterstained using 4′,6-diamidino-2-phenylindole.Images were taken using an upright fluorescence microscope (Nikon Eclipse C1,Tokyo,Japan).The results were analyzed using ImageJ software in accordance with Qianru’s method (2021).Briefly,all slices were taken using the same exposure setting,and all images were quantified using ImageJ software.Each specimen was randomly selected from five 200-fold visual fields for observation and the average number of positive cells in the specimen was calculated.
Behavioral analysis
Behavioral analysis was performed at 10,20,and 30 days (n=6/group) after successfully creating the model.
Gait analysis
The sciatic function index (SFI) was measured to evaluate motor function recovery in animals in all four groups after nerve injury.The rats were pretrained before the test so that they could pass through the gait recording device (self-made).A black nontoxic dye was applied to the soles of the animals’ hind feet,and white paper was placed at the bottom of the walking path to record the rats’ footprints.Then,the animals passed through the gait path,and their footprints were recorded.Five complete and clear footprints were required from each experimental animal to complete the SFI calculation(González Porto et al.,2019).
SFI=[-38.3×(EPL -NPL)/NPL]+[109.5×(ETS -NTS)/NTS]+[13.3×(EIT -NIT)/NIT]-8.8,
where PL is the distance between the heel and the third fingertip,TS is the distance between the first toe and the fifth finger,IT is the distance between the second toe and the fourth toe,E is experimental side or sciatic nerve crush side,and N is the normal side.
Sensory function test
A toe pinch test was performed to evaluate sensory function recovery (pain score).The third,fourth,and fifth toes were clamped with fine forceps.The grade of the avoidance reflex,escape behavior,and vocalization (grade 0:normal,brisk;1:mildly damaged;2:moderately damaged;3:completely damaged) (Okada et al.,2010) were observed by a blinded evaluator to check the test reliability.
Statistical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes are similar to those reported in a previous publication (Liu et al.,2022b).The statistical analysis of all data was performed using SPSS version 22.0 (IBM Corp.,Armonk,NY,USA) and graphed using GraphPad Prism software version 8.0 (GraphPad Software,San Diego,CA,USA,www.graphpad.com).All data are expressed as the mean ± standard deviation (SD).A oneway analysis of variance was used to test for differences between the data in each group using the least significant difference and Duncan method of multiple comparisons.P<0.05 was considered to be a significant difference.
Results
Significantly differentially expressed genes in the injured rat sciatic nerve after AKBA treatment
Differential gene expression analysis showed that 258 genes were significantly differentially expressed at the mRNA level in the AKBA group compared with the genes in the Model group (Figure 2A1).These genes included 189 upregulated genes and 69 downregulated genes.Three hundred fifty-five genes,including 194 upregulated genes and 161 downregulated genes,were significantly differentially expressed at the mRNA level in the AKBA group compared with the genes in the Sham group (Figure 2A2).Four hundred ten genes,among which 171 were upregulated and 239 were downregulated,were significantly differentially expressed between the Sham and Model groups (Figure 2A3).
Enrichment analysis of differentially expressed genes in the injured rat sciatic nerve after AKBA treatment
KEGG results showed that significantly differentially expressed genes in the AKBA and Model groups were enriched in 193 pathways.The ten pathways with the highest enrichment included the phagosome pathways (Figure 2B1).The following ten genes were involved in the phagosome pathway:Tcirg1,Cybb,MPO,Thbs2,Cd209f,Stx7,RT1-M2,C3,LOC100363064,andRGD1564571(Figure 2B2).Among these genes,MPO,Cd209f,LOC100363064,andRGD1564571were downregulated,and the rest were upregulated in the AKBA group compared with the genes in the Model group(Figure 2B3).In addition to the ten most enriched items,the neurotrophic factor signaling pathway was also identified,in which BDNF was significantly upregulated (Figure 2B4) in the AKBA group compared with that in the Model group.The ten pathways with the highest enrichment in the AKBA and Sham groups are shown inFigure 2B5.The neurotrophic factor signaling pathway,in which the upregulated geneNGFRplays a role,was another enriched pathway(Figure 2B4) in the AKBA group compared with that in the Sham group.The ten pathways with the highest enrichment in the Model and Sham groups are presented inFigure 2B6.
We also performed an overall KEGG enrichment analysis on the up-and downregulated genes.The results are shown inAdditional Figure 1.Compared with the genes in the Model group,the up-regulated genes in the AKBA group were mainly enriched in myocardial contraction (Additional Figure 1A) and the main down-regulated genes were enriched in the peroxisome proliferatorsactivated receptors and phagosome signaling pathway (Additional Figure 1B).Compared with the genes in the Sham group,the main up-regulated genes in the AKBA group were enriched in cardiac muscle contraction (Additional Figure 1C),and the main down-regulated genes were enriched in adenosine 5′-monophosphate activated protein kinase signaling pathway (Additional Figure 1D).Compared with the genes in the Sham group,the up-regulated genes in the Model group were mainly enriched in cell adhesion molecules(Additional Figure 1F),and down-regulated genes were mainly enriched in the glucagon signaling pathway (Additional Figure 1E).
In summary,the phagosome pathway was the main enriched pathway that played a role in AKBA’s effects on sciatic nerve damage and repair (Figure 2B2and3),and NGFR and BDNF were significantly differentially expressed genes that were involved in nerve damage repair (Figure 2B4).
Verification of transcription and translation levels of differentially expressed genes in the injured rat sciatic nerve after AKBA treatment
The results inFigure 3A1-3showed thatNGFR,NGF,andBDNFmRNA expression levels in the AKBA group were increased significantly compared with those levels in the Blank and Model groups (allP<0.01).The MPO mRNA expression level in the AKBA group was significantly reduced compared with that in the Model group (P<0.01).The mRNA expression level was reduced in the AKBA group,but it was not significantly different from that in the Blank group (Figure 3A4).The results showed that compared with those in the Model group,NFGR,NGF,and BDNF protein expression levels were significantly increased (P<0.01) and MPO protein expression was significantly reduced (P<0.01) in the AKBA group (Figure 3B).Compared with the levels in the Blank group,the NFGR,NGF,and BDNF protein expression levels were significantly increased in the AKBA group (allP<0.01).
AKBA inhibits inflammation in the injured rat sciatic nerve
CD68 levels were assessed to explore whether AKBA exerted antiinflammatory effects by regulating the phagosome pathway.CD68 levels in the AKBA group were not significantly different from those of the Blank and Sham groups,but they were significantly downregulated compared with those in the Model group (P<0.05;Figure 4A1andA2).Levels of the inflammationrelated proteins tumor necrosis factor-α,interleukin-1β,and pro-interleukin-1β in the AKBA group were significantly downregulated compared with those in the Model group (allP<0.01) but were not significantly different from those in the Blank and Sham groups (Figure 4BandC1-3).
AKBA promotes myelin and axonal repair after sciatic nerve injury
MBP is the main protein that comprises the myelin sheath,and its expression level can indicate myelin sheath damage (Liu et al.,2019a).As shown inFigure 5AandB,the MBP protein expression level in the Model group was lower than that in the Blank and Sham groups (P<0.01),and the MBP expression level in the AKBA group was significantly higher than that in the Model group (P<0.01).There was no significant difference in MBP expression levels among the Blank,Sham,and AKBA groups.This suggests that AKBA could repair sciatic nerve and myelin sheath damage.
As shown inFigure 5CandD,compared with that in the Sham group,β3-tubulin expression in the model group decreased significantly (P<0.01).Compared with that in the Model group,β3-tubulin protein expression in the AKBA group was increased significantly (P<0.01).There was no significant difference in β3-tubulin protein expression levels between the AKBA and Sham groups.These results demonstrated that AKBA could promote axonal repair after sciatic nerve injury in rats.β3-Tubulin protein expression levels in the AKBA group were not significantly different compared with those in the Blank group.These results illustrated that AKBA could promote repair in injured axons.The silver-plated staining results of nerve tissue on day 30 showed that in the Blank and Sham groups,nerve fibers were intact,the axonal thickness was uniform,and the arrangement was uniform and orderly.In the Model group,nerve fibers were incomplete,axons were disordered,the thickness was uneven,and a large number of separated axons were present with broken ends.In the AKBA group,nerve fibers were intact,the axonal thickness was uniform,and a few vacuoles were present.The experimental results showed that AKBA can promote the repair of damaged nerve fiber axons (Figure 5E).
As shown inFigure 5F,the myelin sheath ultrastructure on day 30 in the Blank and Sham groups was regular and thick with a clear lamina and intact microtubules and microfilaments.Most of the myelin sheaths in the Model group were irregular with fuzzy lamina,sparse arrangement,and disordered microtubules and microfilaments.In the AKBA group,the myelin sheath had a regular shape with a clear lamella,dense arrangement,and intact microtubules and microfilaments.Compared with that in the Sham group,the G-ratio in the Model group was significantly lower (P<0.05).Compared with that in the Model group,the G-ratio in the AKBA group was significantly higher (P<0.05).There was no significant difference in the G-ratio between the AKBA and Sham groups (Figure 5G).The myelin sheath thickness in the Model group was greater,but it was not significantly different from the other groups (Figure 5H).
AKBA improves rat mobility after sciatic nerve injury
Damaged nerve function recovery and sensory function are two key clinical evaluation indicators (Liu et al.,2022a).On days 20 and 30,the SFI in the AKBA group was significantly higher than that in the Model group (P<0.01).The SFI in the AKBA group was not significantly different from that in the Blank group (Figure 6A).The pain score is shown inFigure 6B,and the AKBA and the Model groups showed no significant difference on day 10.However,there was a significant difference on days 20 and 30.This suggests that AKBA could significantly improve recovery from sciatic nerve injury,but it did not promote complete recovery of sensory function.

Figure 1|Test timeline.
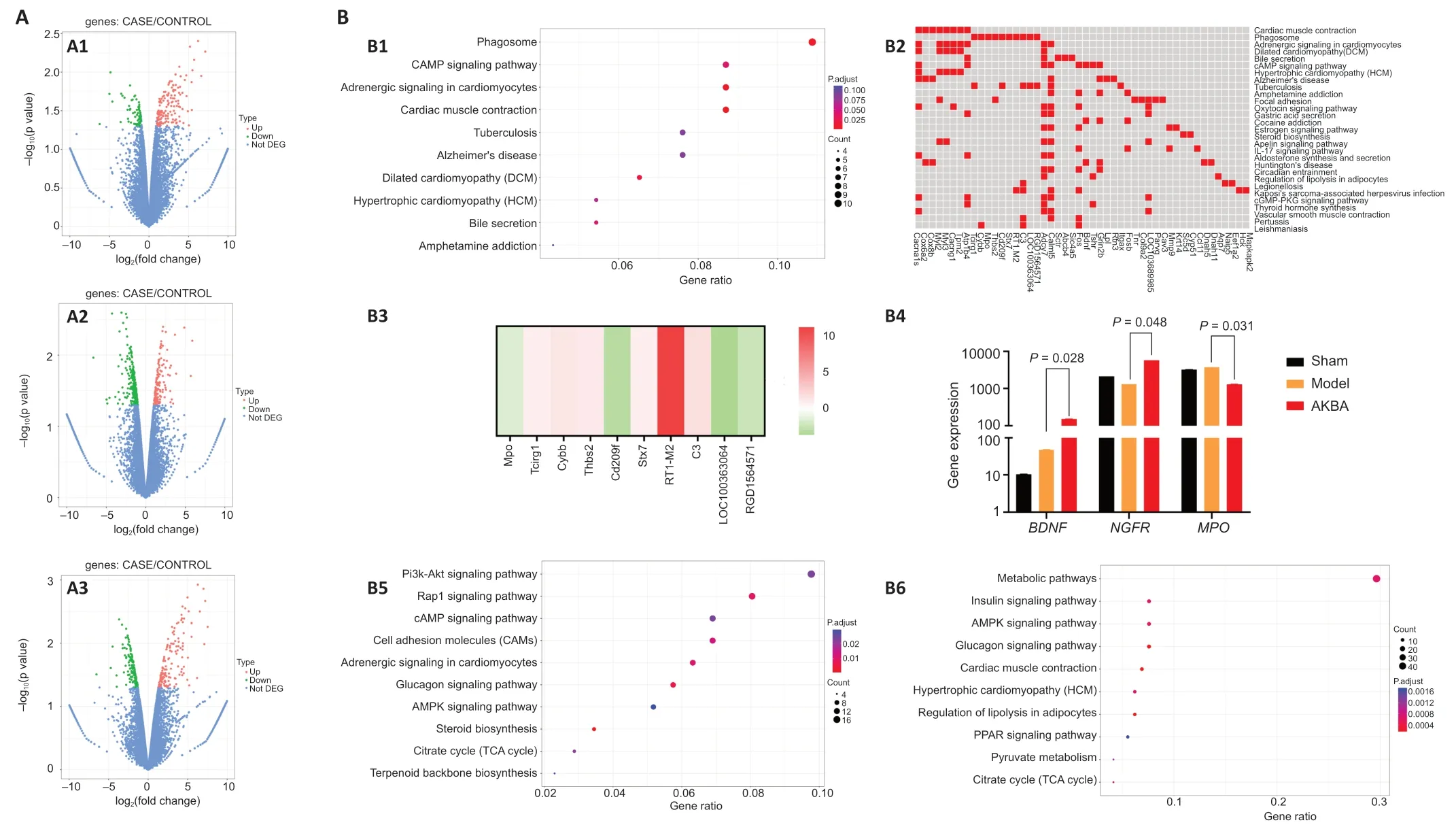
Figure 2|Genomics analysis after sciatic nerve injury treated with AKBA in rats.
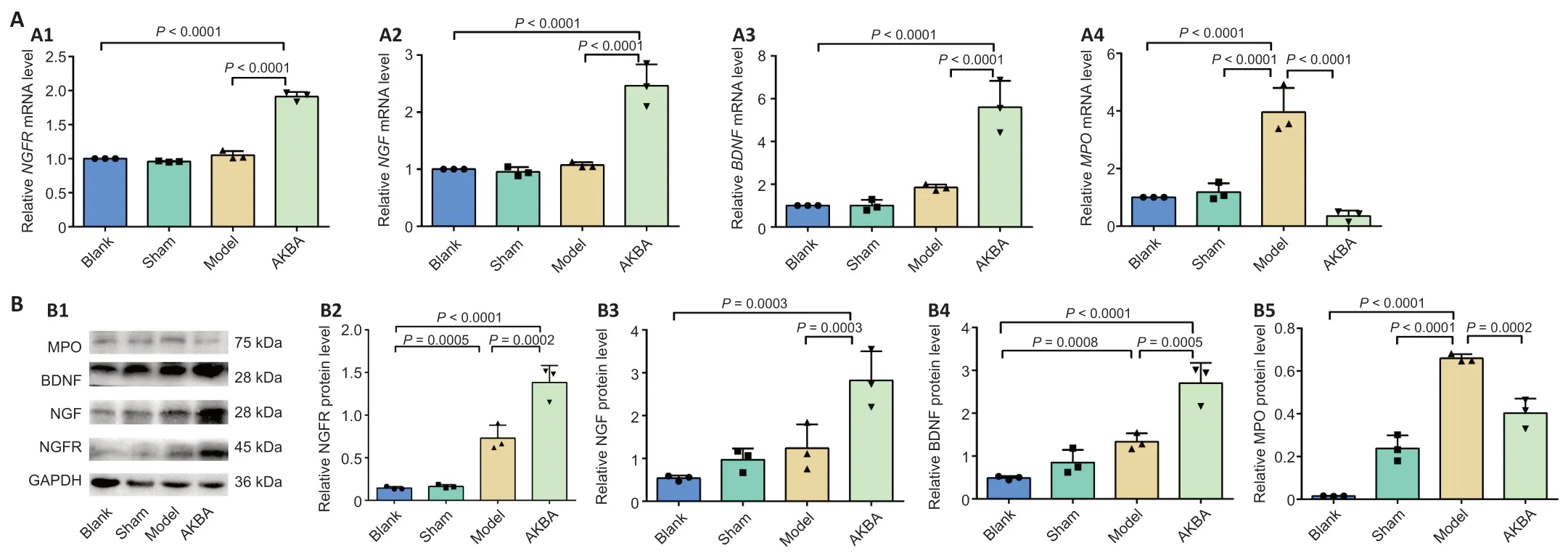
Figure 3|Gene and protein verification in sciatic nerve injury in rats treated with AKBA.
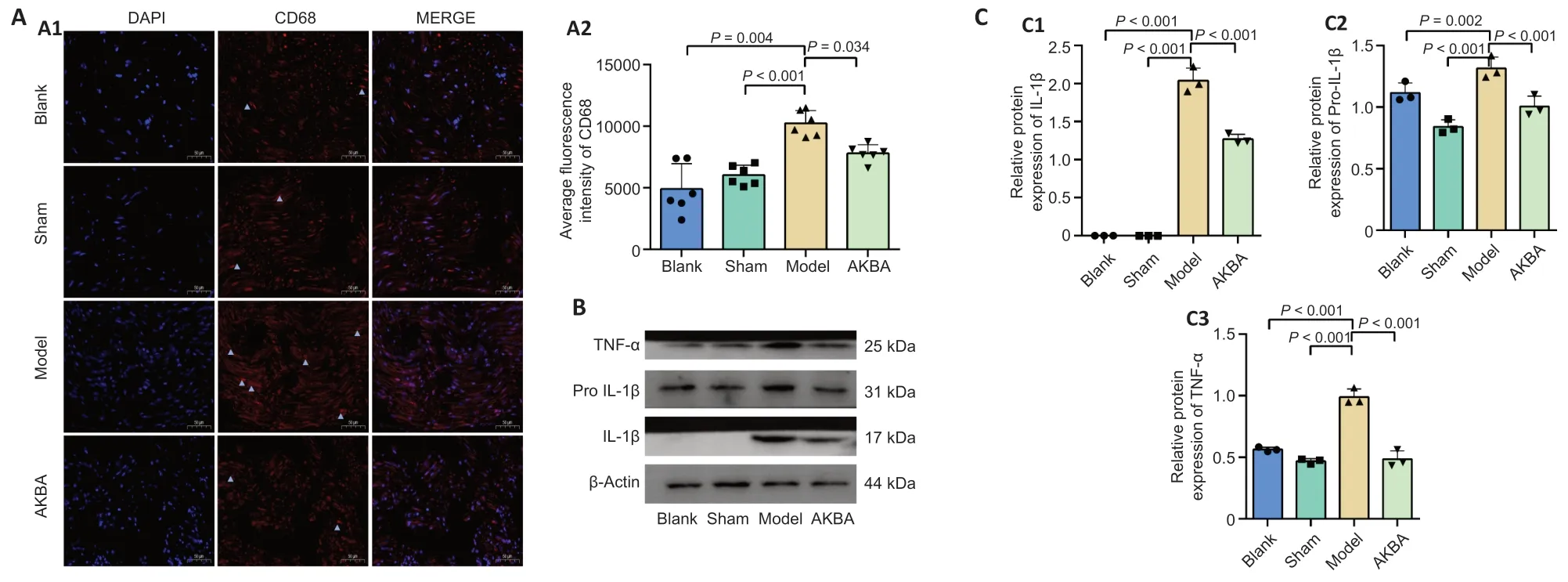
Figure 4|Effect of AKBA on inflammation-related factors in sciatic nerve injury in the rat.

Figure 5|Effects of AKBA on myelin sheath and nerve fiber regeneration in the damaged sciatic nerve in rats 30 days after injury.

Figure 6|Effect of AKBA on behavior of rats with sciatic nerve injury.
Discussion
Previous studies (Jiang et al.,2018,2020) have shown that AKBA can promote Schwann cell proliferation through the extracellular regulated protein kinases signaling pathway to accelerate the repair of sciatic nerve damage.However,the specific mechanism of this effect is unclear.We used whole transcriptomics to sequence and compare all genes in different groups to obtain differentially expressed genes and then performed KEGG enrichment analysis on the differentially expressed genes.We found that AKBA could promote phagosome formation,inhibit inflammatory reactions,and participate in sciatic nerve damage repair.Additionally,AKBA could improve BDNF,NFG,and NGFR protein expression.This provides a theoretical basis for further development of AKBA as a new veterinary drug to treat PNI in small animals.
KEGG analysis aggregates differentially expressed genes into pathways,thus identifying pathways that promote damage repair.KEGG enrichment analysis revealed that genes that showed differential mRNA expression between the AKBA and Model groups were most enriched in the phagosome pathway.Phagocytosis is the process where cells ingest relatively large particles,and it is a central mechanism in inflammation (Brown and Neher,2014).Phagosomes are formed when specific receptors on the phagocyte surface recognize ligands on a particle’s surfaces.The following 10 genes are involved in phagocytosis:Tcirg1,Cybb,MPO,Thbs2,Cd209f,Stx7,RT1-M2,C3,LOC100363064,andRGD1564571.The results of this study showed that among these genes,MPOandCd209fwere downregulated,while the rest were upregulated after nerve injury,and thus,genes are jointly involved in the repair of sciatic nerve injury.Therefore,it is speculated that AKBA could promote phagosome formation,inhibit inflammatory reactions,and participate in sciatic nerve damage repair.Through detecting MPO gene downregulation,AKBA was shown to reduce MPO mRNA and protein expression.This result is consistent with the findings of previous studies that showed that a reduction in MPO can effectively reduce peripheral nerve lesions,protect peripheral nerve cells,and reduce oxidative stress (Chen et al.,2020).AKBA has been shown to reduce CD68 and inflammatory protein expression,such as tumor necrosis factor-α,interleukin-1β,and pro-interleukin-1β,which suggests that AKBA can inhibit the inflammatory response.However,it remains unknown if inhibiting the inflammatory response is directly mediated by MPO,which will be studied in the future.
BDNF can promote peripheral nerve regeneration,protect damaged neurons and nerve cells,and maintain sensitivity to neurons (Lu et al.,2019).NGF is an endogenously produced polypeptide that can promote neuronal differentiation,survival,and repair in the central and peripheral nervous system (Farhadieh et al.,2003;Jones et al.,2019;Nocchi et al.,2019).NGFR is a low-affinity receptor that binds to BDNF and NGF,and it can mediate nerve cell survival (Carito et al.,2014).These results demonstrated that AKBA promoted sciatic nerve injury repair by increasing NFGR,NGF,and BDNF mRNA expression and thereby increasing the corresponding protein expression level.
MBP is an oligodendrocyte marker and a myelin sheath quantity and function marker (Vassall et al.,2015).β3-Tubulin is tubulin that is involved in the specific differentiation of neuronal cell types (Reichert et al.,2019),and an increase in this marker can reflect nerve repair after injury.The experimental results illustrated that MBP expression in the AKBA group was significantly increased compared with that in the Model group.β3-Tubulin expression in the AKBA group was also significantly increased compared with the Model group.This result further verified that AKBA regulated NGFR,BDNF,and NGF protein expression to repair damaged sciatic nerves.The G-ratio is an indicator that reflects the maturity of regenerated myelinated nerve fibers.The most suitable ratio of the axon diameter to the total diameter is approximately 0.6,which is more conducive to a better conduction velocity(Philpott et al.,2017).A myelin thickness analysis also showed that the myelin thickness in the Model group was greater and the axons were smaller.The myelin sheath thickness in the AKBA group was relatively small,and the G-ratio was closer to that of the Blank and Sham groups.Thus,AKBA has the effect of repairing damage to the myelin sheath.The results of silver plating and the G-ratio show that AKBA exerted a good repair effect on damage to the nerve myelin sheath.
The recovery of damaged nerve function and pain perception are two key clinical evaluation indicators.The SFI can be used to judge the capability of nerve regeneration after nerve injury and the degree of nerve motor function(Samadian et al.,2020).If the SFI is high,then the indicators of the nerve surrounding the muscle tissue are also good.Disuse muscle atrophy occurs after PNI (Dyer et al.,2016) and affects nerve sensory function.The toe pinch test was used to assess the recovery of sensory function.The behavioral test results revealed that AKBA could accelerate damaged sciatic nerve function recovery,but it could not completely repair the damage during the experimental period.
However,our research has some limitations.In vivostudies have concluded that AKBA can inhibit inflammation (Wei et al.,2020) and improve neurotrophic factor signaling pathways.However,in vitrostudies are also needed,and we have not performed these studies,which is a research limitation.In this study,AKBA promotes sciatic nerve injury repair in rats by increasing BDNF,NGFR,and NGF mRNA expression,which thereby increases the expression of their corresponding proteins,and by decreasing MPO mRNA and protein expression in the phagosome pathway to inhibit inflammation.
Author contributions:Study design and manuscript draft:YW,XWJ,WHY;experiment implementation:YW,ZLX,XLM,CZ,MHH;data analysis:YW,ZLX,XLM,CZ,MHH,XWJ,WHY;manuscript revision:XWJ,WHY.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that there is no conflict of interest regarding the publication of this paper.
Author statement:All genomics raw sequencing data has been uploaded to GEO (accession number GSE179400).In addition,the datasets used in the project are available from the corresponding author.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:KEGG analysis of differentially expressed genes in rat sciatic nerve after AKBA treatment
- 中国神经再生研究(英文版)的其它文章
- Interleukin 17A deficiency alleviates neuroinflammation and cognitive impairment in an experimental model of diabetic encephalopathy
- NR4A1 agonist cytosporone B attenuates neuroinflammation in a mouse model of multiple sclerosis
- A new anterograde trans-synaptic tracer based on Sindbis virus
- Interleukin-4 promotes microglial polarization toward a neuroprotective phenotype after retinal ischemia/reperfusion injury
- Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss
- A multiple-tissue-specific magnetic resonance imaging model for diagnosing Parkinson’s disease:a brain radiomics study

