NR4A1 agonist cytosporone B attenuates neuroinflammation in a mouse model of multiple sclerosis
Hai-Zhen Yu,Bing-Qing Zhu,Lin Zhu,Shuo Li,Li-Mei Wang
Abstract Nuclear receptor subfamily 4 group A1 (NR4A1) is an orphan nuclear receptor,which is expressed in the majority of cells.NR4A1 expression in peripheral blood mononuclear cells is low during the preclinical stage of multiple sclerosis.Knockout of the Nr4a1 gene in mice can aggravate the symptoms of experimental autoimmune encephalomyelitis (EAE),which is an animal model of multiple sclerosis.In this study,we intragastrically administered the NR4A1 agonist cytosporone B (Csn-B) to mice after inducing EAE.After treatment with Csn-B,the clinical symptoms in the EAE mice were substantially attenuated compared with that in PBS-treated control mice.The percentages of CD4+ T cells and F4/80+ cells in the central nervous system were decreased.In addition,interferon-γ and interleukin-17 production by proinflammatory Th1/Th17 cells in the central nervous system and interferon-γ levels in splenocytes were decreased after Csn-B treatment.These findings suggest that the NR4A1 agonist Csn-B can alleviate nerve injury after EAE induction,and,therefore,may be useful as a potential treatment for multiple sclerosis.
Key Words:cytosporone B (Csn-B);experimental autoimmune encephalomyelitis;macrophages;microglia;multiple sclerosis;NR4A1 agonist;NR4A1;Th1;Th17;treatment
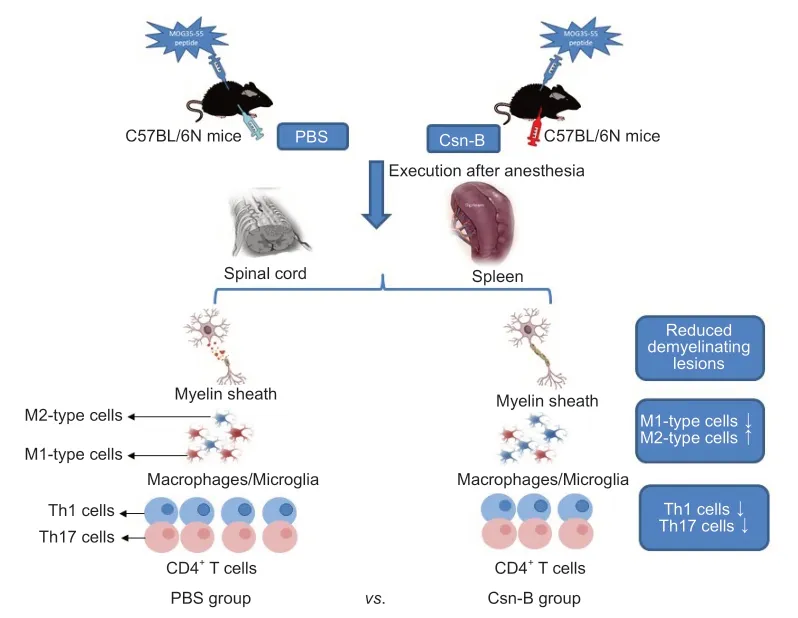
Graphical Abstract Cytosporone B (Csn-B) attenuates inflammation and demyelination in mice with experimental autoimmune encephalomyelitis.
Introduction
The orphan nuclear receptors NR4A1 (NUR77),NR4A2 (NURR1),and NR4A3(NOR1) constitute the NR4A subfamily of transcription factors (Germain et al.,2006).Orphan nuclear receptors are immediate-early response gene products(Maxwell and Muscat,2006),which can regulate glucose metabolism (Fu et al.,2007;Miao et al.,2019),lipid homeostasis (Pols et al.,2008;Miao et al.,2020),cancer development (He et al.,2020),inflammation (Hu et al.,2017;Xiong et al.,2020),and vascular remodeling (Nomiyama et al.,2009;Li et al.,2018;Kang et al.,2019).Additionally,NR4A family members regulate gene expression that is related to cell differentiation,proliferation,and programmed death (Winoto and Littman,2002;Liu et al.,2019;He et al.,2020).The latter process is characterized by receptor migration to the mitochondria and subsequent release of cytochrome c into the cytoplasm(Li et al.,2000).Achiron et al.(2010) conducted a study to compare gene expression profiles of peripheral blood mononuclear cells from nine patients who developed MS (MS2b) and 11 matched patients without MS.The expression of NR4A1 was significantly down-regulated in patients with MS2b(Achiron et al.,2010),and the expression ofNR4A1andNR4A2genes in 31 patients with clinically isolated syndrome was significantly lower than that in 13 healthy subjects matched for gender and age.The expression of the NR4A1 protein in peripheral blood mononuclear cells decreased significantly in patients with MS2b and clinically isolated syndrome (Achiron et al.,2010).Gene-array analysis (Achiron et al.,2010) indicated that NR4A1 was downregulated during the MS pre-disease state.Our previous report showed that the absence of theNr4a1gene in mice increased EAE severity,which was characterized by substantially enhanced inflammation and demyelination(Wang et al.,2018).Judging from the current evidence,it can be inferred thatNr4a1gene expression may be related to the regulatory mechanisms of EAE and MS.
Cytosporone B (Csn-B) was isolated from the endophytic fungusDothiorellasp.HTF3.Csn-B is a natural agonist for NR4A1 (Brady et al.,2000) and demonstrates strong binding affinity that upregulates NR4A1 transactivational activity (Zhan et al.,2008).One study found that Csn-B induced apoptosis by targeting the mitochondria (Zhan et al.,2008).Csn-B administration decreased the formation of atherosclerotic plaques in mice that consumed fat-rich diets (Hu et al.,2014) and mitigated inflammation in a colitis mouse model by reducing pro-inflammatory factors (Wu et al.,2016).By increasing NR4A1-mediated apoptosis,Csn-B suppressed xenograft tumor growth in nude mice (Zhan et al.,2008).Csn-B also inhibited excessive inflammation and improved osteoarthritis by activating NR4A1 (Xiong et al.,2020).Together,these findings suggest that targeting NR4A1 may have a potential curative effect on inflammatory diseases.This study evaluated the functions of the NR4A1 agonist Csn-B and the mechanisms by which Csn-B potentially regulated the development of EAE.
Materials and Methods
Animals
Female mice are more sensitive than male mice,as manifested by a higher incidence of EAE and greater severity of clinical signs (Voskuhl et al.,1996).In previous studies (Zhang et al.,2015;Wang et al.,2020),female mice were mainly used.Six to eight-week-old,female C57BL/6N mice (n=49,weighing 18-20 g) were acquired from Zhejiang Vital River Laboratory Animal Technology Co.,Ltd.,China (license No.SCXK (Zhe) 2019-0001).The mice were maintained in a specific pathogen-free environment at the Zhengzhou University Experimental Animal Center (Zhengzhou,China).Forty-four mice were used for the EAE modeling,and the other five mice were used as a source of naïve CD4+T cells.The experimental procedures and protocols were approved by the Animal Ethics Committee of Zhengzhou University (approval No.2019-KY-142) on June 12,2019.
EAE induction
EAE was induced in the C57BL/6N mice as described previously (Stromnes and Goverman,2006).Briefly,the myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55;250 µg;R&D Systems,Minneapolis,MN,USA)was dissolved in 100 µL phosphate buffer saline (PBS).The peptide was mixed thoroughly with 100 µL Freund′s complete adjuvant (MilliporeSigma,Burlington,MA,USA) containing 5 mg/mL of Mycobacterium tuberculosis H37Ra (Difco Laboratories,Inc.;Detroit,MI,USA) to prepare a water-in-oil emulsion of complete antigen.Each mouse was subcutaneously injected at the neck and back with 100 µL complete antigen/site.Pertussis toxin (200 ng;List Biological Lab;Epsom,UK) was dissolved in 200 µL PBS and injected intraperitoneally at 0 and 48 hours post-immunization as a co-adjuvant to induce EAE.Subsequently,EAE mice were randomly assigned to the Csn-B (n=27) and PBS (n=17) groups.
Drug administration
The NR4A1 agonist Csn-B (MilliporeSigma) was dissolved in 200 µL PBS.The mice with EAE were intragastrically administered 2.5,10,or 20 mg/kg/d Csn-B based on a previous study (Egarnes et al.,2017).Five mice were in each group.Csn-B was administered once per day beginning on day 10 postimmunization as a preventative agent in the dose optimization study or on day 14 post-immunization as a treatment in the follow-up experiments.The Csn-B was administered until day 21.Control mice were intragastrically administered 200 µL PBS at the same time points as the Csn-B administration(Figure 1).
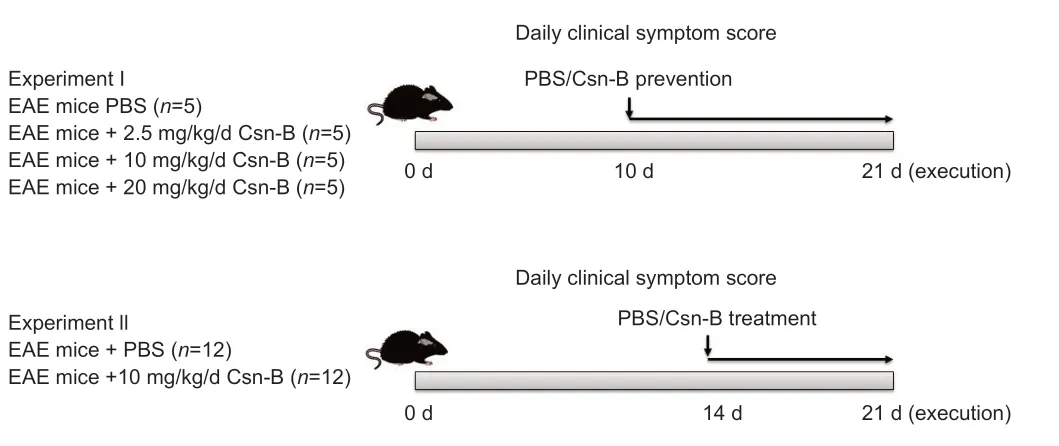
Figure 1|Experimental flow chart.
EAE clinical score
Beginning at the time of EAE induction in the mice,the symptoms were assessed each day based on the Clinical Grading of Mice with Classic Encephalomyelitis (Stromnes and Goverman,2006) by an investigator who was blinded to the groups.Additional Table 1summarizes the criteria for the assessment of clinical symptoms in mice with EAE.A higher score indicates greater severity of symptoms and a worse prognosis for the mice.
Induction of Th1 and Th17 cell differentiation in vitro
Th1 and Th17 cell differentiation was inducedin vitroutilizing protocols that were previously reported (Zhang et al.,2015).In summary,mice were sacrificed after intraperitioneal injection of 2% pentobarbital (40 mg/kg;Notlas Bio Co.,Ltd.;Beijing,China).Naïve CD4+T cells were isolated from the spleens of C57BL/6N mice (n=5) by negative selection with mouse CD4 microbeads (Miltenyi Bio Co.,Somerville,MA,USA).Purified cells were cultured with 1 µg/mL soluble rat anti-CD3 (Abcam,Hong Kong,China,Cat# ab24948,RRID:AB_470375) and 1 µg/mL hamster anti-CD28 (Abcam,Cat# ab25234,RRID:AB_470416) antibodies at 37°C for 3 days.Five ng/mL mouse interleukin-12 (IL-12,R&D Systems) was added to stimulate Th1 differentiation,and 2 ng/mL transforming growth factor-β1 (Beyotime Bio Co.,Ltd.,Nantong,Jiangsu,China),20 ng/mL interleukin-6 (IL-6,R&D Systems),10 ng/mL interleukin-1β (IL-1β,R&D Systems),10 µg/mL rabbit anti-interleukin-4(anti-IL-4,Abcam,Cat# ab62351,RRID:AB_943835),and 10 µg/mL rat anti-interferon-γ (anti-IFN-γ,Miltenyi Biotec,Cat#130-102-388,RRID:AB_2659990)were added to stimulate Th17 differentiation (Zhang et al.,2015).The culture medium also contained Csn-B at different concentrations (5 and 10 µg/mL) for the intervention groups,while the control group was treated with PBS.Flow cytometry was used to evaluate cells that had been incubated for 3 days.
I would ask thee, said Vasilissa, of the men on horse back. When I came to thy hut, a rider passed me. He was dressed all in white and he rode a milk-white horse. Who was he?
Histopathology
On day 21 post-immunization,mice were anesthetized by intraperitioneal injection of 2% pentobarbital,and 30 mL cold PBS was perfused into the heart to clear the blood.The spinal cord tissue was obtained immediately and fixed using 4% paraformaldehyde for 24 hours,then placed in ethanol and xylene.The tissue was then embedded in paraffin and cut into 5-mm-thick sections.The spinal cord sections were stained with hematoxylin and eosin (HE) to evaluate the degree of inflammation and luxol fast blue (LFB) to evaluate the degree of demyelination.For HE staining,the paraffin sections obtained in the previous step were first deparaffinized in xylene,and anhydrous ethanol was used to remove the xylene.The sections were hydrated by immersing in solutions with decreasing concentrations of alcohol.The sections were incubated in hematoxylin and eosin staining solutions and then washed in running water.Finally,the sections were dehydrated with ethanol and xylene and sealed.For LFB staining,the paraffin sections were first deparaffinized as described,stained with LFB staining solution overnight at room temperature,and washed with ethanol and distilled water.The sections were incubated in saturated lithium carbonate solution and washed again.Finally,the sections were dehydrated with ethanol and xylene and sealed.Tissue sections were observed by microscopy.The inflammation and demyelination of spinal cords after HE and LFB staining,respectively,were evaluated under blinded conditions using a scale from 0 to 3 as previously described (Yang et al.,2010).Additional Table 2summarizes the criteria for the assessment of inflammation and demyelination.A higher score indicated that the tissue displayed greater inflammation and demyelination than a lower score.
Flow cytometry
As described above,the mice were perfused,and the spinal cords and the spleen were obtained on day 21 post-immunization.The mononuclear cells infiltrating the spinal cord and spleen were isolated using a 70 µm nylon cell strainer and enzymatic digestion with LiberaseTM(Roche;Basel,Switzerland)for 20-30 minutes at 37°C.Then mononuclear cells from spinal cord were isolated using a Percoll gradient (70/37%).These collected cells were washed,and all surviving cells were counted using a light microscope (CKX31;Huyu Trading Co.,Ltd.;Shanghai,China).Mononuclear cells isolated from the spinal cord and spleen tissues were suspended at a concentration of 2 × 106/mL in complete RPMI 1640 medium containing 10% fetal bovine serum (FBS)and then washed in PBS containing 3% FBS and 0.02% NaN3 for the purpose of surface marker staining.Fc receptors were blocked using Fc receptor Blocker (MK Bio Co.,Ltd.,Shanghai,China).The cells were incubated on ice with the appropriate fluorochrome-conjugated antibodies for 30 minutes.In brief,after washing these cells in fluorescence-activated cell sorter (FACS)buffer,anti-CD4 (Abcam,Cat# ab53420,RRID:AB_2075566),anti-F4/80(MyBioSource,Cat# MBS607610,RRID:AB_10888718),anti-CD40 (LSBio(LifeSpan) Cat# LS-C43310-250,RRID:AB_1051259),anti-CD86 (US Biological,Cat# C2438-01H,RRID:AB_2074980),anti-CD206 (Thermo Fisher Scientific,Cat# MA5-28581,RRID:AB_2745540) or anti-I-region associated antigen(anti-Ia,Abcam,Cat#ab51262,RRID:AB_869913) antibodies were added for surface marker staining.Prior to intracellular cytokine staining,phorbol 12-myristate 13-acetate (50 ng/mL),ionomycin (500 ng/mL),and Golgi-Stop (1 mg/106cells) were added to the cells,which were then incubated in RPMI 1640 medium containing 10% FBS at 37°C for 4 hours.The cells were fixed and permeabilized using Fix/Perm reagents (BD Biosciences) and then incubated with fluorescent-labeled antibodies against intracellular cytokines.Intracellular staining was performed in accordance with the manufacturer’s instructions.M1 macrophages were labeled with anti-IL-12(MBL International,Cat# JM-5162-100,RRID:AB_591999).M2 macrophages were labeled with anti-IL-4 (Abcam,Cat# ab62351,RRID:AB_943835).Th1 cells were labeled with anti-IFN-γ (Miltenyi Biotec,Cat# 130-102-388,RRID:AB_2659990) and Th17 cells were labeled with ani-IL-17 (US Biological,Cat#18439-10B,RRID:AB_2124882).After the last wash,the cells were collected on a FACSAria flow cytometer (BD Biosciences) and analyzed using FlowJoTMsoftware v.10.0 (Tree Star,Ashland,OR,USA).
Cytokine levels in splenocytes detected by enzyme-linked immunosorbent assays
The mice were perfused on day 21 post-immunization.Single-cell suspensions were prepared from the spleen tissues using sterile 70 µm nylon cell strainers.Erythrocytes from the spleens were dissolved by adding NH4Cl-Tris buffer at room temperature for 5 minutes.Then,the single-cell suspensions were washed,re-suspended,and all surviving cells were counted.Splenocytes were incubated in RPMI 1640 (Gibco) with 10% FBS in triplicate in 24-well plates at a concentration of 2 × 106/mL.Cells were re-stimulated for 48 and 72 hours with 25 µg/mL MOG35-55.Cell-free supernatants were collected,and the cytokines interleukin-17 (IL-17) and IFN-γ,were measured using enzymelinked immunosorbent assay kits (R&D Systems) in accordance with the manufacturer’s instructions.
Statistical analysis
The sample size was selected based on previous literature and a prior study by our team (Wang et al.,2018).No animals were missing in any experimental group,and the evaluator assessed disease symptoms in the mice using a blinded method.GraphPad Prism v.9.0 software (GraphPad Software,Inc.;La Jolla,CA,USA,www.graphpad.com) was used for statistical analyses.Data are expressed as the mean ± standard error of mean (SEM).Student’st-tests were used to compare the means of two groups.Comparisons of more than two groups were conducted using one-way analysis of variance with Bonferronipost hoctests.Statistical significance was defined as aP-value less than 0.05.
Results
NR4A1 agonist Csn-B affects the differentiation of Th1 and Th17 cells in vitro
To elucidate the role of Csn-B in the differentiation of CD4+T cellsin vitro,naïve T cells were collected from wild-type mice and induced to differentiate into Th1 or Th17 cells.Under the conditions for Th1 differentiation,the percentage of CD4+IFN-γ+cells significantly decreased after Csn-B treatment compared with that in the PBS control group.Under conditions for Th17 differentiation,the percentage of CD4+IL-17+cells was also significantly decreased compared with that in the control group (Figure 2).These results suggest that Csn-B has a significant impact upon Th1 and Th17 cell differentiationin vitro.
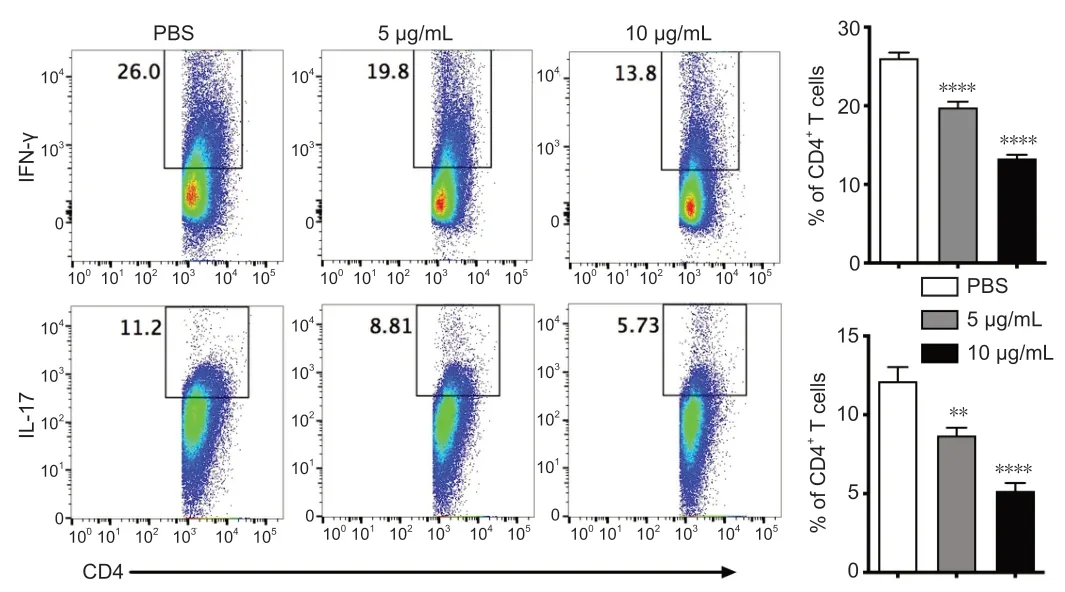
Figure 2|Th1 and Th17 cell differentiation is decreased in vitro after treatment of naïve CD4+ T cells with cytosporone B (Csn-B).
Determination of the optimal doses of Csn-B in EAE suppression
To determine the optimal Csn-B dose for treatment of EAE,the EAE mice were intragastrically administered 2.5,10,or 20 mg/kg Csn-B per day as a preventive intervention.The control group was treated with PBS.Symptom onset was observed beginning on days 12-13 after immunization.The clinical function scores showed no significant difference between the mice treated with 2.5 mg/kg/d Csn-B and the control groups;however,the clinical function scores of the 10 and 20 mg/kg/d Csn-B groups were substantially lower than those in the PBS-treated mice (Figure 3).No difference was observed between the 10 and 20 mg/kg/d Csn-B groups (Figure 3).The results indicated that oral treatment with Csn-B reduced the severity of EAE symptomsin vivo,and the Csn-B dose of 10 mg/kg per day was chosen as the therapeutic dose for all subsequent experiments.
Csn-B attenuates EAE clinical symptoms by reducing demyelination and inflammatory cell infiltration in the spinal cord
To determine the impact of Csn-B on EAE,mice were administered the MOG35-55 peptide to induce EAE.EAE clinical symptoms were observed beginning on days 12-13 after induction.Based on the above study,mice were intragastrically administered 10 mg/kg Csn-B per day as a treatment beginning on day 14 post-immunization.Compared with the scores in the control group,the clinical function scores of the Csn-B group were significantly decreased(Figure 4A).The Csn-B treatment significantly alleviated demyelination and inflammation in L3 spinal cord tissue compared with that in the control group on day 21 (Figure 4B).The pathological scores for inflammation and demyelination were significantly lower in the Csn-B group of mice than in the control group (Figure 4B).Flow cytometry demonstrated that the percentages and absolute numbers of CD4+T cells (P<0.001) and F4/80+macrophages/microglia (P<0.01) isolated from the CNS were significantly decreased in the Csn-B-treated mice compared with those in the PBS control group (Figure 4C).These findings illustrated that the NR4A1 agonist Csn-B attenuated the clinical symptoms of EAE by reducing demyelination lesions and inflammatory cell infiltration in the CNS.

Figure 3|The NR4A1 agonist cytosporone B (Csn-B) can suppress the development of experimental autoimmune encephalomyelitis symptoms in vivo.
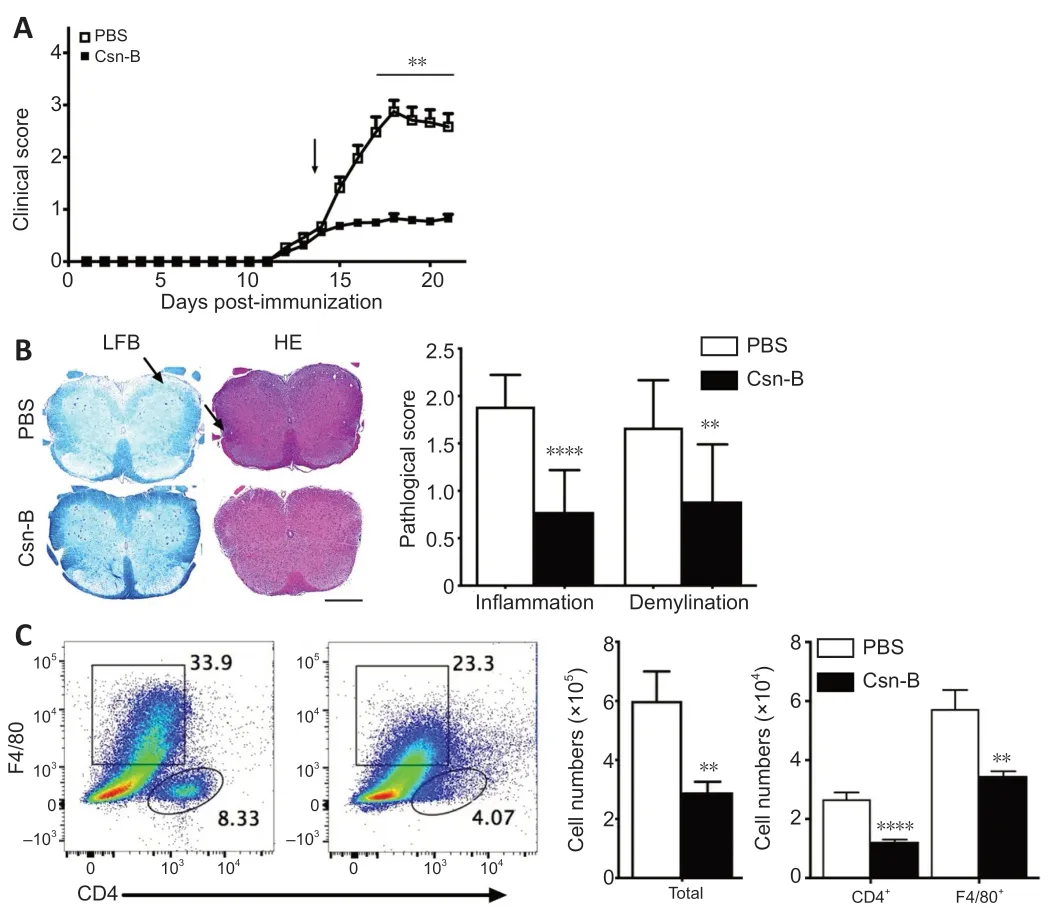
Figure 4|Cytosporone B (Csn-B) attenuates clinical symptoms and reduces demyelination and inflammatory cell infiltration in the central nervous system.
Macrophages/microglia display an enhanced anti-inflammatory cell phenotype in the CNS and spleen after Csn-B treatment
To investigate the immunological mechanisms associated with the reduction in EAE severity in Csn-B-treated mice,we evaluated the polarization of macrophages/microglia that were isolated from the CNS and spleens by flow cytometry.As shown inFigure 5,compared with that in mice given PBS,the Csn-B group showed significantly decreased expression of M1 markers.The expression of Ia,CD86,CD40,and IL-12 in the CNS and Ia and CD86 in splenocytes was decreased in mice treated with Csn-B.In contrast,the expression of the M2 markers CD206 and IL-4 were elevated in the CNSinfiltrating cells and splenocytes of Csn-B treated mice compared with that in the PBS group (Figure 5).These results indicated that macrophages/microglia displayed an enhanced M2 (anti-inflammatory) phenotype after treatment with Csn-B.
Csn-B inhibits Th1 and Th17 cell responses in the CNS and spleens of EAE mice
To explore the effect of Csn-B on differentiation of CD4+T cellsin vivo,T cell phenotypes in Csn-B-and PBS-treated mice were examined.Compared with that in the PBS group,the percentages of IFN-γ+cells and IL-17+cells in the CNS of Csn-B treated mice were significantly reduced.The percentage of IFN-γ+cells was also reduced in the spleen;however,the numbers of IL-17+cells in the spleen were not influenced by Csn-B treatment (Figure 6A).Enzyme-linked immunosorbent assay kits were used to detect the secretion of cytokines from splenocytes culturedin vitrofor 48 and 72 hours with MOG35-55.Splenocytes in the Csn-B group secreted lower levels of IFN-γ and IL-17 than cells in the PBS control group in response to MOG35-55 (Figure 6B).These results were similar to the results using flow cytometry observed above,and,thus,demonstrated that Csn-B effectively reduced secretion of the pro-inflammatory cytokines IFN-γ and IL-17 by Th1/Th17 cells in mice with EAE.
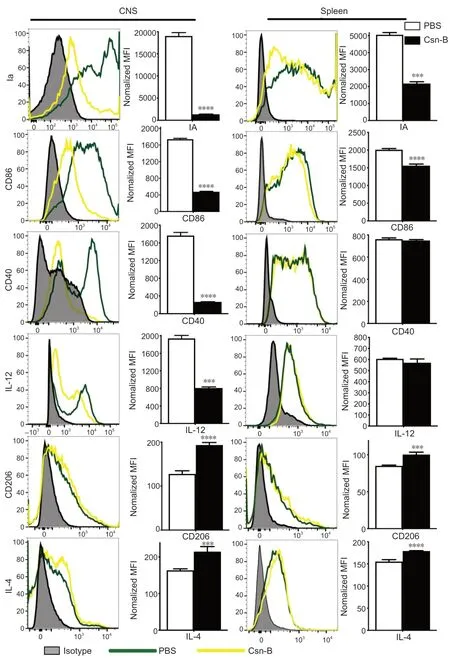
Figure 5|Macrophages/microglia display an enhanced anti-inflammatory cell phenotype after cytosporone B (Csn-B) treatment.
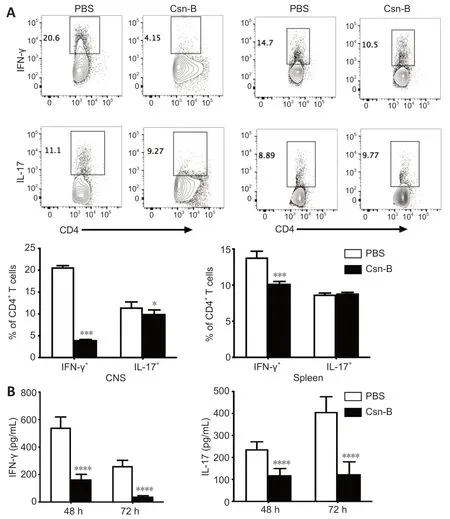
Figure 6|Cytosporone B (Csn-B) decreases T helper (Th)1 and Th17 cell responses in vivo.
Discussion
The present study revealed that Csn-B can attenuate the clinical severity of EAE with a potential therapeutic effect.First,we provided evidence that Csn-B suppressed Th1/Th17 differentiation of CD4+T cellsin vitro.Second,we demonstrated that Csn-B ameliorated the severity of EAE at the pathological level using an appropriate dose compared with that observed in the control group.The reduction in EAE severity was manifested mainly by decreases in inflammatory cell infiltration and formation of demyelination lesions in the CNS.Furthermore,the percentages and absolute numbers of CD4+T cells and F4/80+cells in the CNS were reduced in the Csn-B-treated mice compared with that in the control group.Third,we also discovered that Csn-B decreased proinflammatory Th1/Th17 cell responses and related cytokine production,such as IFN-γ and IL-17.We further validated that macrophage/microglial differentiation was directed toward an anti-inflammatory M2 phenotype after Csn-B treatment.These results suggest that Csn-B may be a potential novel therapy for EAE and may represent a safe and effective candidate drug for MS patients in the future.
In the physiological state,NR4A1 plays a crucial role in regulating inflammatory responsesin vivo;however,reduced expression has been reported in chronic diseases,such as cancer and neurodegenerative disease(Wu and Chen,2018;Yan et al.,2020),and upregulating the activity of NR4A1 can reduce the severity of these diseases (Rouillard et al.,2018;Yang et al.,2020).Over-expression of the Nr4a1 gene suppressed the secretion of pro-inflammatory cytokines mediated by oxidized low-density lipoprotein;however,Nr4a1 knockdown resulted in elevated cytokine secretion in animal models of atherosclerosis (Shao et al.,2010).Inflammatory gene expression was reduced when NR4A1 was upregulated in atherosclerotic lesions,including the expression of IL-1β,IL-6,and IL-8 (Bonta et al.,2006).Thus,correcting NR4A1 dysfunction may be beneficial for improving inflammatory disease prognoses.In accordance with these findings,the current study demonstrated a reduction in inflammation and demyelination in mice with EAE after treatment with the NR4A1 agonist Csn-B.
MS is characterized by multi-focal inflammatory lesions causing demyelination and axonal degeneration (Constantinescu et al.,2011).Therefore,inflammation plays a crucial role in the pathological mechanism of MS.The clinical signs of inflammation in nerve tissue are controlled in part by the infiltration of pro-inflammatory CD4+T cells and activation of macrophages/microglia.Previous studies have shown an imbalance between Th1/Th17 and regulatory T cells in EAE (Becher and Segal,2011;Zhan et al.,2020).Th1/Th17 cells are characterized as pathogenic T cells (Langrish et al.,2005;Park et al.,2005;Weiner,2008),whereas regulatory T cells can modulate the immune system and reduce inflammation in EAE (Danikowski et al.,2017).Th17 cells infiltrating the CNS were observed not only in EAE mice but also in patients with MS (Balasa et al.,2020).In addition,clinical scores and inflammation were significantly reduced when IL-17 receptors were lacking or IL-17-specific inhibitors were used in animal models of MS and rheumatoid arthritis(Pöllinger,2012).Overexpression of NR4A1 can inhibit T cell differentiation,whereas NR4A1 silencing reduces T cell tolerance and increases inflammatory effects in mice;thus,this protein may be a potential target for tumor immunotherapy (Liu et al.,2019).
Macrophages/microglia are important effector cells in EAE lesions (Jack et al.,2005;Lassmann,2010;Rawji and Yong,2013),which release IL-1β and TNF-α resulting in demyelination and axonal injury (Ledeen and Chakraborty,1998;Bogie et al.,2014).However,the mechanism of this process is unclear.Studies have shown that the mechanism may be related to triggering uncontrolled synaptic activation and excitotoxicity in the EAE animal model (Musella et al.,2016;Rizzo et al.,2018).Microglia,as resident macrophages of the CNS,play a role in immune surveillance and facilitating repair mechanisms after brain injury or inflammation-associated tissue damage during CNS diseases when at rest (Benarroch,2013;Yamasaki et al.,2014).Under pathological conditions,microglia are activated and undergo morphological and molecular changes(Wu et al.,2014;Wolf et al.,2017) and participate in the EAE autoimmune reaction via antigen presentation,secretion of various cytotoxic substances,and phagocytosis (Lassmann and van Horssen,2011;Dendrou et al.,2015).Previous studies have demonstrated that microglia/macrophages display two activation phenotypes,M1 (pro-inflammation) and M2 (anti-inflammation)(Gordon,2003),which participate in the pathogenesis of EAE.It has been shown that inhibiting M1 and promoting M2 cells can significantly reduce EAE symptoms (Qin et al.,2012;Liu et al.,2015).M1-type cells secrete a large number of pro-inflammatory cytokines,which lead to the central recruitment of other pro-inflammatory cells,while M2-type cells can secrete antiinflammatory cytokines and promote the differentiation of oligodendrocytes to accelerate the re-myelination process (Kigerl et al.,2009;Mikita et al.,2011;Chu et al.,2018).
Different mechanisms may be involved in Csn-B-mediated reduction in the clinical severity of EAE in mice,but these mechanisms synergistically reduce inflammation.Our previous study showed that NR4A1 inhibited Th1/Th17 cell differentiation (Wang et al.,2018),and the current study demonstrated that upregulating the activity of NR4A1 by Csn-B treatment suppressed proinflammatory CD4+T cell responses as well as cytokine production.It seems reasonable that,in our study,mice treated with Csn-B showed a similar effect.One study has shown that Csn-B treatments affected the regulation of macrophage/microglial activation and polarization in EAE (Rothe et al.,2017) and increased M2-type cells.Our results are in line with the findings of this study and demonstrated that the number of anti-inflammatory cells increased,while pro-inflammatory cells decreased after Csn-B treatment.A previous study illustrated that NR4A1 regulated the expression of proinflammatory genes by controlling the nuclear factor κB pathway,thereby reducing the secretion of inflammatory cytokines in an osteoarthritis model(Xiong et al.,2020).Another study indicated that regulation of the Janus kinase-signal transducer and activator of transcription signaling pathway can prevent the development of EAE by reducing levels of pathogenic Th17 cells and its key effector molecule IL-17A (Ma et al.,2016).However,there is no consensus on these mechanisms.
In conclusion,our study provides several novel findings that indicate Csn-B can effectively alleviate inflammation and demyelination in an animal model of MS during the acute phase.Csn-B may be a novel therapy and may provide a theoretical and experimental basis for the selection of effective drugs that achieve clinical benefits in patients with MS and other autoimmune diseases.This study was a preliminary exploration of the mechanisms by which Csn-B prevented EAE in mice.The limitation is that the study only focuses ontissue,however,the unique signaling pathway(s) downstream of NR4A1 that prevents inflammation at the molecular level and the effect of Csn-B on longterm prognoses for MS and other diseases both require further investigation.
Author contributions:Study conception and design,and manuscript draft:HZY,LMW;experiment implementation and collaboration in manuscript draft:HZY,BQZ;critical input and study supervision:LZ,SL.All authors read and approved the final manuscript.
Conflicts of interest:The authors have no conflicts of interest to disclose.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Clinical grading of mice with experimental autoimmune encephalomyelitis.
Additional Table 2:Criteria for the assessment of inflammation and demyelination.
- 中国神经再生研究(英文版)的其它文章
- Acetyl-11-keto-beta-boswellic acid promotes sciatic nerve repair after injury:molecular mechanism
- Interleukin 17A deficiency alleviates neuroinflammation and cognitive impairment in an experimental model of diabetic encephalopathy
- A new anterograde trans-synaptic tracer based on Sindbis virus
- Interleukin-4 promotes microglial polarization toward a neuroprotective phenotype after retinal ischemia/reperfusion injury
- Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss
- A multiple-tissue-specific magnetic resonance imaging model for diagnosing Parkinson’s disease:a brain radiomics study

