Determination of Sodium Pentachlorophenoxide Residues in Animal-derived Foods by Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
Chengmei WANG Chunxia CHENG Guixia YANG Susu ZHANG Xuan QIAN



Abstract [Objectives] This study was conducted to establish an ultra-high performance liquid chromatography-tandem mass spectrometry for the rapid extraction of sodium pentachlorophenoxide from animal-derived food.
[Methods] The samples were extracted with an acetonitrile water solution (8∶ 2), 0.1 mol/L hydrochloric acid and a purification extraction bag with shaking. Centrifugation was performed to obtain supernatants, which were added to purification tubes containing PSA and C 18 for purification, and then filtered with membranes for determination. Each test solution was separated by a ZORBAX Eclipse plus C 18 column with acetonitrile and 5 mmol/L ammonium acetate as mobile phases, and determined with electrospray ionization and multiple reaction monitoring.
[Results] The method had good linearity in the concentration range of 1.0-50 ng/ml, and the correlation coefficient was 0.999 7. The limit of detection was 0.25 μg/kg and the limit of quantification was 0.75 μg/kg. The recovery was between 87.4% and 112.5%, and the RSD % was between 0.5% and 10.0%.
[Conclusions] The method has simple operation and high sensitivity, and is suitable for trace detection of sodium pentachlorophenoxide in large quantities of animal-derived food.
Key words Sodium pentachlorophenoxide; Ultra-high performance liquid chromatography-tandem mass spectrometry; Animal-derived food; Trace analysis
Sodium pentachlorophenoxide is an organochlorine pesticide, which also has the function of killing leeches, brackish-water crabs, fruit tree pests, fungi, bacteria, etc. It can also be used as a wood preservative and an agricultural herbicide, and is widely used. Because sodium pentachlorophenoxide has high water solubility, it is easy to spread widely with water as a carrier, causing pollution to water sources and soil. After entering into feed plants, it accumulates in animals through the food chain, and remains in food. Sodium pentachlorophenoxide enters human and animal body through the food chain and is decomposed into pentachlorophenol. Pentachlorophenol has the toxicity of organochlorine and phenol, can inhibit the oxidative phosphorylation in the process of biological metabolism, and can cause damage to the liver, kidney and central nervous system of the human body [1] . About 2 g can be fatal. Mild symptoms include nausea, dizziness, and fatigue, and severe symptoms include high fever, coma, and even death. According to the List of Drugs and Other Compounds Prohibited for Use in Food Animals (Announcement No.250 of the Ministry of Agriculture and Rural Affairs), sodium pentachlorophenoxide is a drug that is prohibited to be used in food animals (shall not be detected in animal food). The U.S. Environmental Protection Agency (EPA) has listed sodium pentachlorophenoxide as a suspected carcinogen and restricted its use. In Announcement No.235 Maximum Residue Limits of Veterinary Drugs in Animal Derived Food issued by the Ministry of Agriculture of China in 2002, sodium pentachlorophenoxide is listed as a prohibited drug and shall not be detected in animal food. In recent years, in market sampling activities , sodium pentachlorophenoxide has been detected in animal-derived food from time to time. Therefore, how to detect this substance quickly and accurately is a problem that needs to be studied urgently.
At present, common methods for the determination of sodium pentachlorophenoxide include gas chromatography-tandem mass spectrometry [2-4] and high performance liquid chromatography-tandem mass spectrometry [5-7] . Among them, gas chromatography-tandem mass spectrometry requires derivatization, and the pretreatment process is relatively complicated. Moreover, the reagents used are relatively harmful. High performance liquid chromatography-tandem mass spectrometry is widely used due to its high sensitivity and accuracy. However, the purification process mainly includes solid phase extraction purification method and QuEChERS method, of which the solid phase extraction method has good extraction effect, few impurities and long time. The QuEChERS method has high efficiency, but the impurity interference is strong, and the recovery is not high. Therefore, it is necessary to find a suitable and efficient pretreatment method. In this study, the extraction, purification and mobile phase conditions were further optimized. QuEChERS was combined with high performance liquid chromatography-tandem mass spectrometry to determine the residues of sodium pentachlorophenoxide in animal- derived food (chicken, pork, mutton, beef, fish, etc. ). The method had simple process, good purification, and high extraction rate, and provides technical support for the extraction of sodium pentachlorophenoxide in animal-derived food.
Materials and Methods
Instruments and reagents
Standard sodium pentachlorophenoxide (purity 86.0%, Dr. Ehrenstorfer, USA); acetonitrile (chromatographically pure, MERCK, USA); methanol (chromatographically pure, MERCK, USA); ammonia (analytically pure, Chengdu Kelong Chemical Reagent Factory); acetic acid (chromatographically grade, Cycling); hydrochloric acid (analytically pure, Sinopharm chemical reagent); ammonia water (analytical grade, Sinopharm chemical reagent); sulfuric acid (chromatographic pure, Sinopharm chemical reagent); n-hexane (analytical grade, Sinopharm Chemical Reagent Co., Ltd.); sulfuric acid (chromatographically pure, Sinopharm Chemical Reagent Co., Ltd.); n-hexane (analytically pure, Sinopharm Chemical Reagent Co., Ltd.).
Cleanert PAX solid phase extraction cartridge (150 mg/6 ml, Agela Technologies); QuChERS extraction kit (Qingdao Baierwei Biotechnology Co., Ltd.); QuChERS clean-up kit (Qingdao Baierwei Biotechnology Co., Ltd.); Agilent 1290 Infinity II/6470 high performance liquid chromatograph-tandem mass spectrometer (Agilent Technologies Co., Ltd., USA); 3-18KS high-speed refrigerated centrifuge (SIGMA Technology Co., Ltd.); Multi Reax multi-function oscillator (Heidolph, Germany); pipette (Eppendorf); TGL-16B high-speed desktop centrifuge (Shanghai Anting Scientific Instrument Factory); T25 digital homogenizer (IKA, Germany); GWB-2E pure water machine (Beijing Purkinje General Instrument Co., Ltd.).
The samples were food such as chicken and fish.
Methods
Preparation of standard solutions
The solid standard product was weighed and prepared with methanol to 1 mg/ml, which was diluted step by step to an intermediate standard solution of 1 μg/ml. Then, a certain amount of the intermediate standard solution was pipetted and diluted with water to standard series solutions with concentrations of 1, 2, 5, 10, and 20 ng/ml, respectively.
Pretreatment of samples
First, a (2±0.01) g of sample was weighed into a 50 ml centrifuge tube, and added with 1 ml of 0.1 mol/L hydrochloric acid solution, 14 ml of 80% acetonitrile water solution and QuEChERS extraction bag. The sample was extracted with shaking for 15 min, and centrifuged at 8 000 r/min for 5 min. Next, 1.5 ml of the supernatant was transferred into a QuEChERS purification tube, vortex-mixed for 1 min, and centrifuged at 7 000 r/min for 5 min. The obtained solution was filtered with a membrane for testing.
Instrument conditions
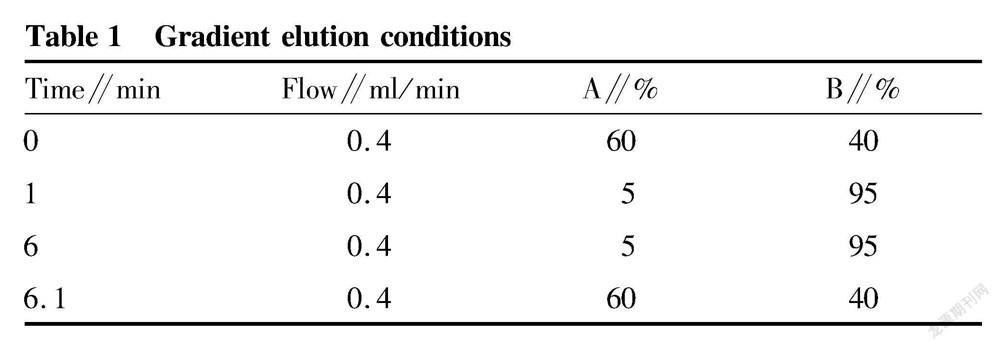
The instrument conditions were as follows: column: ZORBAX Eclipse plus C 18 column (50 mm× 3.0 μm), padding: octadecyl carbon chain, column temperature: 35 ℃, flow rate: 0.4 ml/min, injection volume: 1 μl, and mobile phase: A-5 mmol ammonium acetate solution, B-acetonitrile. The mobile phases and elution conditions are shown in Table 1.
The mass spectrometry conditions were as follows: scanning mode: negative election spray ionization (ESI), detection mode: multiple reaction monitoring (MRM), Gas Temp: 350 ℃, gas flow: 13 L/min, and nebulizer: 40 psi. The monitoring conditions are shown in Table 2.
Preparation of standard working curve
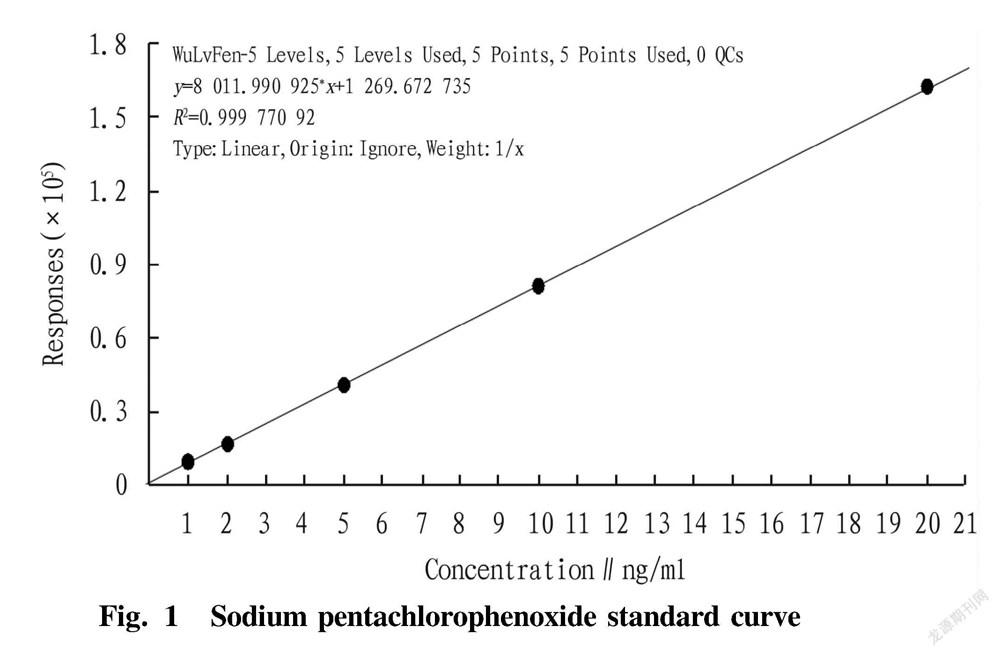
The standard stock solution of the target substance with a mass concentration of 1.0 μg/ml was prepared using the standard substance, and stored in a refrigerator. A sample without sodium pentachlorophenoxide was taken as a matrix sample, which was added with a standard solution having a certain concentration to the concentrations of 1.0 , 2.0, 5.0, 10.0 and 20.0 ng/ml, respectively. The solutions were treated according to the above pretreatment, and the obtained solution was loaded on the machine to make a matrix standard curve. The standard curve had good linearity with a correlation coefficient of 0.999 7, as shown in Fig. 1.
Results and Analysis
Optimization of different extractants
The dissociation constant pKa of sodium pentachlorophenoxide is 4.74. It mainly exists in the form of molecules in acidic solvents, and the extract mainly exists in the form of pentachlorophenol under acidic conditions, and in the form of sodium pentachlorophenoxide in alkaline conditions. In this study, it was found through extraction under different pH conditions that the extraction rate under acidic conditions was higher than that under alkaline conditions, and a relatively large peak appeared after the peak of sodium pentachlorophenolate during extraction under alkaline conditions. In this study, chickens and carps were studied separately, and a variety of extractants were used to treat the samples, including 5% triethylamine in acetonitrile water solution (acetonitrile∶ water=7∶ 3), 5% ammonia solution in acetonitrile water solution (8∶ 2), 1% formic acid in acetonitrile, acetonitrile water solution (7∶ 3), acetonitrile water (8∶ 2) solution containing 0.1 mol/L hydrochloric acid, and n-hexane extraction after sulfonation with sulfuric acid. Six parallel groups were performed for each concentration, and the results were averaged. The extraction efficiencies of various extraction solutions were compared and analyzed, and the results are shown in Table 3. The extraction rates of sodium pentachlorophenoxide under acidic conditions were higher than those under alkaline conditions, and the value was the highest in acetonitrile water solution containing 0.1 mol/L hydrochloric acid. Therefore, this extractant was finally selected as the extraction reagent of sodium pentachlorophenoxide in animal-derived food.
Chengmei WANG et al. Determination of Sodium Pentachlorophenoxide Residues in Animal-derived Foods by Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
Optimization of different extraction methods
The correct selection of extraction methods has a significant impact on the recovery. At present, the main extraction methods include ultrasonic extraction, oscillation extraction, and vortex extraction. These methods are all suitable for the extraction of animal-derived food. Ultrasonic extraction mainly relies on the vibration of ultrasonic waves to make extractants enter samples quickly, but it is easy to cause the samples to form agglomerates which cannot be easily dispersed during the extraction process. Oscillation extraction time is generally longer and milder than vortexing. The vortexing time is short and the frequency is high. Under the same conditions, the recovery of vortex extraction was the best, reaching 95.6%. Therefore, vortex extraction was selected in this study.
Optimization of the amount of extraction solvent
It has been found through experiments that with the increase of the amount of extraction solvents, the recovery of extraction also increases gradually, but the recovery will show a downward trend after increasing the extraction solvents to a certain extent. A small amount of extraction solvents cannot make good contact with samples. Although more extraction solvents have better contact, the cost is high and the extraction time is long. Therefore, an appropriate amount of extraction solvents can achieve higher recovery and save time. In this study, different solvent amounts were compared, and it was found that 15 ml could not only extract adequately, but also save time properly.
Optimization of purification conditions
Animal-derived food contains many oils, proteins, carbohydrates, and organic acids, which have certain impacts on the service life of chromatographic columns and the accuracy of the results. PSA can remove polar components in the matrices, such as organic acids, pigments and sugars, while C 18 has strong adsorption to strongly hydrophobic components such as lipids [8] . The QuEChERS purification tubes contain PSA, C 18 and anhydrous sodium sulfate. In this study, the QuEChERS purification tubes were adopted, which avoided weighing and also removed interfering components.
Linear range, limit of detection, and limit of quantitation for the method
Under the optimized experimental conditions, taking the peak area as the ordinate and the concentration of sodium pentachlorophenoxide as the abscissa, the linearity of sodium pentachlorophenoxide was good in the concentration range of 1.0-50 ng/ml, and the correlation coefficient was 0.997. By gradually reducing the spiked concentration, three times the signal-to-noise ratio (S/N) was used as the limit of detection, and 10 times the signal-to-noise ratio (S/N) was used as the limit of quantification. Finally, the limit of detection was determined as 0.25 ng/ml, and the limit of quantification was 0.75 ng/ml. Calculated according to the sample weight of 2 g in this study, the limit of detection in this method was 0.125 ng/ml, and the limit of quantification was 0.375 ng/ml, which is better than the national standard method [9] .
Recovery and precision tests
Chicken, carp, pork, mutton and beef that did not contain sodium pentachlorophenoxide were selected for standard addition and recovery. In this study, the standard was added at three gradients, and six parallel groups were performed for each gradient. The specific results are shown in the following table. The recovery of sodium pentachlorophenoxide was in the range of 87.4%-112.5%, and the precision was in the range of 0.2%-9.8%.
Conclusions and Discussion
Based on the pretreatment of QuEChERS samples, a method for the extraction of sodium pentachlorophenoxide from animal- derived food was established in this study. The method is simple to operate, avoids purification and derivatization, saves time and cost, and has a lower detection limit than the standard. It can not only be used for the trace determination of sodium pentachlorophenoxide in animal-derived food, but also provides technical support for food safety risk monitoring.
References
[1] HU B, CHEN YZ, HU HM. Research on the harm to humanbeing and livestocks caused by residues of sudium pentachlorophenate in animal foodstuff[J]. Meat Hygiene, 2005(2): 27-29. (in Chinese).
[2] HE X, ZHOU JF, TANG HQ. Determimation of pentachlorophenol in freshwater fish by sold phase extration gas chromstogaphy coupled with triple quadrupole mass spectromety[J]. J Food Saf Qul, 2018, 9(9): 2096-2100. (in Chinese).
[3] SHI YQ, YANG MI, YANG QH, et al. Rapid determination of pentachlorophenol in aqutic products by GC-MS [J]. Chin J Food Hyg, 2015, 27(1): 19-21. (in Chinese).
[4] ZHAO J ,CHENG L, TANG XQ, et al. Determination of pentachlorophenol in chicken and pork by gas chromalography- ion trap tandem mass spectromety(MS) coupled with isotopic intemal standard derivatization[J]. Chin J Health Lab Technol, 2016, 26(19): 2773-2775. (in Chinese).
[5] CUI Y, FANG CG, LI Q, et al. Simultaneous determination of seven organic contaminants residue in drinking water and source water by Ultra performance liquid chromatography-mass/mass spectrometry [J]. Chin J Pubic Health Eng, 2017, 16(2): 125-128. (in Chinese).
[6] ZHUANG L, MIN JQ, CHEN XH, et al. Determination of pentachlorophenol in urine by SPME-LC-MS/MS[J]. Chin J Health Lab Technol, 2017, 27(22): 3209-3211. (in Chinese).
[7] XIA BL,YAN QF, YANG N, et al. Determination of pentachlorophenol residue in animal-origin foods by EMR-lipid-ultra performance liquid chromatography-tandem mass spectrometry[J]. J Food Saf Qual, 2019, 10(14): 4698-4705. (in Chinese).
[8] SUN JY, AN LH, YANG M, et al. Rapid screening and analysis of sodium pentachlorophenoxide residues in food by QuEChERS-high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety & Quality, 2020(14): 4758-4762. (in Chinese).
[9] GB 23200.92-2016 National food safety standards: Determination of pentachlorophenol residue in animal-derived foods liquid chromatography-mass spectrometry[S]. 2016. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Molecular Cloning and Bioinformatics Analysis of crp Gene in Vibrio alginolyticus
- Breeding Technology of Sunflower Inbred Lines with Four Generations in One Year
- Artificial Plant Seeds and Their Application
- Breeding of a Water-saving Drought-resistant Two-line Hybrid Rice Variety Wanliangyou 1008
- Development of Landscaping and Selection of Contemporary Landscaping Plants
- The Register and Characteristics of Chinese Key Protected Wild Plants in Dabie Mountains National Nature Preserve, Hubei Province

