Effects of Grape Seeds and Tea Polyphenols on Serum Enzymatic Indices of Finless Eels
Wenhuan ZHU Zhaohui WEI Tianle HUANG Yangzhi LUO Shaomei YU Yiran LIU Shengyuan ZHANG Ping DENG Guizhen DING Hongliang XU Liqiang ZHANG



Abstract [Objectives] This study was conducted to investigate the possibility of adding grape seeds and tea polyphenols to improve the health of finless eels ( Monopterus albus ).
[Methods] Twelve cages were selected in the same water body and divided into 4 groups, each with 3 parallel groups. The control group was fed with common finless eel feed, and the experimental groups were fed with feed supplemented with grape seeds, tea polyphenols, and grape seed-tea polyphenol compound (with a ratio of grape seeds to tea polyphenols at 1∶ 1), respectively. The feeding amount of each additive was 1%. The experiment was carried out by feeding medicated feed for 3 d and ordinary feed for 3 d, that is, alternately feeding medicated feed and ordinary feed. The experiment lasted for 18 d.
[Results] Six serum enzymatic indices were detected after the breeding. The results showed that compared with the control group, the three groups of samples had a certain protective effect on the liver in terms of liver function indices. In terms of immunity evaluation, the tea polyphenol group and the grape seed-tea polyphenol complex group could increase the immunity of eels to varying degrees. In terms of antioxidant capacity, all three groups of samples could increase the body’s antioxidant capacity. The results of this study showed that adding tea polyphenols and grape seeds to the feed simultaneously could effectively protect the liver of fish, and simultaneously enhance their antioxidant and non-specific immune functions.
[Conclusions] This study provides a reference for the application of the two Chinese herbal medicines, grape seeds and tea polyphenols, in finless eel farming.
Key words Grape seed; Tea polyphenol; Finless eel; Serum
Finless eel ( Monopterus albus ) is an important aquaculture species in China. According to the data of the 2020 China Fishery Statistical Yearbook , the total output of the national finless eel farming in 2019 was 313 790 t. Large-scale finless eel farming has also brought serious disease problems. The diseases of finless eels mainly include bacterial diseases, such as enteritis, haemorrhagic septicaemia and ulcers, and parasitic diseases, such as Trypanosoma monopter, Proterometra guangzhouensis and Neosentis celatus [1-4] .
With the development of aquaculture, concepts such as green farming, food safety, and water environmental protection are increasingly recognized by farmers. Chinese herbal medicine has the advantages of green safety, non-specific immune enhancement, no easy production of drug tolerance, and anti-stress, and more and more attention has been paid to its role in the field of aquaculture [5-9] . Grape seeds are the seeds of grapes. In addition to being rich in protein, minerals, fats, various trace elements and carbohydrates, grape seeds are also rich in polyphenolic compounds, such as tannins and resveratrol. Studies have shown that grape seed extracts have significant antioxidant, hypolipidemic, antibacterial, antitumor and other effects, so they have high medicinal value [10-14] . Tea polyphenols are the general term for polyhydroxy compounds, and their main components are anthocyanins, flavanols, flavonoids, phenolic acids and flavonols, and have high medicinal functions [15-21] .
In this study, in order to investigate the effects of grape seeds and tea polyphenols on the non-specific immunity of finless eels, grape seeds, tea polyphenols and their compound preparation were added into the feed of finless eels at an amount of 1%, aiming to provide a reference for the application in farming.
Materials and Methods
Experimental materials
Materials added to feed included grape seeds (Shaanxi Sciphar Natural Products Co.,Ltd.), tea polyphenols (Hubei Zhongxin Biotechnology Co., Ltd.), and grape seed and tea polyphenol complex (complex of grape seeds and tea polyphenols with a mass ratio of 1∶ 1). The feeding test of eels was carried out in Weixiang Aquaculture Professional Cooperative in Xiantao City, Hubei Province. Wild finless eel fry were caught from the wild field. After domestication in an indoor greenhouse, strong finless eel fry with a body mass of approximately 100 g/eel were selected and placed in nylon rope cages (2 m×2 m). Alternanthera philoxeroides (Mart.) Griseb., commonly known as water peanut, is planted in the cages. The breeding site was a soil pond. The experiment was carried out from July 22 to August 10.
Experimental methods
The experiment was divided into 4 groups, 1 control group and 3 experimental groups, each with 2 replicates. The control group was fed with common finless eel feed, and the experimental groups were fed with feed supplemented with grape seeds, tea polyphenols, and grape seed-tea polyphenol compound respectively. The feed was minced fillet of silver carp surimi and eel feed Jiasheng No.2 mixed at 1∶ 1 (the main ingredients of basic feed were fish, soybean meal, yeast, refined fish oil, flour, chelated minerals, stable compound vitamin, etc. ). The feeding amount of each additive was 1%. In the same water body, 12 cages were selected, and the experiment was carried out by feeding medicated feed for 3 d and common feed for 3 d, that is, alternately feeding medicated feed and ordinary feed. They were fed once in the morning and evening, and the food table was cleaned 1 h after feeding to remove excess feed. The experiment lasted for 18 d.
Index determination
Samples were taken on day 6, day 12, and day 18, respectively. Six finless eels were taken from each group, and the tails were docked to collect blood. After coagulation, centrifugation was performed at 4 000 r/min for 10 min at 4 ℃, and serum was collected and frozen at -20 ℃ for future use.
The investigated serum enzymatic indexes included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate (ALP), acid phosphatase (ACP), nitric oxide synthase (NOS), and superoxide dismutase (SOD). The serum enzymatic indexes were entrusted to Wuhan Servicebio Biotechnology Co., Ltd. for determination. An automatic biochemical analyzer performed automatic detection after the samples were loaded. Generally, the biochemical operation is carried out according to the instructions of the kit, and the experimental operations of different indexes are different.
Data analysis and statistics
One-way ANOVA was performed using GraphPad Prism 8.0.2 software, and multiple comparisons between groups were performed using Duncan’s method, with a significant level of P <0.05.
Results and Analysis
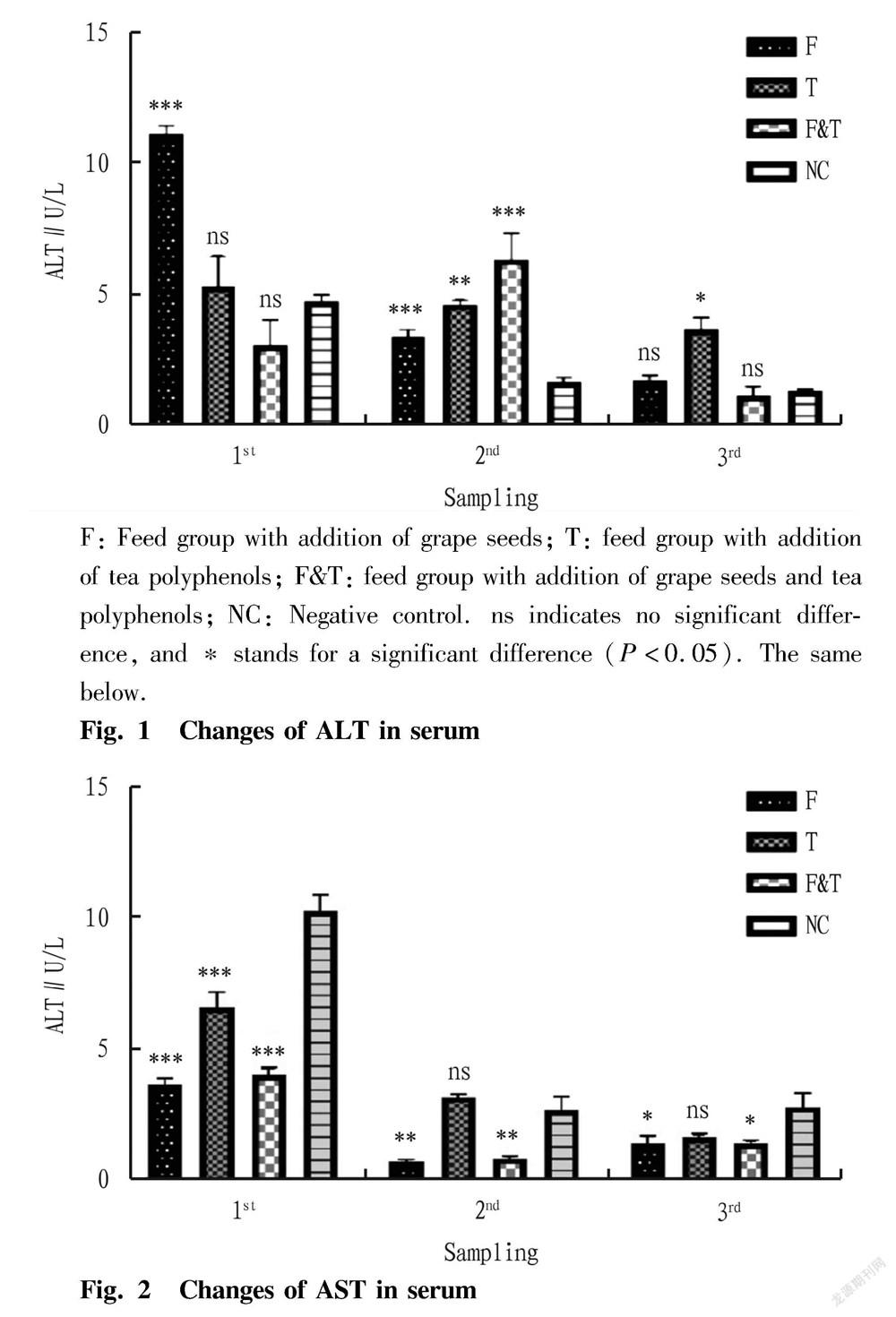
As shown in Fig. 1, the activity of alanine aminotransferase (ALT) in the serum of the eels in the grape seed-added feed group (F) was significantly higher than that in the control group in the first sampling and the second sampling ( P <0.05), while there was no significant difference from the control group in the third sampling ( P >0.05). The tea polyphenol-added feed group (T) showed no significant difference in the activity of serum alanine aminotransferase from the control group in the first sampling ( P >0.05), but was significantly higher than the control group in the second sampling ( P <0.05), and there was also a significant difference in the third sampling ( P <0.05). For the group fed with the feed added with grape seeds and tea polyphenols (F&T), there was no significant difference in the activity of serum alanine aminotransferase from the control group in the first sampling and the third sampling ( P >0.05), but a significant difference from the control group in the second sampling ( P <0.05).
It can be seen from Fig. 2 that the activity of aspartate aminotransferase (AST) in the serum of eels in the grape seed-added feed group (F) was significantly lower than that in the control group in all the first, the second and the third sampling ( P <0.05). The tea polyphenol-added feed group (T) was significantly lower in the activity of aspartate aminotransferase (AST) from the control group in the first sampling ( P <0.05), while no significant differences were observed in the second sampling and the third sampling ( P >0.05). The activity of aspartate aminotransferase in the serum of eels in the group of feed added with both grape seeds and tea polyphenols were significantly lower than that in the control group in all the first, the second and the third sampling ( P <0.05).
F: Feed group with addition of grape seeds; T: feed group with addition of tea polyphenols; F&T: feed group with addition of grape seeds and tea polyphenols; NC: Negative control. ns indicates no significant difference, and * stands for a significant difference ( P <0.05). The same below.
As shown in Fig. 3, the activity of alkaline phosphatase (ALP) in the serum of eels in the grape seed-added feed group (F) was significantly lower than that in the control group in the first sampling and the third sampling ( P <0.05), while no significant difference was observed from the control group in the second sampling ( P >0.05). The tea polyphenol-added feed group (T) was significantly lower in the activity of alkaline phosphatase than the control group in the first and the third sampling ( P <0.05), but it was significantly higher than the control group in the second sampling ( P <0.05). The feed group with the addition of grape seeds and tea polyphenols was significantly higher in the activity of alkaline phosphatase than the control group in the first sampling ( P <0.05), but was significantly lower than the control group in the second and third sampling ( P <0.05).
It can be seen from Fig. 4 that the activity of acid phosphatase (ACP) in the serum of eels in the grape seed-added feed group (F) was significantly lower than that in the control group in the first and second sampling ( P <0.05), while there was no significant difference from the control group in the third sampling ( P >0.05). The tea polyphenol-added feed group (T) and the grape seed and tea polyphenol addition group (F&T) were significantly lower than the control group in the first, second and third sampling, respectively ( P <0.05).
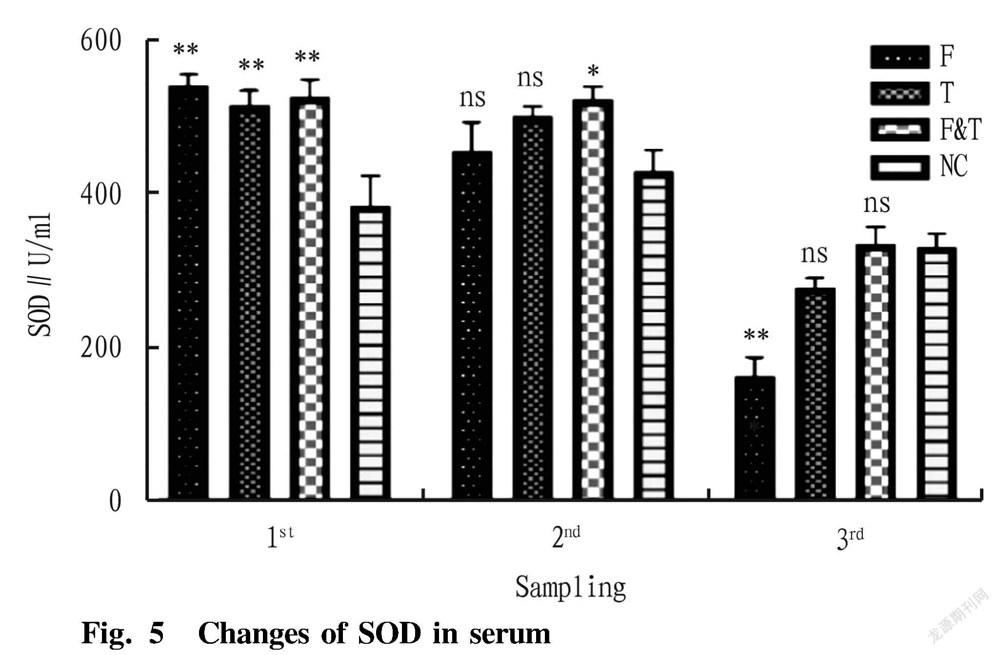
As shown in Fig. 5, the activity of superoxide dismutase (SOD) in the serum of eels in the grape seed-added feed group (F) was significantly higher than that in the control group in the first sampling ( P <0.05), while there was no significant difference from the control group in the second sampling ( P >0.05), and the value was significantly lower than that in the control group in the third sampling ( P <0.05). The activity of superoxide dismutase in the serum of eels in the tea polyphenol-added feed group (T) was significantly higher than that in the control group in the first sampling ( P <0.05), while there were no significant differences from the control group in the second and third sampling ( P >0.05). For the feed group with the addition of both grape seeds and tea polyphenols (F&T), the activity of superoxide dismutase (SOD) was significantly higher than that in the control group in the first and second sampling ( P <0.05), while there was no significant difference from the control group in the third sampling ( P >0.05).
It can be seen from Fig. 6 that there was no significant difference in the activity of serum nitric oxide synthase (NOS) in eels in the grape seed-added feed group (F) from the ontrol group in the first sampling ( P >0.05), while in the second and third sampling, the activity of serum nitric oxide synthase was significantly lower than that in the control group ( P <0.05). The tea polyphenol-added feed group (T) had no significant differences in the activity of serum nitric oxide synthase from the control group in the first, second and third sampling ( P >0.05). The grape seed and tea polyphenol addition group showed on significant differences in the activity of serum nitric oxide synthase from the control group in the first and second sampling ( P >0.05), while in the third sampling, the activity of serum nitric oxide synthase was significantly lower than that in the control group ( P <0.05).
Wenhuan ZHU et al. Effects of Grape Seeds and Tea Polyphenols on Serum Enzymatic Indices of Finless Eels ( Monopterus albus )
Conclusions and Discussion
ALT and AST are key enzymes in amino acid metabolism, and their activity also reflects the normality of liver function. When hepatocytes are damaged, the permeability of the hepatocyte membrane increases, and ALT and AST in the cytoplasm are released into the blood, resulting in increases in the activity of serum ALT and AST. However, they are not organ-specific, and many diseases can cause its increase [22] . Serum ALT and AST are indicators reflecting liver cell damage, and the increase of the activity of non-specific enzymes is a common clinical phenomenon [23] . Increased ALT and AST are associated with fat deposition in the liver [24] . In this study, the addition of grape seeds and tea polyphenols to the feed could effectively reduce the ALT and AST values in the serum of eels, indicating that both grape seeds and tea polyphenols have a certain protective effect on the liver.
The immunity of aquatic animals is evaluated by the activity of ALP and ACP [25] . The activity of NOS is positively correlated with the non-specific immunity of fish, and after fish receives external stimuli, the enzyme can express NO in large quantities, thereby killing pathogenic bacteria through the non-specific immunity of NO, so as to increase the non-specific immunity [26] . In this study, the grape seed-added feed group had no significant effect on the three indicators of ALP, ACP and NOS, while the tea polyphenol-added feed group could increase fish immunity to a certain extent, though the duration was short and the degree of increase was limited. It indicates that tea polyphenols can enhance the non-specific immunity of fish to a certain extent.
The oxygen-containing free radicals and peroxides generated during the redox reaction are called reactive oxygen species (ROS). Due to the strong oxidative properties of ROS, oxidative stress will occur when excessive ROS are produced in the body, such as oxidizing the cell membrane to produce lipid peroxides, which thereby destroys the cell structure and leads to cell dysfunction, apoptosis or necrosis [27-28] . ROS in general organisms are in a state of dynamic equilibrium [29] . Superoxide dismutase (SOD) reduces the damage to cells by disproportionating superoxide anion, and has a certain repairing effect on damaged cells. SOD is the first barrier against oxidation in organisms [30] . In this study, both the grape seeds-added feed group and the tea polyphenol-added feed group could significantly increase the level of SOD in the body, indicating that they can effectively enhance the antioxidant capacity of organisms.
Grape seeds and tea polyphenols have various biological activity. Tea polyphenols have antioxidant, bacteriostatic and mutagenic effects [31] , and grape seeds have anti-oxidation, blood vessel elasticity-enhancing , and liver-protecting effects [32] . The results of this study showed that the simultaneous addition of tea polyphenols and grape seeds to fish feed could effectively protect the liver of fish, and simultaneously enhance their antioxidant and non-specific immunity functions.
References
[1] WEN ZR. Advances in researches of main diseases and prevention and control technologies of ricefield eel[J]. Hubei Agricultural Sciences, 2018, 57(14): 10-14. (in Chinese).
[2] LI MF, WANG RY. Progress on study of Monopterus alba (Zuiew) disease[J]. Modern Fisheries Information, 2004(6): 6-8. (in Chinese).
[3] ZENG MH, ZHANG ZP, GAO S, et al. Isolation, identification and drug sensitivity test of ricefield eel white head pathogens[J]. Journal of Yangtze University: Natural Science Edition, 2016, 13(9): 29-32, 3-4. (in Chinese).
[4] REN HM, HE Z, YANG DY, et al. Isolation, identification and pathogenicity of causative pathogen for muscular ulcer in Monopterus albus [J]. Acta Hydrobiologica Sinica, 2010, 34(6): 1106-1112. (in Chinese).
[5] CHI MM. The function and application of grape seeds[J]. Food Research and Development, 2016, 37(20): 221-224. (in Chinese).
[6] SUN XL, YANG SY, CHEN SQ, et al. Effects of compound Chinese herbal medicine on antioxidant, non-specific immunity and digestive enzyme activity in Salvelinus pluvius and Salmo trutta fario[J]. Chinese Journal of Fisheries, 2020, 33(3): 7-12. (in Chinese).
[7] DIAO J, WANG YH, WANG SX, et al. Effects of a compound chinese herbal immune enhancer on the immunity and disease resistance in Japanese flounders Paralichthys olivaceus [J]. Journal of Guangxi Academy of Sciences, 2020, 36(2): 137-144. (in Chinese).
[8] LU SX, LUAN XB, WANG D, et al. Effect of intermittent administration of three compound Chinese herbal medicines on growth and immunity in amur sturgeon[J]. Chinese Journal of Fisheries, 2020, 33(1): 13-18. (in Chinese).
[9] FU Y, FU B, GUO XQ, et al. Effect of Chinese herbal medicine compounds on growth and non-specific immune response of Litopenaeus vannamei [J]. South China Agriculture, 2020, 14(3): 163-166. (in Chinese).
[10] SHI HR, HE Q, LYU QQ, et al. Effect of compound Chinese medicine and nucleotide additives on growth and immunity of hybrid grouper ( Epinephelus fuscoguttatus ♀× E. lanceolatus ♂) [J]. Chinese Journal of Fisheries, 2019, 32(6): 41-47. (in Chinese).
[11] ZHAO KS, SHI Q, LI XC. Research progress on chemical constituents and pharmacological effects of grape seeds[J]. Journal of Taishan Medical College, 2019, 40(9): 718-720. (in Chinese).
[12] SHAN WW, ZHAI ZH, DING YS, et al. Effect of grape-seed proanthocyanidins extract on renal tubular epithelial cells apoptosis induced by iohexol and its mechanism[J]. Acta Universitatis Medicinalis Anhui, 2019, 54(12): 1859-1864. (in Chinese).
[13] DU YM, HAN HD, GUO ZY, et al. Effect of grape seed proanthocyanidin extract on cisplatin-induced apoptosis of mouse testicular sertoli TM4 cells[J]. Food Science, 2020, 41(9): 98-104. (in Chinese).
[14] TU X, LUO L, LIU YL, et al. The effects of grape seed proanthocyanidin extract on oxygen-glucose deprivation injury in HT22 cells [J]. Chinese Journal of Neuroanatomy, 2020, 36(1): 57-64. (in Chinese).
[15] TIAN FZ, WANG YL, ZHAO GH, et al. Research progress on the antibacterial effect of tea polyphenols[J]. Modernizing Agriculture, 2020(5): 29-32. (in Chinese).
[16] CAI J, YE R, JIA K, et al. Review on extraction and antibacterial activity of tea polyphenols[J]. Chemical Reagents, 2020, 42(2): 105-114. (in Chinese).
[17] LIU YH, LI J, LI K, et al. Tea industry situation: Tea polyphenols[J]. University Chemistry, 2019, 34(8): 102-106. (in Chinese).
[18] ZHANG XF, CHEN RF, LIU LJ, et al. Study on the application of tea polyphenols in food[J]. Chinese Journal of Bioprocess Engineering, 2019, 17(4): 424-429. (in Chinese).
[19] MA H, RU X, WANG J, et al. Study on the antioxidant capacity of four tea water extracts and tea polyphenols in vitro [J]. Food Research and Development, 2019, 40(8): 65-70. (in Chinese).
[20] ZHOU J. Application and research progress of tea polyphenols in animal production[J]. Guangdong Feed, 2019, 28(3): 27-29. (in Chinese).
[21] YAN ZM, GUO QP, ZHONG YZ, et al. Tea polyphenols functions and their application in animal production[J]. Chinese Journal Of Animal Nutrition, 2019, 31(4): 1525-1532. (in Chinese).
[22] HAN XS. Correlation between serum adiponectin, alanine aminotransferase and metabolic syndrome[D]. Taiyuan: Shanxi Medical University. (in Chinese).
[23] HU G, QIAO Q, TUOMILEHTO J, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic european men and women[J]. Archives of Internal Medicine, 2004.
[24] HOFFMAN E L, VONWALD T, HANSEN K. The metabolic syndrome[J]. South Dakota Medicine the Journal of the South Dakota State Medical Association, 2014, spec no: 24-28.
[25] SARLIN PJ, PHILIP R. Efficacy of marine yeasts and baker’s yeast as immunostimulants in Fenneropenaeus indicus : A comparative study[J]. Aquaculture, 2011, 321(3/4):173-178.
[26] LIU HL, GUO WJ, WANG CG, et al. Effects of breeding density on growth, metabolism and immunity of Penaeus merjimin[J/OL]. Fisheries Science: 1-10[2021-06-10]. https://doi.org/10.16378/j.cnki.1003-1111.20062. (in Chinese).
[27] HALLIWELL B, GUTTERIDGE JC. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy[J]. Lancet, 1984, 323(8391): 1396-1397.
[28] NORDBERG J, ARNR ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system[J]. Free Radic Biol Med, 2001, 31(11): 1287-312.
[29] SIES H. Role of reactive oxygen species in biological processes[J]. Klinische Wochenschrift, 1991, 69(21): 965-968.
[30] MRUK DD, SILVESTRINI B, MO MY, et al. Antioxidant superoxide dismutase a review: Its function, regulation in the testis, and role in male fertility[J]. Contraception, 2002, 65(4): 305-311.
[31] YANG JX. Research and application of tea polyphenols[J]. Nongjia Keji, 2011(7): 94-94. (in Chinese).
[32] SUN BM. Study on the mechanism of tea plant MYB transcription factor CsAN1 regulating grape seed[D]. Guangzhou: South China Agricultural University. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Molecular Cloning and Bioinformatics Analysis of crp Gene in Vibrio alginolyticus
- Breeding Technology of Sunflower Inbred Lines with Four Generations in One Year
- Artificial Plant Seeds and Their Application
- Breeding of a Water-saving Drought-resistant Two-line Hybrid Rice Variety Wanliangyou 1008
- Development of Landscaping and Selection of Contemporary Landscaping Plants
- The Register and Characteristics of Chinese Key Protected Wild Plants in Dabie Mountains National Nature Preserve, Hubei Province

