The Phragmites Root-Inhabiting Microbiome: A Critical Review on Its Composition and Environmental Application
Donglin Wng, Yohui Bi*, Jiuhui Qu
a Key Laboratory of Drinking Water Science and Technology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
b College of Resource and Environment, University of Chinese Academy of Sciences, Beijing 100049, China
Keywords:Phragmites Rhizosphere microbiome Microbial community composition Pollution remediation Constructed wetlands
ABSTRACT As widespread wetland plants,Phragmites play a vital role in water purification and are widely utilized in constructed wetlands (accounting for 15.5% of applied wetland plants) as a natural alternative to wastewater treatment. However, despite such common applications, current understanding of the basic composition of the Phragmites root-inhabiting microbiome and the complex functions of each member of this microbiome remains incomplete, especially regarding pollution remediation. This review summarizes the advances that have been made in ecological and biochemical research on the Phragmites root microbiome,including bacteria,archaea,and fungi.Based on next-generation sequencing,microbial community compositions have been profiled under various environmental conditions. Furthermore,culturebased methods have helped to clarify the functions of the microbiome, such as metal iron stabilization,organic matter degradation, and nutrient element transformation. The unique community structure and functions are highly impacted by Phragmites lineages and environmental factors such as salinity.Based on the current understanding of the Phragmites root microbiome,we propose that synthetic microbial communities and iron-manganese plaque could be applied and intensified in constructed wetlands to help promote their water purification performance.
1. Introduction
Wetlands are often considered to be the ‘‘kidneys of the Earth”due to their unique ecological role in water purification [1].Wetlands constructed for wastewater treatment are engineered systems inspired by natural processes, which transform and/or stabilize pollutants in contaminated water within a more controlled environment [2]. Constructed wetlands (CWs) have developed rapidly over the last two decades due to their superior performance in comparison with traditional wastewater treatment plants and have thus drawn increased attention from researchers(Fig. 1). CWs are robust, have low external energy requirements,and are easy to operate and maintain, which makes them suitable for decentralized wastewater treatment in areas without public sewage systems or that are economically underdeveloped [3,4].
The removal of contaminants in CWs is a complex process that mainly involving the combined roles of soil particles,microbes,and plants. These three parts of the CWs play different roles in pollution remediation: Soil particles filter out and adsorb pollutants, thereby providing greater opportunities for their degradation and transformation by plants and microorganisms [5].Enriched microorganisms around the plant roots, resulting from the rhizosphere effects of plants, play a critical role in the biodegradation of pollutants [6]. These root-inhabiting microbes also provide transformed substances (e.g., nitrate) for uptake and assimilation by plants [7]. Therefore, interactions between plants and microbes are critical for CW performance [6,8] and have garnered increasing attention in recent years [9,10]. Most previous studies have focused on how to manipulate the root microbiota to promote crop production [11-13], avoid diseases or pathogens[10,14,15], and resist stress such as drought [16-18]. However,only a few reports have explored wetland plant root-associated microbiomes and their relevant functions [8,19]. To improve the water purification capacity of CWs,it is critical that we understand how wetland plants interact with microbes and contribute to pollution remediation.
As perennial gramineous emergent aquatic plants with strong adaptability and wide ecological amplitude, Phragmites can form dense dominant communities in aquatic ecosystems and are among the most common plants found in wetlands [2,20]. Moreover, Phragmites are one of the most commonly used plants in CWs, accounting for 15.5% of applied wetland plants over the last two decades (Fig. 1) and even higher in actual engineering treatment plants. Given their wide distribution in natural wetlands and large-scale application in CWs, Phragmites and their rootinhabiting microbiome exhibit unique potential for contaminant remediation and improved wetland function.
To determine how microbial-based control strategies can be enhanced in CWs using a collective impact approach,effort should be expended on understanding the symbiosis between reed roots and microbes. In this review, we summarize previous research on the exploration of the reed root-associated microbiome,including:①the role of the Phragmites root microbiome in pollution remediation; ②the contribution of each member in the microbiome,including bacterial, archaeal, and fungal communities; and ③the wide application of Phragmites in CWs, in which potential bioaugmentation approaches can be applied to enhance pollutant removal. Given the above aspects, we also summarize the strengths and deficiencies of current research and propose future developments in this area.
2.Roles of the Phragmites root microbiome in metals,organics,and nutrients removal
As CWs are impacted by different types of contaminants, the reed root microbiome must be versatile under different situations—that is, when interacting with metal ions, organic matter(OM), or nutrient elements.
2.1. Metal ions
The removal of metal ions mainly relies on the plant rhizosphere environment [21,22]. For some metal ions, such as zinc(Zn), iron (Fe), and manganese (Mn) ions, which are attributed to plant growth and photosynthesis, assimilation by plants is of first consideration,especially when metal ions are present at relatively low concentrations [23]. The ions can be absorbed by plants and are then transferred to the shoots and leaves. Nevertheless, these metal ions are generally precipitated in anaerobic and anoxic environments,given their redox potentials.Plant root exudates,which are associated with root microbes, can influence metal ion bioavailability and anti-aggregation by secreting secondary compounds to mediate dissolution. For example, siderophores are known to play a role in the chelation and dissolution of various elements [22,24].
Other metal ions that are harmful to human health and the environment, such as arsenic (As) and chromium (Cr), especially at high concentrations, can be immobilized and transformed by roots and their microbes.This is regarded as the primary approach for metal ion stabilization in heavy metal wastewater treatment[25-27]. Phragmites can form Fe plaque to strengthen the wetland load under high-concentration metal ion stress [28]. Fe plaque is generated under root radial oxygen loss [29,30] and with the help of Fe-oxidizing bacteria, which are common root-inhabiting microbes [31]. The plaque is characterized as amorphous oxide or hydroxides, which provide sufficient sites for microbial congregation and metal ion absorption[32,33].Therefore,high concentrations of metal ions can be sequestrated by Fe plaque in reed root systems.Certain harmful ions such as As(III)can be adsorbed onto the Fe plaque and oxidized into less harmful As(V)by As-oxidizing root-inhabiting bacteria [34,35]. The same process has been reported for selenium and mercury [36]. A more detailed discussion is provided later in this work.
Collectively, the above processes can transfer metal pollution from aggregations in the soil to microbes and plants, thereby alleviating soil pollution and enhancing the performance of CWs.
2.2. Organic matter
OM in wastewater treatment is commonly characterized by chemical and biochemical oxygen demand (COD and BOD, respectively). In CWs treating artificial wastewater, the establishment of Phragmites exhibits a higher removal efficiency of COD and organic nitrogen (N) than areas without Phragmites [37]. Phragmites can absorb a certain amount of soluble OM (octanol/water partition coefficient, logKowbetween 0.5 and 3.5), such as trichloroethene(TCE) [38], and show a low adsorption efficiency for perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS) [39], and ibuprofen [40]. Nevertheless, certain OM pollutants can be removed by root microbes[41].In addition,OM can serve as a carbon (C) and N source for the root-inhabiting microbiome [38]. For example, the Phragmites root microbe Sphingobium fuliginis uses 4-tert-butylphenol as the sole C source for growth [42].
The reed root microbiome is like a‘‘black box”in which interactions among microbes and microbes, and among microbes and plants, are complex. During rhizoremediation, plant roots secrete exudates, which enhance or stimulate the growth and activity of the rhizosphere microbial community, resulting in the effective degradation of OM pollutants [8]. However, future research needs to explore the pollution remediation potential against more complex and harmful OM, which could broaden the application of reed-bed CWs.
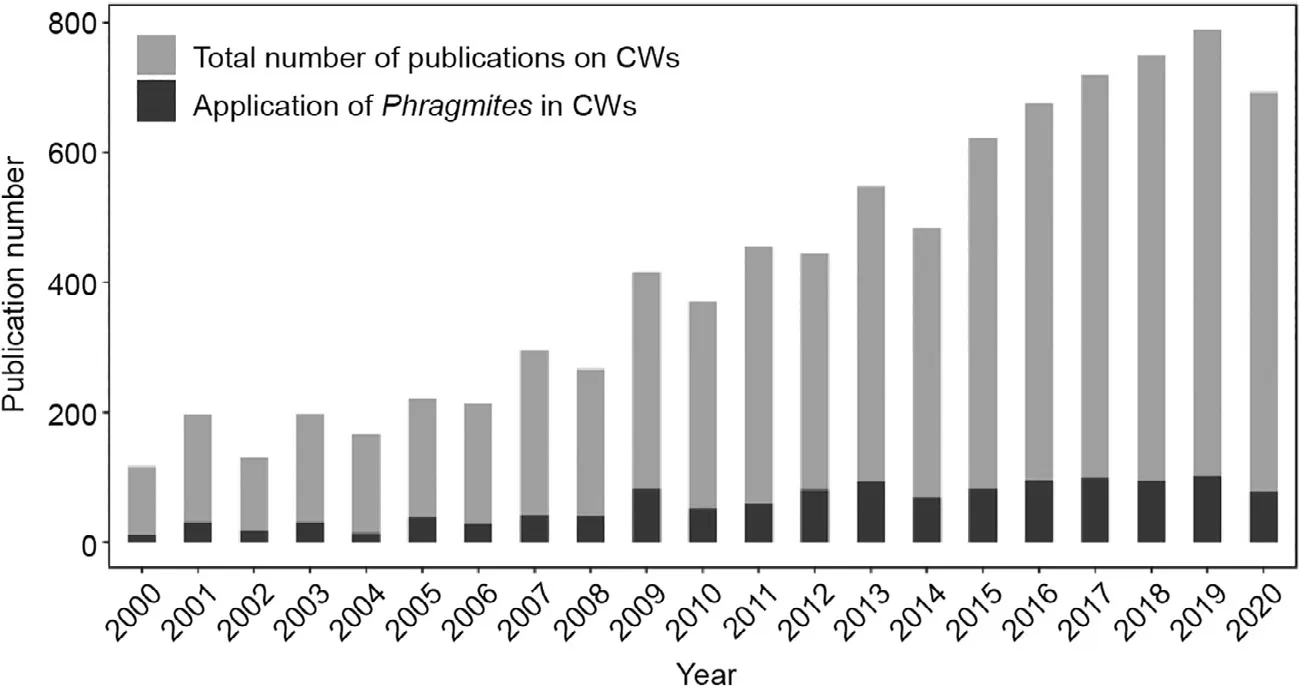
Fig. 1. Proportions of applied plants in CWs in articles published from 2000 to 2020. Data were obtained from the Web of Science Core Collection using ‘‘constructed wetlands”as the topic words in articles from 2000 to 2020.The grey bar represents the total number of publications using‘‘constructed wetlands”as topic words(contained in title or abstract or keywords); the black bar represents studies that applied reeds (Phragmites).
2.3. Nutrient elements
Both nitrogen (N) and phosphorus (P) are essential growth factors for Phragmites species and their rhizosphere microbes. The removal of nitrogen and phosphorus by Phragmites is effective when the plants are harvested and removed from the system;otherwise,nitrogen and phosphorus can be reintroduced following plant decomposition[43].In contrast,root microbes may be able to convert or mobilize these nutrient elements in situ,which benefits the long-term and sustainable operation of CWs.
It has been found that the planting of Phragmites promoted the mineralization of organic nitrogen,nitrification,and denitrification in the resulting sediments compared with sediments that had not previously been planted. The microbes inhabiting the roots of Phragmites were responsible for almost half of the total N removal[37]. The rhizosphere environment of the Phragmites provided the necessary conditions for microbial N cycling: ①a repeating cycle of oxidizing and reducing conditions to cope with different environments for the transformation of the varied forms of nitrogen;②sufficient C source for denitrification or dissimilatory nitrate reduction to ammonium (DNRA), which is provided by the exudates of Phragmites,while OM can serve as a C source for microbes;and ③most importantly, a specific microbiome with functional genes, which is commonly composed of bacteria and archaea.The abundances of bacterial 16S ribosomal RNA (rRNA), archaeal 16S rRNA [44], ammonia-oxidizing bacterial ammonia monooxygenase (coding by the gene amoA), ammonia-oxidizing archaealamoA [40,41], denitrifying bacterial nitrite reductase (nirK) genes[44], and even the anaerobic ammonium oxidation (anammox)process [45,46] increased significantly in sediments planted with Phragmites, and promoted the mineralization of organic N, carried out nitrification and denitrification in the sediments, and eventually facilitated the N removal. Therefore, it is obvious that reed roots provide a suitable platform for root-inhabiting microbes to complete N removal.
For phosphorus, as a basic growth element for plants and microbes, assimilation is the primary process that occurs during wastewater treatment in CWs.Compared with nitrogen,the existing forms of phosphorus (e.g., PO43-) can also precipitate with metal ions [44]. Given that Fe plaque on the reed root surfaces gathers metal ions as well as different forms of phosphorus, the reed rhizosphere environment forms a phosphorus pool for both the plant itself and its root microbes [48]. In addition, some root microbes can further transform or absorb phosphorus for plants or themselves, resulting in purification [49].
To conclude, the Phragmites root-inhabiting microbiome is a vital part of pollution remediation in CWs. Therefore, understanding the Phragmites root microbiome is essential for successful contaminant removal and immobilization, and includes an understanding of: ①microbial composition and existence in the root environment; ②microbial performance under various situations;and ③potential microbial functions for further applications.
3. Phragmites root microbiome members and their performance
Investigation of the magnitude and distribution of plant microbiome inhabitants—which include fungi,bacteria,archaea,protists,and viruses—over the past several decades has had a transformative effect on plant-microbe research [11]. The development of nextgeneration sequencing (NGS), a culture-independent method, has allowed researchers to study the composition profiles of microbiomes under different environments.Among different NGS methods, amplicon sequencing has been widely used for the composition profiling of bacteria,archaea(e.g.,16S ribosomal DNA(rDNA)), and fungi (e.g., internally transcribed spacer (ITS), 18S rDNA). Therefore, we reviewed recent culture-independent and culture-dependent research profiling the root microbiomes and their contributions to pollution remediation. As current research on protists and viruses in the Phragmites root environment has not directly contributed to contaminant transformation due to their specific ecological roles [50,51], separate discussions are only provided for the bacterial,archaeal,and fungal communities.
3.1. Bacterial community composition
Bacterial community composition (BCC) has been extensively studied in previous research using 16S rDNA gene-based highthroughput sequencing. Studies on the root-inhabiting bacterial microbiota have focused on the‘‘bulk soil compartment”(i.e.,bacteria in unplanted soil); ‘‘rhizosphere compartment” (i.e., bacteria attached to roots and collected by the centrifugation of root washings); and ‘‘root compartment” (i.e., epiphytic bacteria found in root tissue depleted of soil particles by sequential washing and sonication) [52]. In Phragmites roots, these three compartments generally exhibit different structures and compositions. He et al.[53] examined the BCC of the common reed (Phragmites australis(P.australis))and observed higher α-diversity(indicating richness)and lower β-diversity (indicating stability across samples) of the BCC in the rhizosphere compared with that in the bulk soil and root compartments. These findings indicate that the assembly of the Phragmites root microbiome is recruited under the synthetic effects of plants and the environment,which is consistent with the finding that deterministic processes determine the bacterial assemblages of the Phragmites rhizosphere microbiome from the perspective of microbial community ecology [54]. In addition, the Phragmites rhizosphere BCC can affect the composition of the root compartment bacteria[53].Due to their distinct roles,the rhizosphere bacterial community is of particular interest in research, as shown in Table 1 [54-63].
3.1.1. Compositional variation
Taxonomically,bacteria are strongly represented by Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes at the phylum level[64].However,the BCC structure can vary in different studies,as Phragmites are well adapted to a wide variety of soil (or sediment) environments, which exhibit considerable differences in salinity (0-25 part per trillion (ppt)), pH, nutrient content, and hydrological regime [65]. As shown in Table 1, geographical sampling sites in previous wetland research can be classified into shoreline and riparian zones, in which salinity plays an important role in driving BCC [55]. Furthermore, the water quality of CWs exerts an important influence on the structural and functional characteristics of Phragmites BCC [66]. In addition to environmental effects, Phragmites lineage and genotype are important. Bowen et al.[56]explored different lineages and compositions of the root microbiota of P.australis and showed that within-lineage bacterial communities are similar but among-lineage bacterial communities are distinct.In contrast,Yarwood et al.[67]reported that bacterial biomass and composition do not differ between native and invasive P. australis lineages. The reasons for these different results may be related to the effects of the environment, which differed considerably between the garden control test used by Bowen et al. [56] and the field test used by Yarwood et al. [67]. Eventhough P. australis lineages can cause composition differences, the outer environment might have masked the effects brought by lineages.Furthermore,genotype-driven influence could also be superseded by environmental factors. For example, Phragmites karka (P.karka), another genotype of Phragmites, shows an overrepresentation of Proteobacteria, Firmicutes, Actinobacteria, and Chloroflexi in rhizosphere communities, which is in agreement with existing studies on P. australis [55]. In fact, the composition of the exudates produced by different species or lineages and the accompanying changes in soil C and N compounds might stimulate or inhibit specific species of bacteria [68]. Moreover, under the same habitat, P. australis and Typha latifolia tend to converge toward a common taxonomic composition [57].
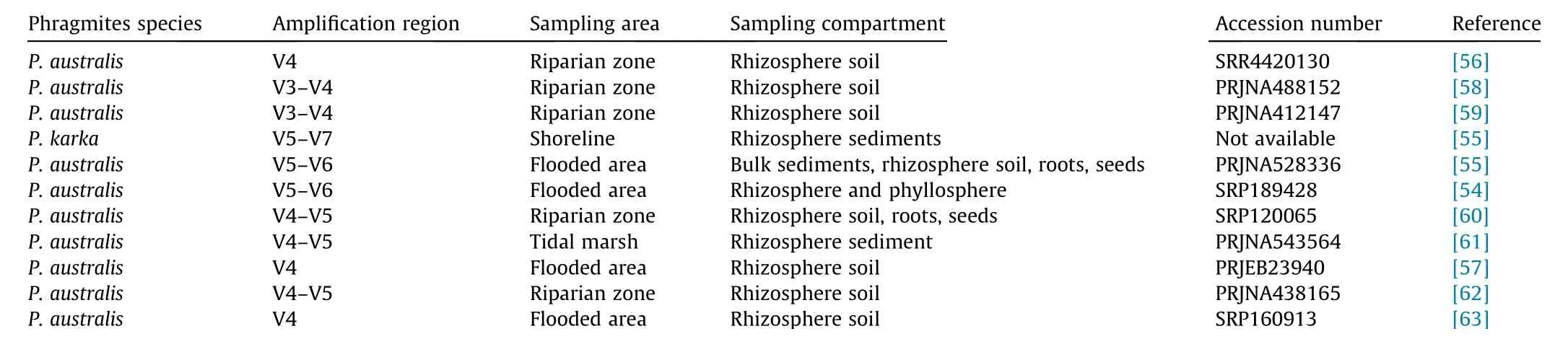
Table 1 Research applying 16S rDNA sequencing on the Phragmites root microbiome.
In general, environmental factors exert a strong influence on Phragmites BCC, and may even masked the ‘‘rhizosphere effects”caused by host plants. Nevertheless, the current methods limit our understanding. Even under the same amplicon sequencing method,primer bias or variation in amplification region can differ,which makes cross-validation between studies difficult to carry out. Therefore, sampling and sequencing methods with uniform procedures should be established [69]. In addition, shotgun metagenomic/metatranscriptomic sequencing could be further considered in order to profile the functional compositions of the Phragmites root-inhabiting microbiome.
3.1.2. Potential roles in pollution remediation
The aim of BCC profiling is to thoroughly understand the special functions fulfilled by the bacterial community. Zhang et al. [58]used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) to predict the potentially functional composition of the Phragmites root bacterial microbiome from 16S rDNA gene sequences.The results showed that xenobiotic biodegradation and metabolism,which are related to benzoate and aminobenzoate degradation, were the most dominant functional groups. The functional categories involved in the degradation of chloroalkane,chloroalkene,caprolactam,naphthalene,and toluene were also plentiful.
In addition to culture-independent methods,culture-dependent methods can be used to confirm the distinct functions of a bacterial community.Toyama and colleagues carried out a series of degradation experiments using P.australis rhizosphere sediments to biodegrade technical nonylphenol (tNP) [70] and pyrene and benzo[a]pyrene [71], and found results similar to those predicted using PICRUSt above. The researchers could not only profile the functional composition in combination with the isotopic tracer technique, but also quantify the contribution of the Phragmites bacterial community to nitrate reduction in different habitats(i.e., water sediment or land soil) [47]. Thus, bioinformatics can provide a relatively complete profile of functional composition,and can therefore help in future experiments to verify the proposed functions of interest.
3.2. Phragmites root-inhabiting archaea and fungi
Although they are active members of microbial communities,archaea and fungi remain poorly characterized in the roots of wetland plants,and their functional roles are largely unreported.However, they still contribute to and play important roles in microbe-Phragmites interactions. Most previous research has examined archaea and fungi in regard to the root compartment, where they attach closely to the root surface of Phragmites,as discussed below.
3.2.1. Archaeal community
Archaeal community composition has been studied based on 16S rDNA gene sequencing, in the same way as bacteria has been studied [72]. However, as only a few archaeal species have been successfully cultivated using culture media, culture-independent methods(e.g.,NGS)are more commonly utilized to study archaeal diversity[73].Based on current research,the most commonly documented archaeal phyla are Crenarchaeota and Euryarchaeota,among which ammonia-oxidizing and methanogenic archaea are the dominant types and play significant roles in the N cycle and methane production in Phragmites rhizosphere soil, respectively[72,74,75].
Archaeal community composition is influenced by many factors. The composition of Phragmites root archaea is more sensitive to environmental factors compared with the host-plant-driven effect from the genotypes of Phragmites, as the same dominant archaeal composition in the Phragmites root is also present in the rice root system [76]. However, unlike bacterial communities,endophytic archaeal communities are more vulnerable to soil salinity [74]. The different distribution patterns of Phragmites root bacterial and archaeal endophytes along a salinity gradient imply that these two groups play different roles in plant-microbe interactions. Furthermore, archaea may have the ability to promote Phragmites growth and resistance to salinity, as archaeal community composition is strongly associated with water salinity [75].
Unfortunately,due to a lack of available databases and effective cultural methods, the structure and functions of Phragmites root archaea are beyond current research. From the perspective of bioinformatics, a total of 19.1% of the sequences in the Phragmites root environment are unclassified,suggesting that many unidentified archaea are actively involved in the reed wetland ecosystem[72]. Therefore, more unique functions of Phragmites root archaea could be discovered with the development of more advanced methods.
3.2.2. Fungal community
Integrated cultivation and molecular identification approaches are commonly used to explore fungal community structure [77].Based on molecular analysis of the rDNA internal transcribed spacer(ITS)region,only a few fungal species are reported to dominate the highly diverse community structures associated with Phragmites[68].Endophytes have been recovered from the roots of P. australis, showing that Hypocreales and Sarocladium strictum
dominate the isolated fungal community[77,78].Although the different climatic and geophysical conditions at sampling sites may influence the composition of fungal communities, these dominant species contribute to certain functions associated with Phragmites,such as the ability to resist salinity [72]. Phragmites root fungal communities under high-salt circumstances show higher resistance to Zn,mercury,and salt stress compared with fungal species found under low-salt conditions. These endophytes also show a greater propensity for the growth promotion of rice seedlings (a model species) under salt stress [79,80].
Apart from salinity,lineage is also important in fungal community composition [20]. Recently, Gonzalez Mateu et al. [81]assessed the role of dark septate endophytes (DSEs) in the fungal endophyte communities of native and invasive lineages of P. australis and found that native and invasive lineages had distinct fungal endophyte communities. Studies on the differences between invasive and native lineages of P. australis are consistent with the‘‘habitat-adapted symbiosis” hypothesis—that is, that endophytic microbes may help plants adapt to extreme habitats [64,74,80].The co-evolution of plants and microbes, along with the capacity of plants to establish symbiotic relationships with diverse endophytic microbes, appears to enhance plant tolerance to abiotic stress [80]. These results could indicate a factor that contributes to plant invasiveness,which can cause both economic and ecological damage [82].
Each member of the Phragmites root microbiome is closely related to the host plant as well as to the environment, which can exert varied influences on different members. During the operation of CWs, changes in temperature, pH, and dissolved oxygen and redox potential (ORP) influence the biomass, microbial composition, and intensity of microbial interactions, which can be indicated by network complexity [83]. A variety of key pollutant-removal processes such as sedimentation, precipitation,and volatilization would be subsequently influenced [84]. All members of the microbiome exhibit a high potential for pollution remediation, which makes Phragmites the most widely applied plant in CWs. However, as a compact pollution remediation platform,CWs show great strength in integrating state-of-the-art techniques for performance promotion.Based on the current advances in microbial functional composition, further effort is required regarding how to apply biological augmentation processes in CWs in order to control and promote performance precisely.
4. Bioaugmentation application in CWs
Each member of the root microbiome plays a role in pollution remediation. Nevertheless, interactions among members of the microbiome can amplify certain functions,such as N transformation and stubborn OM degradation [85,86]. Therefore, based on our understandings of the exact functions of each member in the rhizosphere microbiome and the complex interactions among them,we can design and assemble a microbial community according to specific purposes,which are known as synthetic microbial communities(SynComs)[87].Moreover,aside from living microbes,extracellular polymer substances that are generated by microbes,as well as root iron plaque, also contribute to pollution remediation [14]. For example, iron plaque has been shown to participate in harmful heavy metal ion (e.g., As) stabilization, phosphate immobilization,N cycling, and micropollutant removal [88-92]. The components of the iron plaque not only included Fe oxides,but also Mn oxides,which were generated by microbial processes [92]. Therefore, we propose intensifying the generation of Mn oxides by stimulating the functional microbes,which can eventually lead to the enhanced performance of CWs.Based on the two aspects described above,two methods can be applied to enhance the performance of CWs: the application of synthetic microbial communities, which may promote the microbial removal of pollutants; and intensification of the generation of biogenic Fe-Mn oxides.
4.1. Synthesizing rhizosphere microbiomes
According to the National Human Genome Research Institute(National Institutes of Health (NIH), USA), synthetic biology is defined as ‘‘a field that involves redesigning organisms for useful purposes by engineering them to have new abilities.” This technique can be applied to give host plants growth-promoting advantages and disease resistance [93]. For a new CW, the Phragmites rhizosphere microbiome can be engineered using bottom-up or top-down approaches. In bottom-up approaches, microbes associated with special functional traits and abilities are isolated from environmental microbiomes.After direct assembly or genetic engineering (e.g., phage integrases, integrative and conjugative elements (ICEs), or chassis-independent recombinase-assisted genome engineering (CRAGE) to ensure that the microbes carry desired physiological traits and functions (e.g., degrading specific pollutants)), the selected microbes are reassembled as SynComs[87,94]. The selection of microbes in SynComs should consider the unique traits and abilities of each member in order to ensure that they complement each other; functional redundancy of Syn-Com members can increase the resilience of inoculants, especially in wetland environments[95].Plants are then inoculated with the SynComs, which can robustly recolonize the hosts. In top-down approaches, mobile genetic elements (e.g., plasmid-harbored specific degradation genes)are incorporated into a conjugal donor strain. Then, ideally through horizontal gene transfer, the donor strain can deliver the mobile genetic element to a broad range of rhizosphere microbes in situ [93]. At present, the application of SynComs has helped clarify the roles of the rhizosphere microbiome (e.g., plant-microbe interactions) under different environmental conditions; however, the use of SynComs to reinforce the rhizosphere effect(e.g.,bioremediation)is still largely unexplored.Furthermore, whether SynCom members can survive and exert their functions in situ is unpredictable because plant-microbe and microbe-microbe interactions among rhizobial plants,indigenous microbes,rhizobial inoculants,and SynComs are complex and may hinder successful colonization (Fig. 2).
4.2. Intensifying the generation of biogenic Fe-Mn oxides
Precipitation of Fe hydroxides and oxyhydroxides in reed rhizosphere environments can lead to the formation of Fe plaque, which can, in turn, act as a barrier to heavy metal uptake[96]. Mn plaque is usually co-generated or sub-generated with Fe plaque [97]. Fe plaque not only shows a high affinity to Mn ions,but can also provide growing sites for Fe and/or Mn oxidizing bacteria (FeOB and MnOB) [32,98]. Unlike Fe oxides, which rely heavily on chemical reactions between Fe(II) and oxygen [31],bacterially mediated oxidation dominates the abiotic oxidation of Mn(II) by several orders of magnitude [99]. Thus, bacterial Mn(II) oxidation is considered to be a primary driver for Mn(III) and Mn(IV) oxide formation within the environment. Like Fe hydroxides, biogenic Mn oxides have a large specific surface area, which results in high absorption rates of OM and of heavy metal ions such as As, Cr, and lead [100]. Due to the high redox potential of Mn oxides, the adsorbed contaminants can be oxidized, and the generated Mn(II) is subsequently re-oxidized by MnOB [101-103].
Active Mn cycling shows great potential in wastewater treatment in CWs [104]. The removal efficiencies of nitrate, phosphate polycyclic aromatic hydrocarbon (PAH), and micropollutants in CWs are significantly increased with the application of Mn oxides[105-109]. As the abovementioned Mn-amending research did not detect Mn(II) in the effluent, the researchers speculated that Mn oxidation occurred within their systems; however, they underestimated the Mn(II) oxidation processes and plantmicrobe reactions (Fig. 2). Xie et al. [110] noted that Mn oxidation activity is more intense at depths of 10-20 cm below the CW surface, where plant-microbe interactions are also relatively more intense. Thus, it is reasonable to believe that the plant rhizosphere environment favors Mn(II) oxidation and MnOB enrichment.
In natural environments, Fe-Mn plaque accumulation can account for up to 10% of reed root dry weight and extends as much as 15-17 μm into the rhizosphere, thus providing an ecological foundation for wastewater treatment [111]. However, the average concentration of Mn in wetlands is approximately 100 mg·kg-1[97]. This is a critical factor in regard to the application of the Mn-amending method to promote the removal of pollutants in CWs, as shown in Fig. 2. Key enzymes in Mn biooxidation processes are closely related to litter decomposition[112]. Plant litter can accelerate Mn oxidation by enriching MnOB in the Phragmites root environment [113]. In addition, microbial Mn oxidation may be a stress response to the molecular arms secreted by plants [114,115]. Phragmites usually successfully compete with other plants to form dense dominant communities,and are considered to be invasive species in North America; furthermore, it has been found that their root exudates can act as‘‘molecular arms” [116]. It is reasonable to believe that planting Phragmites together with other wetland plants in CWs could stimulate the generation of Phragmites’ molecular arms to win the competition, which could, in turn, contribute to the generation of Mn oxides (Fig. 2).
5. Summary
In general, the ecologically meaningful application of Phragmites depends on interactions between reed roots and their associated microbes. Each root-inhabiting microbe, including bacteria,archaea, and fungi, directly or indirectly contributes to metal ions sequestration, OM degradation, and nutrient element transformation.The composition of the microbiome varies with environmental factors (e.g., salinity) as well as plant genotype. While current culture-dependent and culture-independent methods have profiled the structure of the microbial communities, advances in genetic sequencing technology and the development of cultivation media will increase our capability to detect and identify new microbial taxa. To better understand the complex interactions involved in the root microbiome, basic scientific research in combination with improved technologies will enable CWs to be used on a broader scale. Designing and applying Phragmites root Syn-Coms and bio-generating Fe-Mn plaque(oxides)could help to balance and strengthen the current shortfalls and functions of CWs.In future research, we consider it essential to explore the detailed mechanisms of pollutants’ transformation and degradation in the rhizosphere environment and recognize the exact groups of functional microbes that contribute to pollution remediation. Based on that knowledge, SynComs could be assembled more suitably for application in CWs. In addition, substrate materials of CWs(e.g., Mn oxides) could be modified to possess the ability to selectively accumulate functional microbes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51778603) and the Chinese Academy of Sciences(QYZDY-SSW-DQC004).
Compliance with ethics guidelines
Donglin Wang,Yaohui Bai,and Jiuhui Qu declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Science and Technology for Combating Global Water Challenges
- Brain-Computer Interface Speaks Up
- Solar Geoengineering to Reduce Global Warming—The Outlook Remains Cloudy
- Pandemic Scrambles the Semiconductor Supply Chain
- A Multi-Stage Green Barrier Strategy for the Control of Global SARS-CoV-2 Transmission via Cold Chain Goods
- Next-Generation Imaging: New Insights from Multicolor Microscopy in Liver Biology and Disease

