Recent advances in the electroreduction of carbon dioxide to formic acid over carbon-based materials
LI Wen-bin, YU Chang*, TAN Xin-yi, CUI Song, ZHANG Ya-fang, QIU Jie-shan*
(State Key Lab of Fine Chemicals, School of Chemical Engineering, Liaoning Key Lab for Energy Materials and Chemical Engineering,Dalian University of Technology, Dalian 116024, China)
Abstract: The electroreduction of carbon dioxide (CO2) driven by renewable energy is an important route for CO2 conversion and utilization. Formic acid (HCOOH), as an important chemical and safe hydrogen storage material, is one of the main and promising materials for CO2 electroreduction. The physical and chemical properties of CO2 and the reaction mechanisms for its electroreduction to HCOOH are outlined and the recent development of carbon-based catalysts, including metal-free carbon catalysts and carbonsupported catalysts, for CO2 electroreduction to HCOOH is reviewed. The design of reactors for HCOOH production and strategies for their optimization are summarized and discussed. Hybrid CO2 electrolysis technology is analyzed, such as electroreduction coupled with the methanol electrooxidation reaction. Lastly, key challenges and development trends for CO2 electroreduction to HCOOH are presented, which are expected to provide guidance for the development of this technique.
Key words: Carbon-based materials;CO2 electroreduction;Formic acid;Reactor
1 Introduction
The constant burning of fossil fuels results in large amounts of greenhouse gases that are released into the atmosphere, which causes an increase of the global carbon dioxide (CO) concentration continuously, and also causes the imbalance of the carbon cycle. To deal with climate change problems that was caused by massive COemissions, including global warming, ocean acidification and ecosystem destruction, the conversion and utilization of COhave attracted the wide attention of researchers.Electrochemical COactivation and catalytic conversion driven by renewable energy has become a significant technical route for COconversion and utilization due to its advantages of green, mild operating conditions, and energy saving. Electrochemical COreduction reaction (CORR) is a proton-coupled multi-electron transfer process, and the reaction is relatively complex, which involving multiple reaction intermediates and different reduction products. COis linearly symmetric in structure and has chemical stability. In thermodynamics, the activation of COrequires a large energy input with the C―O bond energy of 803 kJ mol. In terms of kinetics, the catalytic conversion of COis competitive with hydrogen evolution reaction (HER). Therefore, the development of electrocatalysts with high activity, selectivity and stability is the key to achieve the COactivation and conversion.
Electrochemical COconversion involves multielectron transfer, which can produce a variety of reduction products, including carbon monoxide(CO), formic acid (HCOOH), methane(CH), ethylene (CH), ethanol (CHOH),etc. Among the COelectroreduction products,HCOOH, as an important chemical basic raw material, is the key reaction intermediate for synthesis of down-stream products such as HCOOH ester, formamide and other products. HCOOH is also an important hydrogen storage material, which can safely and efficiently store hydrogen energy in the form of liquid and ensure the safety of hydrogen storage and transportation. At present, the industrial production method of HCOOH is mainly methanol carbonylation,which belongs to energy-intensive industry with the issues of environmental pollution problems. Therefore, the concerned electrochemical technology to convert the COinto HCOOH is a significant and promising technology, where the COconversion to HCOOH is a two-electron transfer process with an initial potential of −0.19 V vs. RHE. In contrast to the multi-electron transfer and multiple-step process of COreduction to Cproducts, the electrocatalytic COreduction to HCOOH has a relatively low overpotential, featuring high energy efficiency and low cost in terms of theory.
Currently, the electrocatalytic materials that are used for CORR mainly include carbon-based materials, metal and metal oxides, metal-organic skeleton (MOF) and covalent organic skeleton(COF), as well as molecular catalyst. Among them, the carbon-based materials are highly concerned and have made great progress in the field of COelectroreduction to HCOOH by virtue of their advantages of unlimited sources, controllable morphology, large specific surface area and high stability, indicative of superiority and well development potential.
In this review, the recent advances of carbonbased electrocatalytic materials in electrochemical COreduction to HCOOH is summarized. Firstly, the physical and chemical properties of COmolecule are introduced. The reaction mechanism for electroreduction of COto HCOOH and common carbon-based catalysts are reviewed and discussed. Furthermore, the design and optimization strategy of reactors for HCOOH production was summarized and commented. Then the hybrid COelectrolysis technology was analyzed by taking the COelectroreduction coupled methanol electrooxidation reaction as an example. Finally, the current challenges and future industrial prospects for COelectroreduction to HCOOH are discussed and proposed.
2 Mechanism of CO2 electroreduction to HCOOH
As for COmolecule, the outermost four electrons of C atom are configurated by sp hybridization,which are filled in the form of two sp hybrid orbitals and 2 unhybridized orbitals (and). The two sp orbitals of C atom overlap with the 2p orbitals of O atoms to formbonds. While the two unhybridized p orbitals overlap in parallel with the p orbitals provided by each of the two O atoms to form large π bonds, respectively. Therefore, the molecular linearity of COis stabilized by the unique π-conjugated system of three centers with four electrons. On the basis of unique stability, the activation of COmolecule is a critical step during CORR process. In view of this, it is necessary to input electrons to activate COinto excited state of CO, and further achieve the COelectroreduction. Various products are produced, being dependent on the different reduction reaction mechanisms derived from the multiple electron transfer. Electroreduction of COto HCOOH is a two-electron transfer reaction which can be divided into the two reaction mechanisms. The first reaction mechanism is schemed as follows:

In general, the presented mechanisms involve several processes, including COadsorption, activation, reaction on the catalyst surface and desorption of the intermediates from the surface of catalyst. The active sites will bond with the C or O atoms of COmolecule, which depends on the properties of adopted and developed catalyst. Finally, the two reaction intermediates (COOH, OCHO) follow the different reaction pathways to proceed the COelectroreduction.
At present, the development of efficient and stable electrocatalysts is one of the keys to realize the COelectroreduction to HCOOH. Carbon-based electrocatalysts are highly concerned due to the low cost and adjustable structure stability. Of course, metalfree carbon materials can not only be directly used as electrocatalysts for COelectroreduction, but also can be adopted as suitable supports to construct the highperformance electrocatalysts via coupling with other active components.
3 Metal-free carbon catalyst
The carbon materials have been ideal electrocatalysts for CORR because of superior conductivity and high stability under electrochemical conditions.Moreover, the active sites can be constructed easily by virtue of the characteristics of adjustable defect sites,surface chemistry and electronic structure of carbon materials, resulting in the improvement of performance of metal-free carbon catalysts. For electroreduction of COto HCOOH, Xu et al.prepared a series of monolayer graphene nanodisks (GNDs) with abundant oxygen functional groups by a simple hydrothermal reaction under different temperatures,where graphene oxide and nitric acid were used as the raw materials. The GNDs-160 can realize a supreme current density and Faradaic efficiency (FE) (Fig. 2a,b). The density functional theory (DFT) revealed that the synergetic effects between carboxyl groups on graphene surface and other adjacent oxygen-containing groups (hydroxyl, carbonyl, epoxy, etc.) on the surface of GNDs were responsible for the high performance, which could significantly reduce the energy barriers for COtoCOOH (Fig. 2c, d). Meyer et al.firstly synthesized N-doped carbon nanotubes by treating the carbon nanotubes in an ammonia plasma atmosphere. Then polyethylenimine modified Ndoped carbon nanotubes were prepared by an impregnation method, where the N-doped carbon nanotubes were immersed into polyethylenimine aqueous solution. The as-made metal-free carbon material as the electrocatalyst exhibited a FEof 87% with a current density of 9.5 mA cmfor COelectroreduction to HCOOH. As a cocatalyst, the coated polyethyleneimine and doped nitrogen species in carbon nanotubes resulted in the formation of a favorable microenvironment, promoting the enrichment of COon the electrode surface, contributing to the adsorption and activation of CO, and improving the production rate of HCOOH.
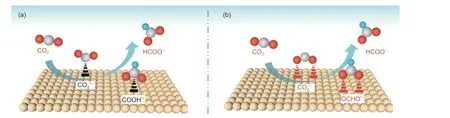
Fig. 1 Two possible mechanisms for electroreduction of CO2 to HCOO−, where the C, O, H atoms and catalyst are represented by red, gray, blue, yellow colors.

Fig. 2 (a) Linear sweep voltammetry curves over different GNDs catalysts for CO2RR. (b) FEHCOO− over different GNDs catalysts for CO2RR. (c) Models of carbon catalysts with different configurations containing oxygen functional groups. (d) Gibbs free energy of CO2RR and HER over carbon catalysts with different configurations of oxygen-containing functional groups[31]. Reprinted with permission by American Chemical Society.
4 Carbon-supported catalysts
Besides as electrocatalysts for COelectroreduction directly, the carbon materials are also used as the low-cost and scalable supports for catalyst for CORR, including zero-dimensional carbon quantum dots, one-dimensional carbon nanotubes, two-dimensional graphene etc. Various metal or metal oxides are supported or anchored on the various carbon matrix,producing a series of electrocatalysts such as carbonsupported metal catalysts, heteroatom-doped carbonsupported metal catalysts, MOF-derived carbon-based catalyst. As a result, the electronic structure, the Lewis acidity/alkalinity of catalyst surface, Fermi level etc. can be regulated, finally resulting in the improved COadsorption and activation, accompanied by the decreased reaction energy barrier and the promotion of activity and selectivity of the catalysts for electroreduction of COto HCOOH.
4.1 Carbon-supported metal catalysts
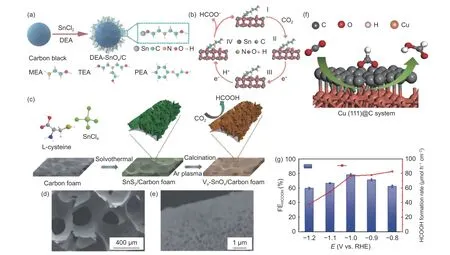
For carbon-supported metal catalysts, Kang et al.synthesized a carbon black-supported diethanolamine-modified tin oxide catalyst by using carbon black, SnCland diethanolamine as raw materials(Fig. 3a). The catalyst exhibited an excellent performance with a FE of 84.2% for HCOOH at a potential of−0.75 V vs. RHE. The surface amine functional groups on the catalyst were beneficial to capture and collect the COmolecule, and accelerate the formation of HCOO, thereby regulating the distribution of products (Fig. 3b). Our group designed a monolithic carbon foam-supported SnOnanosheet with rich oxygen vacancies(Fig. 3c), which achieved a maximum FEof 86% for CORR. It can be observed from the scanning electron microscopy (SEM) images that the three-dimensional porous carbon foam catalyst featured the abundant interconnected channels(Fig. 3d, e), which greatly accelerated the mass transfer in the reaction. While the excellent electrical conductivity was in favor of the electron transfer, as expected. The oxygen vacancies of SnOwere conducive to adsorb the COmolecules and provided the more opportunities for exposure of active sites as well as enhancement of the electrocatalytic performance.Deng et al.prepared a Cu@C catalyst with uniformly distributed copper nanoparticles embedded in a carbon substrate by using a simple and fast ball milling method. The DFT results indicated that the high activity of catalyst derived from the synergistic effects between the abundantly exposed Cu (111) surface and the carbon substrate. The charge transfer from Cu to the carbon substrate induced the formation of a charge-rich local microenvironment, which enhanced the adsorption of *OCHO intermediates andpromoted the formation of HCOOH (Fig. 3f). At a potential of −1.0 V vs. RHE, the FE of the Cu@C catalyst reached up to 78% and the yield of HCOOH can be up to 82.8 μmol hcmat −1.2 V vs. RHE(Fig. 3g).
4.2 Heteroatom-doped carbon-supported metal catalysts
Heteroatom doping is an effective strategy to regulate the intrinsic electronic structure of the carbon substrates and further improve their catalysis activity. Further, the introduction of heteroatoms can alter the electronic structure between metal and carbon substrates, adjust the Fermi level of the electrocatalyst as well as change Lewis acidity and alkalinity. Moreover, these heteroatoms can enhance the adsorption and activation of COmolecules on the active centers and promote the formation of the target products. Luo et al.synthesized S-doped BiO-CNT composite material (Fig. 4a). The as-synthesized S-BiO-CNT catalyst can achieve a HCOOH FE of 97.06% (Fig. 4b). The S doping can realize the electron delocalization of Bi atoms and enhance the adsorption of COon the Bi sites, as well as stabilize the intermediates of CO* and HCOO*and hinder the HER. Therefore, the ability of COelectroreduction to HCOOH was enhanced and the HER was inhibited (Fig. 4c). Hu et al.prepared N,S co-doped SnO/NSC composites using mesoporous carbon as substrate (Fig. 4d). The introduction of N and S species can effectively enhance the metal-support interaction and promote the rapid transfer of electrons from the support to the SnOnanoparticles. This endowed the surface of SnOwith negative charge and promoted the adsorption of CO, thus enhancing the activity of CO. The experimental results showed that the maximum FE of SnO/NSC for HCOOH can reach up to 94.4% with a current density of 56 mA cm.Zhang et al.synthesized an atomically dispersed indium-nitrogen-carbon catalyst (In-N-C, Fig. 4e). The strong interaction between atomically dispersed indium and adjacent N atoms on the carbon skeletons can improve the performance of the electrocatalyst for CORR. The In-N-C catalyst can achieve a FE of 80%for HCOOH with a high TOF value (26 771 h).
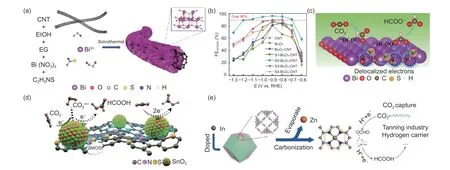
Fig. 4 (a) Schematic fabrication process for S-doped Bi2O3-CNT. (b) FEHCOOH over a series of catalysts at different applied voltages. (c) Schematic diagram of the S species-promoted effects on CO2RR to HCOOH[36]. Reprinted with permission by American Chemical Society. (d) Mechanism diagram for CO2RR to HCOOH over N, S-doped SnO2/NSC[37]. Reprinted with permission by American Chemical Society. (e) Schematic fabrication process for In-N-C[38]. Reprinted with permission by American Chemical Society.
4.3 MOF-derived carbon-based catalysts
MOFs made of metal nodes and functional molecular building blocks are widely used as precursors or templates to synthesize the morphologically and dimensionally controllable catalysts. In addition, the inherent pore structure of the MOFs materials is also helpful for forming the carbon with large specific surface areas and abundant pore channels, which will facilitate the adsorption and uniform distribution of COon active sites. Xia et al.prepared a carbon-coated rodlike BiOcomposite (BiO@C) by high temperature carbonization with Bi-MOF as the precursor(Fig. 5a). The transmission electron microscopy(TEM) image exhibited that the BiOspecies uniformly dispersed within the carbon matrix (Fig. 5b).The synthesized BiOhad excellent electrocatalytic performance, suggesting that carbon matrix contributed to improve the activity and current density, and the bismuth oxide in composites contributed to improve the reaction kinetics and selectivity. For CORR, the catalyst had an initial potential of −0.28 V vs. RHE, and can achieve a maximum FE of 93% for HCOOH with the HCOOH partial current density greater than 200 mA cm(Fig. 5c). Wu et al.synthesized carbon-supported CuBi bimetal catalyst by carbonizing the CuBi-MOF precursor (Fig. 5d). The catalyst showed an excellent CORR performance,with the FE remaining above 93.7% in a wide potential window range of 900 mV for HCOOH, achieving 100% FE of HCOOH at −0.77 V vs. RHE (Fig. 5e).
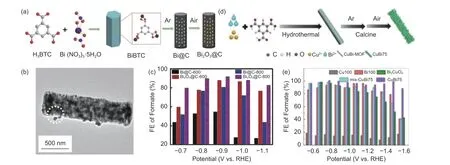
Fig. 5 (a) Preparation diagram of carbon nanorods-encapsulated Bi2O3 (Bi2O3@C). (b) TEM image of Bi2O3@C. (c) FEHCOOH over catalyst at different potentials vs. RHE[40]. Reprinted with permission by WILEY-VCH. (d) Schematic fabrication process for CuBi-C.(e) FEHCOOH over different catalysts under various voltages[41]. Reprinted with permission by Elsevier.
5 Reactor design and optimization
In the conventional H-type reactor, COgas is directly injected into the electrolyte. The COmolecules firstly dissolve in the electrolyte and then take part in reduction reaction at the interface of catalyst.Due to simple equipment and easy operation, H-type reactor is widely used in CORR. However, the solubility of COin the electrolyte will affect the rate of CORR, resulting in limited mass transfer in the whole system, which further hinders the current density boosting for the industrial application of COelectroreduction. Moreover, it is more difficult to understand the CORR system, involving the complex three-phase reaction interface. HCOOH, as a liquid product, will be dissolved in the electrolyte, which will undoubtedly bring about a series of problems,such as product collection, separation and recovery. In this way, the cost of HCOOH production via electrochemical CORR technology would greatly increase,which further hinders the industrial development of HCOOH production. Moreover, to realize the industrialization of HCOOH production, the current density should reach up to industrial level (>200 mA cm) at first, which is hard to be achieved in the traditional Htype reactor. Therefore, the new reactors are urgent to be designed and optimized for achieving an industrialgrade current density and high energy conversion efficiency. This section mainly outlines and introduces the flow reactor, including membrane electrode reactor (MEA)and all-solid-state reactor.There are several new and enlightened reactors/devices for the production of HCOOH via CORR, as schemed in Fig. 6. The H-type reactor as the traditional and most commonly applied reaction device, has been studied in most of review papers. Therefore, the H-type reactor will not be introduced in the follow section.

Fig. 6 Scheme and comparison of four different reactors for producing HCOOH via CO2RR.
5.1 Flow reactor
In order to achieve the high current density and energy conversion efficiency, the flow reactors without mass transport limitations have been widely concerned and applied in CORR. The flow reactor is a three-electrode reaction system, in which the gas diffusion electrode (GDE) is coated with catalyst on one side as cathode. The side of GDE coated by catalyst directly contacts with the catholyte, while COis directly introduced from the back of GDE to participate in the reaction. Moreover, the anode and cathode are separated by an ion exchange membrane, including anion/cation exchange membrane. The COand electrolyte contacts with the catalyst from different sides in the flow cell reactor, respectively, thus avoiding the mass transfer limitation caused by the low solubility of COin the electrolyte. Therefore, the overall current density of CORR can be significantly enhanced in the flow reactor and meet the requirements of industrialization. At the same time, the catholyte and anolyte are continuously circulating in the flow reactor. Thus, the liquid product can be taken away from surface of the catalyst to ensure the full exposure of active sites. Furthermore, the continuous flow of the electrolyte is also conducive to the desorption and release of the gas phase products, finally ensuring the stability of the system.
Wu et al.constructed a MOF-derived leafshaped bismuth nanosheet with a hybridized Bi/Bi-O interface by an-electroreduction method. DFT results revealed that the existence of O atoms at the Bi/Bi-O interface can beneficial to reduce the free energy barrier of CORR. The catalyst was assembled in a flow reactor to evaluate the COelectroreduction activity. At a current density of >200 mA cm, the bismuth nanosheets can achieve HCOOH FE of more than 90% and stably run for more than 10 h, showing an excellent activity and stability. Li et al.synthesized the highly dispersed InOnanoparticles loaded on carbon nanorods. The catalyst exhibited an excellent activity for COelectroreduction in a flow reactor with a FEof 90% and a current density of 300 mA cm. Zhu et al.evaluated and compared the COelectroreduction performance of a bismuthene catalyst in the H-type and flow reactors, respectively. In H-type reactor, the catalyst exhibited a current density of 130 mA cmat −1.17 V vs. RHE.The corresponding current density reached to 560 mA cmin the flow reactor, which is five times higher than that in H-type reactor. This showed that the problems of COdiffusion and mass transport limitation would be well solved in a flow cell, and the activity of catalyst would also be significantly improved.
5.2 MEA reactor
In order to achieve a high current density and high energy conversion efficiency in an industrialscale, MEA reactor is also designed and applied to electrochemical CORR. In the MEA reactor, the cathode, anode and the ion exchange membrane are pressed together, which greatly reduce the resistance of the reactor and improve the energy efficiency of the overall device. At the same time, COis introduced into the reactor accompanied by water vapor to participate in the reaction, which is beneficial to enhance the mass transport and improve the current density.Zhong et al.assembled the as-prepared BiSn catalyst into a MEA reactor. At a current density of 120 mA cm, the FEwas as high as 97.8% at a cell voltage of 3.6 V, and the energy efficiency can reach up to 36%. Xia et al.constructed an aminofunctionalized indium-organic framework material.The catalyst can achieve a commercial current density of 800 mA cmat a cell voltage of 3.4 V in a MEA reactor.
5.3 All-solid-state reactor
The as-obtained liquid product of HCOOH in the above-mentioned reactors will dissolve in the electrolyte. Notably, the final product exists in the form of formate in the alkaline electrolyte rather than the target product of HCOOH. Therefore, to obtain the pure HCOOH, the downstream processes of separation and recovery steps are required, which will inevitably increase the cost of industrial applications, and these downstream processes are energy-intensive and require the complicate production infrastructures. Wang et al.designed an all-solid-state reactor with a porous solid electrolyte layer instead of the traditional liquid electrolyte. During the CORR test, the humid COgas was supplied to the cathode side. On the anode side, hydrogen oxidation reaction was used to replace the water oxidation reaction. The Hgas was passed into the anode side to realize the hydrogen oxidation reaction on the anode catalyst to generate the protons. The generated protons transfer to the cathode through the anion and cation exchange and react with the generated HCOOon cathode was reacted with the protons from anode, which had passed, respectively under the driving of an electric field, finally producing the pure HCOOH. In addition, Nwas used to bring the pure HCOOH vapor out of the reactor,which can avoid the accumulation of HCOOH on the surface of the catalyst and facilitate to maintain the process stability. Finally, a high-concentration pure HCOOH solution was obtained, and Ncan be recycled and continue to enter the reactor. In the all-solid-state reactor, the bismuth-based catalyst can achieve a current density of >440 mA cmwith a FE of >97% and a stability of >100 h for producing HCOOH. At the same time, the generated HCOOH reached a concentration close to 100 wt.%. The allsolid-state reactor simultaneously is fed COand Has gas feedstocks to produce the pure HCOOH, which will have a great application prospect in safe hydrogen storage technology.
6 Hybrid CO2 electrolysis systems
In the section of all-solid-state reactor, it has been mentioned that the valuable products can be obtained by coupling the products from the anode and cathode. Meanwhile, the energy consumption of the entire reaction system can be reduced and the energy conversion efficiency can be improved by using hydrogen oxidation reaction instead of water oxidation reaction at the anode. Recently, a lot of work has been devoted to hybrid COelectrolysis. Other anodic oxidation reactions are replaced by water oxidation reactions, and valuable chemicals can be produced on the anode side. Hybrid COelectrolysis is also concerned, which can significantly improve the economic benefits of the CORR system and promote the industrial development of COelectroreduction technology. Zhu et al.designed a new hybrid COelectrolysis system, in which the COelectroreduction reaction and the methanol oxidation reaction (MOR) are coupled. In this reaction system, the voltage of the overall reaction can be greatly reduced, and the HCOOH product was simultaneously produced at both anode and cathode (Fig. 7a). By using methanol oxidation to replace the water oxidation reaction, the cell voltage of the entire device was declined to 213 mV when the current density reached to 10 mA cm. This proved that this strategy can effectively reduce the power consumption of the overall reaction and improve the overall energy conversion efficiency (Fig. 7b). The as-designed Ni-MOF nanosheet arrays (Ni-NF-Af) and bismuthenes (Bi-ene) electrocatalysts were applied in the cathode and anode, respectively, to configure hybrid COelectrolysis reactor, which can efficiently produce the HCOOH in a wide voltage range (FEs of the both cathode and anode are close to 100%, Fig. 7c). This work provides an inspired idea to reduce energy input and improve production efficiency for realizing the co-production of HCOOH at both cathode and anode.

Fig. 7 (a) Schematic of CO2RR and MOR. (b) LSV curves of CO2RR and MOR. (c) FEformate of CO2RR and MOR[16].Reprinted with permission by WILEY-VCH.
7 Conclusions and perspectives
In general, HCOOH, as a stable liquid product, is valuable and promising, which can be produced on the basis of COelectroreduction technique. In addition,the HCOOH is expected to be widely used as a safe and reliable hydrogen storage material. Consequently,electroreduction technique of COto HCOOH is very significant and needs to be explored and developed,which is full of opportunities and challenges. Of course, some problems/issues and challenges that will be faced and solved, still exist in such a novel system.
(1) Electrocatalytic COreduction to HCOOH is dependent on the design and development of electrocatalyst with high activity, selectivity, stability.Therefore, rational design and construction of carbonbased electrocatalyst is still one of the main focuses in future research. For efficiently and stably electrochemical reducing CO, it is one of the keys to give full play to the advantages of carbon-based materials,such as high conductivity, high specific surface area and acid and alkali resistance. Further, these carbon materials need to be coupled with other active components to construct the advanced carbon-based composite catalysts. In addition, the gradually decreased pH value in the system derived from the produced HCOOH changes the local microenvironment and has an important influence on the finally reaction rate and selectivity of COreduction to HCOOH. As a result,the electrolyte and the separation of HCOOH from the system are also needed to be considered. As for the most popular alkaline electrolyte, high solubility of COleads to a good mass transfer capability and fast reaction rate. Nonetheless, reduction product is in the form of formate rather than HCOOH in the alkaline electrolyte. While as for the acid electrolyte, the HCOOH can be directly produced and avoid the formation of formate. However, the competitive HER is a tough challenge for COreduction to HCOOH. Besides, organic electrolyte, the emerging seawater electrolyte and solid-state electrolyte, as well as external ingredients in electrolyte have been employed in CORR system and are of great potential, which need to be further explored and optimized.
(2) The reaction mechanism of CORR over carbon-based materials remains to be further explored and detected. The tunable intrinsic structure of carbon-based materials such as multiple heteroatomsdoped rich-defective-electron effects, abundant pore channels and functional groups, as well as multi-dimensional characteristics should be correlated with their electrochemical performance towards CO. The adsorption, activation and reduction processes of COon the surface of catalysts should be monitored at molecular scale by the advanced in-situ characterization methods. In addition, DFT is also used to simulate the catalyst structure and catalytic process in the atomic scale, so as to clarify the real active site of the reaction, which helps us to understand the reaction mechanism deeply and design the efficient carbon-based electrocatalyst accurately.
(3) Various obstacles/issues still exist and hinder the industrial application of electroreduction COto HCOOH. Although the current density has reached the industrial level through the design of new devices such as flow reactor and MEA reactor, the reaction overpotential and energy conversion efficiency of the device still need to be further improved, and the utilization rate of COis still very low. Future development needs to focus on innovative catalyst engineering and gas diffusion electrode design, optimization of reaction device, coupling other anode reactions to prepare value-added chemicals, and further exploration the industrial development route of COconversion technology.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (51872035,22078052) and Innovation Program of Dalian City of Liaoning Province (2019RJ03).

