Synthesis of Novel Analogues of Matrine and Anti-Tumor Activity Assessment
QIAN Mingcheng, JIANG Xinyu, QI Ying, LIU Huimin, ZHOU Kuo, ZHAO Shuai, CHEN Xin
(School of Pharmacy, Changzhou University, Changzhou 213164, China)
Abstract:In this article, a series of matrine analogues with ring-opening in the lactam portion of the molecule were synthesized and assessed against four cancer cell lines. The in vitro cytotoxicity study exhibited that analogue C4 with a naphthyl ring displayed the best antiproliferative activity compared to its parent compound matrine and other lactam ring-opening analogues. Taken together, the anti-tumor potential lead compound C4 was discovered, which could be used for further study.
Key words: matrine; structure activity relationship; anti-tumor activity
Matrine (1), an alkaloid extracted from the roots ofSophoraflavescens, shows wide pharmacological activities, such as anti-cancer, anti-inflammation, anti-viral, anti-nociceptive, and anti-arrhythmic effects[1-6]. Recently, matrine has been extensively studied as a potential anti-tumor agent against various cancer cell lines. Nevertheless, the activity of matrine is restricted owing to its low efficacy and short half-life[7]. To overcome these drawbacks, some studies have focused on modifying the structure of matrine to improve its pharmacodynamics and pharmacokinetics[7].
Recently, several matrine analogues without a lactam ring (Fig.1) have been synthesized and displayed moderate to higher potency against HCV and tumors[1,6,8-10]. Among them, the carboxylic acid group in the analogues plays a vital role in anti-HCV activity[8]. However, matrinic acid (2) and its corresponding esters lack antiproliferative activity[1]. Interestingly, when the acid was converted into an amide, analogue3regained moderate anti-tumor activity[9]. Moreover, structure-activity relationships (SARs) indicated that the amide bond is essential for the anti-cancer activity of matrine[1].
We have focused on structure modification of natural products and synthetic methodology for long time[10-12]. Our previous study discovered that some matrine analogues derived from the lactam ring-opening exhibited good anti-tumor activity[10]. Especially, the compoundB3with ameta-bromide on the phenyl ring showed the best antiproliferative activity. Furthermore,B3induced cell cycle arrest in G1 phase and cell apoptosis in a dose-dependent manner on A549 cells. Herein, we report a series of matrine analogues with ring-opening in the lactam portion and with various substituents in the phenyl ring to further study their potential structure-activity relationships.

Fig.1 Structures of matrine and lactam ring-opening analogues of matrine[7-10]
1 Experimental Section
1.1 Chemistry

C1
C1is 4-((1R,3aS,3a1S,10aR)-2-benzoyldecahydro-1H,4H-pyrido[3,2,1-ij][1,6]naphthyridin-1-yl)-N-(m-tolyl)butanamide.
To a solution of compoundB1(200 mg,0.57 mmol) in 10 mL dichloromethane was added triethylamine (59 μL). Then, benzoyl chloride (78 μL) was added to the reaction solution dropwise and stirred at room temperature for 1 h. After reaction, the solvent was removed under reduced pressure to give the crude product, which was further purified by silica column chromatography (eluting withV(DCM)∶V(MeOH)=50—40) to furnish a white solidC1(225 mg) in 87% yield. M.p.79—85 ℃.1H NMR (400 MHz, CDCl3):δ1.17 (t, 1H,J=1.8 Hz), 1.36 (s, 1H), 1.44—1.53 (m, 4H), 1.58—1.70 (m, 3H), 1.77—1.91 (m, 4H), 2.11 (s, 1H), 2.25 (s, 3H), 2.32 (t, 2H,J=2.5 Hz), 2.46—2.49 (m, 2H), 3.00 (q, 1H,J=1.8 Hz), 3.10 (t, 2H,J=2.5 Hz), 3.43 (dd, 1H,J1=3.5 Hz,J2=1.7 Hz), 3.61—3.67 (m, 1H), 4.18 (s, 1H), 6.85 (d, 1H,J=7.5 Hz), 7.11 (t, 1H,J=2.0 Hz), 7.35—7.50 (m, 7H).13C NMR (100 MHz, CDCl3):δ1.14, 8.67, 19.96, 21.59, 22.31, 26.82, 27.09, 35.58, 36.39, 38.46, 46.66, 56.31, 63.98, 117.04, 120.58, 124.72, 127.23, 128.31, 128.69, 128.81, 129.80, 130.35, 132.07, 136.66, 138.57, 138.69, 172.14, 173.53. HRMS (ESI):m/z[M+H]+calcd for C29H38N3O2: 460.295 9; found: 460.296 0.
C2is 4-((1R,3aS,3a1S,10aR)-2-benzoyldecahydro-1H,4H-pyrido[3,2,1-ij][1,6]naphthyridin-1-yl)-N-(3-fluorophenyl)butanamide.
Following the same procedure asC1,C2was obtained as a white solid in 98% yield. M.p. 165—167 ℃.1H NMR (400 MHz, CD3OD):δ1.29—1.59 (m, 7H), 1.60—1.79 (m, 6H), 1.89 (s, 1H), 1.99 (d, 3H,J=11.64 Hz), 2.42 (s, 2H), 2.83 (q, 2H,J=10.99 Hz), 3.36—3.47 (m, 1H), 3.57—3.73 (m, 1H), 6.80 (t, 1H,J=7.72 Hz), 7.24—7.31 (m, 2H), 7.44 (s, 5H), 7.57 (d, 1H,J=11.40 Hz).13C NMR (100 MHz, CD3OD):δ21.59, 21.95, 23.52, 29.42, 35.75, 37.34, 41.02, 57.37, 57.48, 63.92, 107.79, 108.06, 111.14, 111.35, 116.28, 127.81, 129.54, 130.74, 131.09, 131.18, 141.73, 141.84, 163.04, 165.45, 174.27. HRESIMS:m/z[M+H]+calcd for C28H35FN3O2: 464.270 8; found: 464.270 4.
C3is 4-((1R,3aS,3a1S,10aR)-2-benzoyldecahydro-1H,4H-pyrido[3,2,1-ij][1,6]naphthyridin-1-yl)-N-(3-bromophenyl)butanamide.
Following the same procedure asC1,C3was obtained as a white solid in 98% yield. M.p. 111—113 ℃.1H NMR (300 MHz, CD3OD):δ1.29—1.43 (m, 3H), 1.51—1.85 (m, 9H), 2.44—2.48 (m, 3H), 2.88 (s, 1H), 3.07—3.16 (m, 2H), 3.48—3.62 (m, 2H), 4.12 (s, 1H), 7.20—7.23 (m, 2H), 7.44—7.51 (m, 6H), 7.94—8.02 (m, 1H).13C NMR (75 MHz, CD3OD):δ20.86, 21.03, 23.30, 27.79, 36.03, 37.12, 39.70, 55.46, 57.00, 57.06, 64.59, 119.42, 123.28, 123.71, 127.79, 128.16, 129.34, 129.75, 130.64, 131.29, 131.37, 137.92, 141.51, 174.33, 174.91. HRESIMS:m/z[M+H]+calcd for C28H35BrN3O2: 526.188 7; found: 526.188 9.
C4
C4is 4-((1R,3aS,3a1S,10aR)-2-benzoyldecahydro-1H,4H-pyrido[3,2,1-ij][1,6]naphthyridin-1-yl)-N-(naphthalen-2-yl)butanamide.
Following the same procedure asC1,C4was obtained as a white solid in 95% yield. M.p. 94—96 ℃.1H NMR (400 MHz, CDCl3):δ1.27—1.52 (m, 7H), 1.62—1.74 (m, 4H), 1.85 (s, 3H), 2.49 (d, 2H,J=6.40 Hz), 2.89 (q, 2H,J=10.84 Hz), 3.35—3.41 (m, 1H), 3.58—3.64 (m, 1H), 4.18—4.21 (m, 1H), 7.37—7.46 (m, 7H), 7.37—7.46 (m, 7H), 7.74—7.80 (m, 3H), 8.25 (s, 1H).13C NMR (75 MHz, CD3OD):δ21.21, 21.49, 23.57, 28.65, 30.07, 30.73, 32.74, 35.85, 37.31, 40.37, 55.07, 57.14, 57.21, 64.08, 117.64, 121.24, 125.93, 127.47, 127.93, 128.52, 128.59, 129.52, 129.59, 130.91, 135.21, 137.49, 138.28, 174.33. HRESIMS:m/z[M+H]+calcd for C32H37N3O2: 496.295 9; found: 496.295 4.
C5is 4-((1R,3aS,3a1S,10aR)-2-acetyldecahydro-1H,4H-pyrido[3,2,1-ij][1,6]naphthyridin-1-yl)-N-(naphthalen-2-yl)butanamide.

C5
Following the same procedure asC1,C5was obtained as a white solid in 98% yield. M.p. 72—74 ℃.1H NMR (400 MHz, CDCl3):δ1.26—1.52 (m, 7H), 1.62—1.86 (m, 9H), 1.98 (s, 1H), 2.10 (s, 3H), 2.45 (t, 2H,J=6.84 Hz), 2.66 (d, 2H,J=10.20 Hz), 2.84—3.01 (m, 0.5H), 3.40 (s, 1H), 3.62—3.65 (m, 0.5H), 4.19—4.26 (m, 1H), 7.35—7.45 (m, 2H), 7.56(dd, 1H,J1=8.80 Hz,J2=1.92 Hz), 7.77(dd, 3H,J1=15.24 Hz,J2=8.56 Hz), 8.23 (s, 1H).13C NMR (75 MHz, CD3OD):δ21.92, 22.44, 23.59, 29.09, 30.09, 30.31, 34.82, 37.64, 40.91, 45.56, 53.45, 57.35, 63.46, 117.68, 121.23, 125.93, 127.45, 128.52, 128.57, 129.51, 132.01, 135.25, 137.45, 172.81, 174.38. HRESIMS:m/z[M+H]+calcd for C27H36N3O2: 434.280 2; found: 434.280 4.
1.2 Cell culture and MTT assay
The cell lines of human non-small celllung cancer (A549), human hepatocellular carcinoma (HepG2), human large cell lung cancer (H460) and human breast cancer (MCF-7) cells were cultured separately in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/L glucose supplemented with 10% FBS and 1% glutamine. All cell lines were maintained at 37 °C in a humidified atmosphere with 5% CO2.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assaywas carried out in 96-well plates. Cancer cells at the log phase of their growth cycle (1.2×104cells/mL) were added to the 96-well plates (100 μL/well) and incubated for 24 h at 37 ℃ in a humidified atmosphere with 5% CO2. After aspirating medium, 100 μL fresh medium containing the test compound at different concentrations was added to the well. After 72 h incubation, 50 μL of 10% MTT solution per well was added and continually incubated for another 4 h. Then, MTT solution was removed and 100 μL DMSO was added to the well. After 10 min at RT, the OD value of each well was measured on a microplate reader (MULTISKAN GO, Thermo Scientific) at 570 nm. In this assay, the negative reference was 0.1% DMSO and 5-Fu as the positive control.
2 Results and discussion
2.1 Synthesis
The synthesis of matrine amide analogues is described in Fig.2. The key intermediateBiwas obtained in 6 steps according to reported protocols[1,10]. Briefly, the lactam ring in matrine was hydrolyzed to give matrinic acid2, followed by esterization with methanol to afford ester4. Subsequently, protection of the amine with Boc yielded5. Then, hydrolysis of ester5in the presence of NaOH furnished the carboxylic acid6. The intermediate acid6was then coupled with various amines in the presence of HATU as the coupling reagent to give the amidesAi. Subsequently, the deprotection of Boc groups with HCl gas afforded compoundsBi. Finally,Biwas coupled with acyl chloride to give the final compoundsCiin good yields.

Fig.2 Synthesis of final compounds
2.2 Anti-proliferative activity evaluation
All newly prepared matrine analogues (C1—C5) were assessed for cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against four cancer cell lines (HepG2, A549, H460, and MCF-7). 5-Fluorouracil (5-Fu) was selected as a positive control. Cytotoxicity for all compounds was assessed at 50 μmol/L each for 72 h against four cancer cells (Table 1). Unpredictably, most final compounds showed low anti-proliferative activities, exceptC4, indicating that the amide bond at the N position of matrine unfavorably affected anti-proliferative activity. Our previous study revealed that analogueB3with a meta-bromide on the phenyl ring showed the best anti-proliferative activity[10]. However, in this study a benzoyl group was introduced to the N position ofB3to give analogueC3, which lost anti-proliferative activity for all tested cancer cells. Remarkably,C4having the same benzoyl group asC3displayed moderate cytotoxicity against cancer cells, suggesting that the naphthyl ring of the molecule contributed significantly to its anti-proliferative activity.
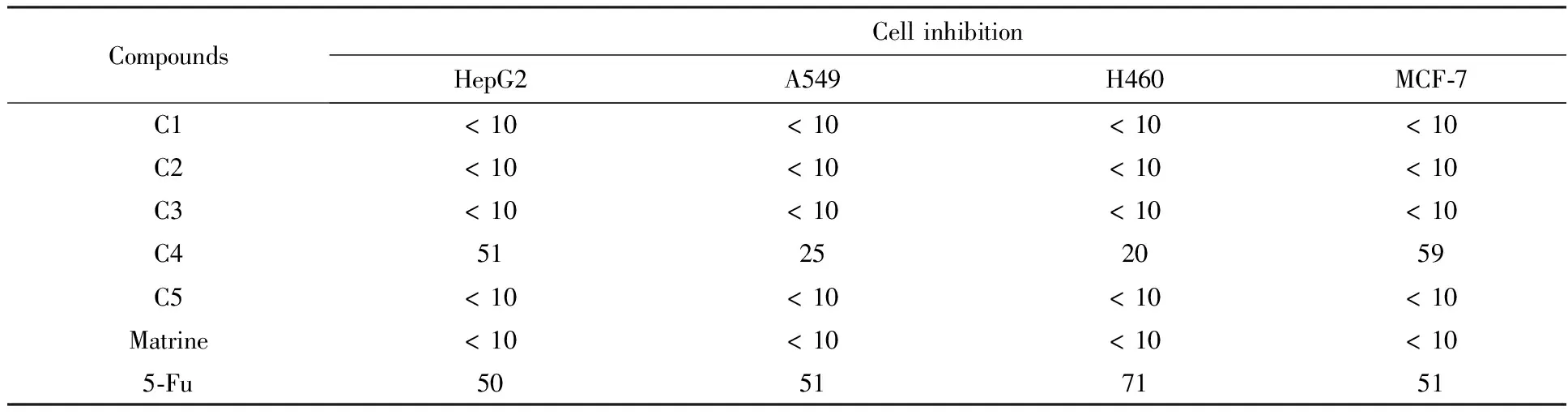
Table 1 Inhibition rates of matrine analogues at 50 μmol/L %
3 Conclusions
In conclusion, we designed and synthesized a series of matrine ring-opening amide analogues (C1—C5). The in vitro cytotoxicity study displayed that analogueC4with a naphthyl ring showed the best anti-proliferative activity compared to its parent compound matrine and other analogues (C1—C3andC5). Together, these data indicate thatC4is a potential anti-cancer candidate, which could be used as a lead compound for further anti-cancer study.

