Synthesis of Highly Luminescent LnMOFs through Structural Regulation①
YU Xiao-Bo CHEN Zhao MA Yuan-Jie LI Ling CHANG Wen-Ting LI Bo ZENG Cheng-Hui②
a (College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, China)
b (Jiangxi Yuean Superfine Met Co Ltd, Ganzhou, Jiangxi 341000, China)
ABSTRACT Two series of three dimensional (3D) lanthanide metal-organic frameworks (LnMOFs) of[Ln(tftpa)1.5(phen)(H2O)]n (Ln = Sm 1a, Eu 1b, Tb 1c, Dy 1d, H2tftpa = tetrafluoroterephthalic acid, phen =1,10-phenanthrolin) and [Ln(tftpa)1.5(bpy)(H2O)]n (Ln = Sm 2a, Eu 2b, Tb 2c, Dy 2d, bpy = 2,2′-bipyridine) are obtained by structural regulation. Results reveal that the 3D LnMOFs show high water- and thermal-stability.Interestingly, through selecting the perfluorinated ligand, and using bpy as an auxiliary ligand to hold back the solvents near to the lanthanide ions, 2b, and 2c show high luminescence quantum yield (QY) of 74.50% and 60.03%, respectively. In order to further improve the luminescence QY, the auxiliary ligand of phen with larger conjugation and more rigid structure is synthesized to replace bpy, and fortunately, higher luminescence QY of 80.73% (1b) and 75.17% (1c) are realized.
Keywords: highly luminescent, LnMOFs, structural regulation, luminescence quantum yield;
1 INTRODUCTION
Metal organic frameworks (MOFs) are self-assembled by coordination bonds between metal nodes and organic ligand linkers[1]. The large specific surface area and micro-porous natures of MOFs make their excellent performances in gas adsorption and separation[2], sensing[3], magnetism[4], proton conduction[5], imaging[6], catalysis[7], and so on[8]. Because the lanthanide ions have a low absorption coefficient, it is necessary to introduce “antenna” ligand to sensitize photo’s energy and transfer it to lanthanide ions[9]. The luminescence of LnMOFs materials has notable features of line-like emission bands, long luminescence lifetime, large Stokes shift,and high color purity[10]. Nevertheless, the luminescence QY of LnMOFs is still unsatisfactory.
Because the usage of the luminescent lanthanide complex highly depends on its luminescence QY[11], numerous studies have been reported to enhance the luminescence QY of the lanthanide complex. The low luminescence QY is mainly ascribed to three factors[12]: firstly, the energy on Ln3+is easily de-excited if N-H, C-H, or O-H group is in a radius of 20 Å[13]; secondly, the energy of triplet excited state of the“antenna” ligand should match very well with the emitting level of Ln3+[14]; thirdly, thef-ftransition of lanthanide ion is parity forbidden, and the absorption coefficient of lanthanide ion is usually very low. Thus, suitable “antenna” ligand with an aromatic group or diketone is usually selected to construct lanthanide complex to improve the energy transferring efficiency from “antenna” to the lanthanide ions[15]. Some works have addressed these problems and documented lanthanide complexes with high QY over 80%[16].
In this work, by selecting the perfluorinated ligand of H2tftpa[17], which does not contain oscillation groups of N-H,O-H, or C-H, as the main ligand, using phen/bpy as an auxiliary ligand to hold back the solvents that contain oscillation groups near to the lanthanide ions, and selecting auxiliary ligand that with larger conjugation and more rigid structure, two new series of highly luminescent 3D LnMOFs were synthesized and fully characterized. These LnMOFs have formulas of [Ln(tftpa)1.5(phen)(H2O)]n(Ln = Sm 1a, Eu 1b, Tb 1c, Dy 1d) and [Ln(tftpa)1.5(bpy)(H2O)]n(Ln = Sm 2a,Eu 2b, Tb 2c, Dy 2d), and the π-π stacking in these structures contributes to their thermal-stability. Interestingly, 1b, 1c, 2b and 2c show quite high luminescence quantum yield (QY) of 80.73%, 75.17%, 74.50%, and 60.03%, respectively.
2 EXPERIMENTAL
2. 1 Synthesis of [Ln(tftpa)1.5(phen)(H2O)]n
18.0 mg Ln(NO3)3·6H2O (0.04 mmol), 4.8 mg (0.02 mmol)H2tftpa, and 8.0 mg (0.044 mmol) phen were mixed in 2.0 mL H2O and 1.0 mL DMF. The solution was transferred to a glass bottle, and then placed in an 80oC oven for 2 days to obtain colorless crystals. The crystals were washed with 5 mL H2O three times and dried in a 60oC oven.
[Sm(tftpa)1.5(phen)(H2O)]n(1a): Yield: 20.63% based on Sm3+. Anal. Calcd. (%): C, 41.02; H, 1.49; N, 3.99. Found(%): C, 41.56; H, 1.69; N, 3.82. FT-IR (Fig. S1) (KBr pellet,cm-1): 3498 (m), 1681 (s), 1600 (s), 1515 (w), 1473 (m), 1388(s), 1261 (w), 1143 (w), 1095 (w), 1000 (s), 846 (s), 775 (w),725 (s), 640 (w).
[Eu(tftpa)1.5(phen)(H2O)]n(1b): Yield: 21.65% based on Eu3+. Anal. Calcd. (%): C, 41.02; H, 1.43; N, 3.98. Found(%): C, 40.69; H, 1.37; N, 3.85. FT-IR (Fig. S1) (KBr pellet,cm-1): 3504 (m), 1677 (s), 1604 (s), 1521 (w), 1467 (m), 1396(s), 1263 (w), 1147 (w), 1103 (w), 1000 (s), 838 (s), 773 (w),723 (s), 640 (w).
[Tb(tftpa)1.5(phen)(H2O)]n(1c): Yield: 20.63% based on Tb3+. Anal. Calcd. (%): C, 41.02; H, 1.43; N, 3.94. Found(%): C, 40.89; H, 1.76; N, 3.79. FT-IR (Fig. S1) (KBr pellet,cm-1): 3511 (m), 1679 (s), 1598 (s), 1517 (w), 1467 (m), 1400(s), 1265 (w), 1145 (w), 1108 (w), 991 (s), 846 (s), 777 (w),742 (s), 642 (w).
[Dy(tftpa)1.5(phen)(H2O)]n(1d): Yield: 19.71% based on Dy3+. Anal. Calcd. (%): C, 41.02; H, 1.43; N, 3.92. Found(%): C, 40.03; H, 1.22; N, 3.75. FT-IR (Fig. S1) (KBr pellet,cm-1): 3513 (m), 1685 (s), 1596 (s), 1519 (w), 1463 (m), 1461(s), 1265 (w), 1147 (w), 1099 (w), 993 (s), 838 (s), 775 (w),719 (s), 640 (w).
2. 2 Synthesis of [Ln(tftpa)1.5(bpy)(H2O)]n
18.0 mg Ln(NO3)3·6H2O (0.04 mmol), 4.8 mg (0.02 mmol)H2tftpa and 6.2 mg (0.04 mmol) bpy were mixed in 3.0 mL H2O. The solution was transferred to a glass bottle, and placed in an 80oC oven for 2 days to obtain colorless crystals.Then these crystals were washed with 5 mL H2O three times and dried in a 60oC oven.
[Sm(tftpa)1.5(bpy)(H2O)]n(2a): Yield: 21.13% based on Sm3+. Anal. Calcd. (%): C, 38.93; H, 1.49; N, 4.13. Found(%): C, 39.72; H, 1.44; N, 4.17. FT-IR (Fig. S1) (KBr pellet,cm-1): 3438 (m), 1670 (m), 1608 (m), 1481 (w), 1400 (m),1270 (w), 1174 (w), 1068 (w), 993 (s), 740 (s), 640 (w).
[Eu(tftpa)1.5(bpy)(H2O)]n(2b): Yield: 18.89% based on Eu3+. Anal. Calcd. (%): C, 38.93; H, 1.49; N, 4.12. Found(%): C, 38.57; H, 1.35; N, 4.03. FT-IR (Fig. S1) (KBr pellet,cm-1): 3446 (m), 1675 (m), 1604 (m), 1484 (w), 1400 (m),1263 (w), 1170 (w), 1068 (w), 995 (s), 746 (s), 640 (w).
[Tb(tftpa)1.5(bpy)(H2O)]n(2c): Yield: 21.02% based on Tb3+. Anal. Calcd. (%): C, 38.93; H, 1.49; N, 4.08. Found(%): C, 39.05; H, 1.43; N, 4.05. FT-IR (Fig. S1) (KBr pellet,cm-1): 3442 (m), 1681 (m), 1592 (m), 1479 (w), 1384 (m),1263 (w), 1170 (w), 1062 (w), 977 (s), 738 (s), 642 (w).
[Dy(tftpa)1.5(bpy)(H2O)]n(2d): Yield: 19.32% based on Dy3+. Anal. Calcd. (%): C, 38.93; H, 1.49; N, 4.06. Found(%): C, 40.22; H, 1.51; N, 4.11. FT-IR (Fig. S1) (KBr pellet,cm-1): 3461 (m), 1685 (m), 1594 (m), 1482 (w), 1388 (m),1268 (w), 1170 (w), 1062 (w), 997 (s), 738 (s), 649 (w).
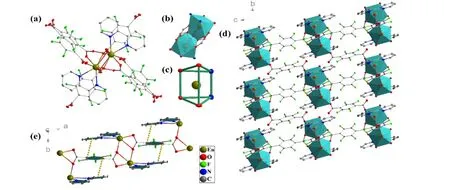
Fig. 1. Structure analysis of [Ln(tftpa)1.5(bpy)(H2O)]n (Ln = Sm 2a, Eu 2b, Tb 2c, Dy 2d): (a) Dinuclear SBU;(b) Dinuclear cluster structure in the SBU; (c) Four O and two N that coordinate to Eu3+ are arranged in twisted triangular prism mode; (d) 3D structure; (e) π-π stacking structure
3 RESULTS AND DISCUSSION
3. 1 Crystal structure description
[Ln(tftpa)1.5(phen)(H2O)]n(Ln = Sm 1a, Eu 1b, Tb 1c, Dy 1d) are isomorphism structures and are similar to the reported results[18,19]. More bond length and bond angle data are in normal ranges, which can be found in Table S1-S4[20].
The structure analysis of [Ln(tftpa)1.5(bpy)(H2O)]n(Ln =Sm 2a, Eu 2b, Tb 2c, Dy 2d) is explained by taking 2b as an example. 2b crystallizes in the triclinicP1space group. Two symmetric Eu atoms in the dinuclear SBU structure are connected by four carboxyls, with the distance between the two central atoms to be 4.234 Å (Fig. 1b). The SBU is built by six tftpa2-, two bpy, and two water molecules (Fig. 1a).Each central metal atom is eight-coordinated by six oxygen and two nitrogen atoms to form a triangular prism configuration (Fig. 1c). Benzene rings in tftpa2-and bpy are connected by π-πstacking to stabilize the MOFs structure(Fig. 1e)[3b]. Different from 1b, tftpa2-in 2b has three coordination modes of µ4-η1-η1-η1-η1, µ4-η2-η1-η1-η2, andµ2-η1-η0-η0-η1(Fig. S3). Each dinuclear SBU is connected by tftpa2-in three directions to form a 3D structure (Fig. 1d).
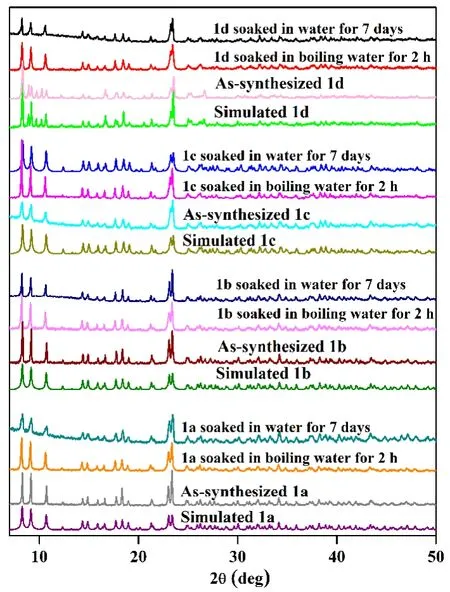
Fig. 2. PXRD patterns of simulated and as-synthesized 1a-1d when soaked in water for 7 days and immersed in boiling water for 2 h
3. 2 Stability of 1a-1d and 2a-2d
TGA data show small weight loss of crystalline water,proving that 1a-1d remain stable below 120oC[18,19], and 2a-2d keep stable below 280oC (Fig. S4 and S5). After soaking the as-synthesized 1a-1d and 2a-2d in water for 7 days and immersing them in boiling water for 2 h, the samples were collected and dried for PXRD test. They competed well with their as-synthesized samples and simulated single-crystal data(Fig. 2 and S6), revealing high water- and thermal-stability of the bulk samples of 1a-1d and 2a-2d. Therefore, they are confirmed to be stable materials[21].
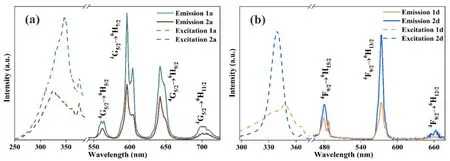
Fig. 3. (a) Luminescent spectra of 1a and 2a; (b) Luminescent spectra of 1d and 2d
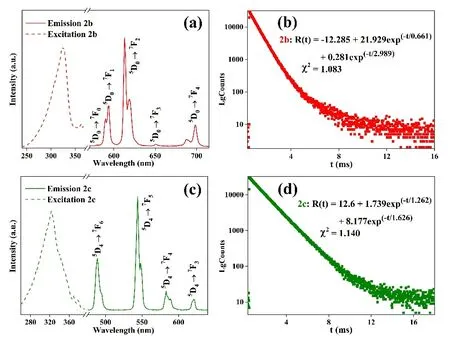
Fig. 4. (a) Photophysical spectra of 2b, (b) Photophysical spectra of 2c,(c) Luminescence decay curves of 2b, (d) Luminescence decay curves of 2c
3. 3 Solid-state photophysical studies
Luminescence spectra of LnMOFs 1a-1d and 2a-2d were recorded in the solid state at room temperature. With the respective excitation at 347 and 324 nm, 1a and 2a show four obvious peaks at 562, 596, 642, and 701 nm (Fig. 3a), which are arising from the4D5/2→6HJ/2(J = 5, 7, 9 and 11) transition of Sm3+[22]. Under the same excitation as 1a and 2a, respectively,2b displays five obvious peaks at 578, 593, 613, 650, and 698 nm, arising from the5D0→7FJ(J = 0, 1, 2, 3, and 4) transition of Eu3+(Fig. 4a)[23]. In luminescence spectra of 2c (Fig. 4c),under the same excitation as 1a and 2a, respectively, four peaks appear at 489, 543, 582, and 621 nm due to the5D4→7F6(J = 6,5, 4, and 3) transition of Tb3+[24]. In luminescence spectra of 1d and 2d (Fig. 4b), under 347 and 338 nm excitation, respectively,three peaks at 479, 573, and 661 nm result from the4F9/2→6HJ/2(J = 15, 13 and 12) transition of Dy3+[25].

Fig. 5. CIE chromaticity diagram of 1a, 1d, and 2a-2d
The luminescence decay curves (Fig. 4b and 4d) are wellfitted with double-exponential function, with the lifetimes of 0.789 and 1.4 ms for 1b, 1c, 2b, and 2c, respectively. These results show LnMOFs contain two luminous points, competing well with their single crystal structure. Interestingly, by selecting the perfluorinated ligand that does not contain oscillation groups as the main ligand, and phen or bpy as an auxiliary ligand to hold back the solvents which contain oscillation groups near to the lanthanide ions, the triplet excited energy state of main ligand H2tftpa is 27 465 cm-1[14],and the energy gaps of the triplet state of ligand and excited state of Tb3+and Eu3+ions are larger than 3500 cm-1,suggesting these two “antenna” ligands in the structures match very well with the emitting level of Tb3+and Eu3+. 1b,1c, 2b, and 2c display very high luminescence QYs of 80.73%, 75.17%, 74.50%, and 60.03%, respectively[26].Interestingly, it is found that by replacing the auxiliary ligand of bpy by phen with larger conjugation and more rigid structure of phen, higher luminescence QYs of 80.73%, 75.17%are realized. CIE chromaticity diagram (Fig. 5) indicates 1a(0.5134, 0.3478), 2a (0.4183, 0.3104), and 2b (0.6165, 0.3611)emit red luminescence, while 2c (0.3244, 0.5424) shows green luminescence[27]. 1d (0.3293, 0.3609) is white light emission,and 2d (0.3661, 0.3989) shows yellow light emission.
4 CONCLUSION
In short, two new series of 3D LnMOFs (1a-1d and 2a-2d)were synthesized by structural regulation. Detailed characterizations show they are high water- and thermostability materials. Interestingly, through selecting the perfluorinated ligand that does not contain oscillation groups,and selecting an auxiliary ligand with larger conjugation and more rigid structure to hold back the solvents near to the lanthanide ions, ultra-high luminescence QY of 80.73% is realized.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

