Atomic Layer Coated Al2O3on Nitrogen Doped Vertical Graphene Nanosheets for High Performance Sodium Ion Batteries
Zhiheng Wu,Xiangdan Zhang,Lijun Deng,Yongshang Zhang,Zhuo Wang ,Yonglong Shen*,and Guosheng Shao
Heteroatom doped graphene materials are considered as promising anode for high-performance sodium-ion batteries(SIBs).Defective and porous structure especially with large specific surface area is generally considered as a feasible strategy to boost reaction kinetics;however,the unwanted side reaction at the anode hinders the practical application of SIBs.In this work,a precisely controlled Al2O3coated nitrogen doped vertical graphene nanosheets(NVG)anode material has been proposed,which exhibits excellent sodium storage capacity and cycling stability.The ultrathin Al2O3coating on the NVG is considered to help construct an advantageous interface between electrode and electrolyte,both alleviating the electrolyte decomposition and enhancing sodium adsorption ability.As a result,the optimal Al2O3coated NVG materials delivers a high reversible capacity(835.0 mAh g-1)and superior cycling stability(retention of 92.3% after 5000 cycles).This work demonstrates a new way to design graphene-based anode materials for highperformance sodium-ion batteries.
Keywords
Al2O3,nitrogen doped vertical graphene nanosheets,reaction kinetics,sodium-ion batteries
1.Introduction
Sodium-ion batteries(SIBs)have aroused tremendous attentions for large-scale energy storage due to its abundant reverse and low cost.[1-4]Sodium possesses similar chemical/physical properties with lithium,and the working mechanism of SIBs resemble that of lithium-ion batteries(LIBs).Thus,carbonaceous materials,involving hard carbon,graphene,and expanded graphite,are extensively explored to act as anode for SIBs,[5-7]which are successful materials for LIBs.[8,9]However,the ionic radius of sodium is~34% larger than that of lithium and the sodium ion is also heavier than lithium ion.These features both affect the transport properties and structural stability of SIBs,[7,10]which lead to quite low capacity and unsatisfied rate capability for carbonaceous materials.Therefore,it is imperative to explore anode materials possessing good sodium storage properties,fast sodium ions diffusion kinetics,and accommodating large volume expansion during long cycling process.
Heteroatom doped graphene materials have been considered as promising anode for SIBs to enhance their reversible capacity,charge transfer,and electrode/electrolyte interaction.[11,12]Notably,nitrogen doping is one of the most developed strategies to enhance their surface wettability,electrical conductivity,introduce defect sites,and enlarge interlayer spacing towards the graphene-based materials,resulting in the enhanced performance of SIBs.Qiao and coworkers reported a tunable enlarged interlayer spacing up to 0.51 nm of N-doped carbon materials through thermal treatment of g-C3N4.Finally,it delivers superior Na+storage performance and ultrahigh rate capability.[13]The 3D N-doped graphene structure was also proposed with high nitrogen content,large surface area,and high pore volume,exhibiting a high reversible capacity(305 mAh g-1at 0.2 A g-1)and a long cycle life at a current density of 5 A g-1after 5000 cycles.[14]Besides the heteroatom doping,the microstructure of anode material is also an important aspect relating with surface area and porous structure,affecting the interaction between active sites and electrolyte.[15,16]Mesoporous soft carbon was reported fabricating from mesophase pitch with enlarged interlayer distance and mesoporous structure,exhibiting high rate capacity and cycling performance.[17]Waste cork-derived hard carbon was also reported possessing a novel hierarchical porous structure,endowing the carbon anode with high specific capacity(360 mAh g-1)and high energy density in full cells.[15]Thus,heteroatom doped graphene materials with beneficial structure are highly attractive for attaining high-performance anode for SIBs.
Vertical graphene nanosheets(VG)and nitrogen doped VG have been reported being promising material for LIBs and SIBs,owing to its beneficial structures such as vertically oriented morphology,high conductivity,high porosity,large surface to volume ratio,and none-stacking between separated nanosheets.This beneficial structure provides fast electron transport network,accessible active sites,and fast diffusion pathway of ions;thus,it has been introduced as support backbone for active materials[18-20]or directly used as anode for batteries.[21,22]However,the defective and porous structure with large specific surface area(SSA)of these carbon materials always suffer from extremely low initial Coulombic efficiency(ICE),low reversible capacity,and unsatisfied cycling stability due to uncontrollable decomposition of the electrolyte and poor interface construction between electrolyte and electrode.[23,24]Therefore,it is highly desired to deactivate the surface defects while maintain the sodium storage capacity of vertically oriented structure.
Surface coating of the electrode with electro-inert material is an effective way to prohibit the side reaction of defectively porous structure and forms favorable interface between electrode and electrolyte.[25,26]Compared with other physical and chemical vapor deposition in surface coating technique,atomic layer deposition(ALD)possesses several superior features such as atomic-scale controllability,conformal coverage,high uniformity,and low substrate temperature,owing to its layer by layer deposition of the self-limiting surface reaction.[27]Therefore,ALD is a powerful technique for material surface modification in energy storage field.Zhou and coworkers produced an ultrathin amorphous SnO2coating via ALD on garnet,which greatly improves the cycling stability of Li/garnet/Li symmetric cells.[28]Cao and coworkers reported ultrathin Al2O3coated hard carbon materials using ALD technique.The material is served as anode in SIBs showing a high reversible capacity(355 mAh g-1),high ICE(75% ),and superior cycling stability.[24]Hu and coworkers demonstrated ultrathin Al2O3coated Na metal by plasma-enhanced ALD.The ultrathin Al2O3serves as a stable artificial SEI and contributes to a uniform deposition/stripping process of Na metal.[29]However,it has been rarely reported about the ALD-Al2O3coated nitrogen doped vertical graphene nanosheets as anode for SIBs.Inspired by this,optimal thickness of Al2O3coated nitrogen doped vertical graphene nanosheets using ALD technique is expected to possess defective and porous structure with advantageous interface between electrode materials,which could both alleviate the electrolyte decomposition and boost kinetics of ion transportation,resulting in enhanced sodium storage performance.
Herein,we proposed an ALD-Al2O3coated nitrogen doped vertical graphene nanosheets(NVG-ALD)directly as the binder-free anode material with remarkable capacity and cycling stability for SIBs.NVG-ALD sample was fabricated on the copper foam substrate by a two-step approach firstly fabricating nitrogen doped vertical graphene nanosheets(NVG)in our laboratory-built high- flux plasma-enhanced chemical vapor deposition(H-PECVD)system and subsequently coating with Al2O3using ALD technology.The NVG-ALD film was firstly demonstrated possessing defective,porous, and vertically oriented structure,which is beneficial for enhancing sodium adsorption and ion diffusion during electrochemical process.Furthermore, the ex situ characteristics reveal the beneficial interface between NVG-ALD electrode and electrolyte.The 3D interface of NVG-ALD was presented being beneficial to sodium storage than the 2D interface of NVG(Figure 1b).As a result,the NVG-ALD with optimal thickness of Al2O3as anode for SIBs delivers a high reversible capacity of 835.0 mAh g-1at a current density of 0.1 A g-1and superior cycling stability(retention of 92.3% after 5000 cycles).
2.Results and Discussion
The morphology and structure of anode materials play important roles relating with active sites exposing and transport kinetics.[15,30,31]Our previous work has demonstrated the potential application of vertically standing graphene films(VSG)directly as the anode in a sodium-ion battery.[22]In order to obtain defective,porous,and vertically aligned graphene nanosheets with a compact arrangement for an enhancement of sodium storage capability,we introduced and modulate the nitrogen plasma via varying the nitrogen gas flow rate from 0 sccm to 10 sccm during the fabrication process in our laboratory-built H-PECVD system,as shown in step 1 in Figure 1a.The fabricated NVG shows apparently different morphology as seen in Figure S1.By increasing the amount of introduced nitrogen gas,the NVG turns into narrowly separated morphology.Besides,the Raman spectra(Figure S2)show that the intensity ratio of D peak to G peak(ID/IG)increases with the increase of nitrogen gas,reaching the highest value when N2flow rate reached 10 sccm.In addition,the interval between 2D peak and G peak becomes smaller with the increase of N2flow rate,showing the largest interval changes value of 13.5 cm-1narrower than that of undoped sample.This indicates that the NVG possesses the most defective structure with thinnest graphene layer when the N2flow rate rises to 10 sccm.After obtaining the NVG with optimal morphology and structure,ALD technique was employed to precisely control the Al2O3coating on the surface of NVG electrode,as shown in step 2 in Figure 1a.
Figure 2 exhibits the morphology of the NVG and ultrathin Al2O3(ALD cycle number of 5)coated NVG(NVG-ALD5)fabricated on copper foam.The NVG shows a vertically and closely connected morphology as seen in the scanning electron microscopy(SEM)images(Figure 2a,b).The vertically oriented graphene nanosheets separate from each other with a distance around 30 nm without agglomeration.This non-stacking feature of NVG is favored for adsorption/desorption of Na ion.Because the separated vertical graphene nanosheets could expose active sites on the surface and provide accessible ion diffusion pathway,ensuring sufficient interaction between electrolyte and electrode.[32]The unique structure of NVG is further characterized by high-resolution transmission electron microscopy(HRTEM)as shown in Figure 2c.The highly extended edges are clearly observed with an enlarged interlayer spacing of(002)plane(0.37 nm).Notably,the NVG contains five layers of the basal plane,confirming the few-layer graphene feature with good crystallization.Similarly,the NVG-ALD5 shows almost intact morphology after ALD coating of Al2O3,as seen in Figure 2d,e.The successful coating of Al2O3was verified by HRTEM,as shown in Figure 2f.It is easy to notice that there is an ultrathin layer with amorphous phase at the outer layer of crystalline graphene.The average thickness of amorphous layer is 0.65 nm,which is consistent with the deposit rate of~1.2 °A cycle-1determined by an ellipsometer.Figure 2g shows the scanning transmission electron microscopy(STEM)image and corresponding element mapping of C,Al,and O for NVG-ALD5.The uniformly distribution of Al and O elements further confirm the fully coating of ultrathin Al2O3using ALD technology.

Figure 2.Morphology characterization of NVG and NVG-ALD5.SEM images of a,b)NVG and d,e)NVG-ALD5.HRTEM images of c)NVG and f)NVGALD5.The insert in(c)is the intensity line profile along the red line.g)STEM image and corresponding elements mapping of NVG-ALD5.
Raman spectroscopy was employed to characterize the structure of graphene material.The Raman spectra in Figure 3a of NVG and NVGALD5 both show three primary peaks of D,G,and 2D peak,locating at 1348.6,1585.8,and 2685.1 eV(Figure 3a).The intensity of D band versus G band(ID/IG)is calculated to be 1.75 for NVG and 1.76 for NVG-ALD5,suggesting a high level of defects in the nitrogen doped vertical structure both for NVG and NVG-ALD5.The apparent D’peak around G peak is also an indicative of disorder structure.In addition,the enlarge interlayer spacing of graphene material was also confirmed by XRD analysis,as seen in Figure 3b.The obvious shift towards low angle of two NVG samples represent the enlarged d-spacing of(002)plane of 0.368 nm compared with 0.336 nm for graphite.This is consistent with the TEM observation in Figure 2c,suggesting that the NVG samples almost reach the minimum separation required for insertion of Na ions(0.37 nm).[33]The enlarged interlayer spacing is beneficial for reducing the energy barrier of Na ion intercalation and deintercalation and thus improve electrochemical performance.[5]The chemical bonding of NVG and NVG-ALD5 were investigated in XPS measurements,as seen in Figure 3c,d.The XPS survey of NVG-ALD5 shows apparent signal of Al and increased intensity of O peak.Meanwhile,the fine scan of Al 2p shows symmetric peak located at around 74 eV,confirming the presence of Al-O bond type and successful coating of Al2O3on the surface of NVG-ALD5.The Al2O3coating also introduce abundant oxygen-containing groups evidenced in the O 1s spectrum(Figure S3a),which would ultimately contribute to the wettability of NVG-ALD5 electrode toward the electrolyte and is beneficial for electrochemical process.

Figure 3.a)Raman spectra of NVG,NVG-ALD5,and graphite for reference.b)XRD pattern and c)XPS survey spectra of NVG and NVG-ALD5.d)Al 2p high-resolution XPS spectra of NVG-ALD5 and NVG.e)N2adsorption/desorption isotherm curves and f)pore size distributions of NVG and NVG-ALD5.
The pore structure and surface area of NVG and NVG-ALD5 was evaluated in N2adsorption-desorption analysis.As can be seen in Figure 3e,two samples both exhibit type IV isotherms.The Brunauer-Emmett-Teller(BET)surface areas are 333.1 and 312.8 m2g-1for NVG and NVG-ALD5.This large specific surface area is beneficial to increase the binding sites for Na ions.Meanwhile,the steep increase of adsorption at low relative pressure and a hysteresis loop at high relative pressure indicates the presence of micropores and mesopores.For comprehensive characteristics of the pore structure,we conducted the density functional theory(DFT)pore-size distributions.Figure 3f shows the pore size distributed at around 40 nm,verifying the mesopore dominant porous structure of NVG and NVG-ALD5.The mesoporous structure can facilitate the transportation of ions toward the active sites on the electrode.[34]As expected,the beneficial pore structure of NVG is well preserved even coated with ultrathin Al2O3using ALD technique and is promising for sodium-ion storage.
To investigate the influence of the Al2O3coating on electrochemical performance,we performed cyclic voltammetry (CV) (at 0.2 mV s-1) and galvanostatic discharge/charge cycling(at 0.1 A g-1)in a half-cell configuration.As can be seen in Figure 4a,an irreversible cathodic peak is observed at 0.8 V in the first cycle and disappears in the second and third cycle,suggesting the formation of SEI.Accordingly,the NVG-ALD5 shows a quite weak peak,revealing the suppressed decomposition of electrolyte(Figure 4b).Compared with NVG,a pair of reversible weak peaks are observed at around 2.0 V in the first cycle and turn to broaden in the subsequent cycles.This imply that redox reactions occur between Al2O3and electrolyte in the first cycle and then form a quite stable interface on the surface of electrode.This is further evidenced by the XPS spectra O 1s fine scan of NVG-ALD5 charged to 3 V(Figure S3a).The O 1s spectra can be deconvolved into three peaks located at 533.1,531.7,and 530.5 eV,corresponding to C-O,Al-O and Na-O,respectively,[24]suggesting the formation of Na-Al-O bonding between coated Al2O3and electrode.After the first cycle,the CV curves tend to overlap each other,showing excellent cyclic stability of the NVG-ALD5.This excellent stability may be ascribed to the Al2O3coating on the surface of NVG-ALD5.The presence of Al-F and Al-O-C bonding denote that the Al2O3is stably bonded with NVG and serve as F ion scavenger,which can avoid the direct damage to the electrode.[23]This robust and beneficial interface formed between electrode and electrolyte finally contribute to the stable electrochemical process.Figure 4c shows galvanostatic charge-discharge profiles of NVG and NVG-ALD5 at a current density of 0.1 A g-1for the 1st and 5th cycles.The curves present dominate sloping region and nearly absent of low-potential plateau region,with a sloping capacity of 784.5 mAh g-1(87.2% of total capacity)for NVGALD5 and 197.3 mAh g-1(85.6% of total capacity)for NVG.From an electrochemical point of view,the high potential slope represents a homogeneous reaction with the applied potential,which is associated with Na+adsorption,while the low-potential plateau indicates a heterogeneous reaction,such as metal plating or insertion into carbon structure.[35-37]This sloping region dominate capacity in the chargedischarge process indicates the dominate capacitive behavior in the sodium storage process,which is also in agreement with the CV curves.Meanwhile,the NVG electrodes deliver a large irreversible initial discharge/charge capacity of 1458.8/245.4 mAh g-1,with relatively low initial Coulombic efficiency(ICE)of 16.8% for NVG for the first cycle.The large capacity loss results from the irreversible reaction between electrolyte and the surface functional groups of electrodes.Even so,the NVG electrode still exhibit superior sodium storage performance to that of undoped vertical graphene nanosheets(VG),revealing the importance of heteroatom doping in sodium storage process(Figure S4).In stark contrast to the poor performance of NVG,the NVG-ALD5 electrode shows an enhanced ICE of 38.2% with the initial discharge/charge capacity of 2565.2/979.9 mAh g-1.More strikingly,the NVGALD5 exhibits a large reversible capacity of 835.0 mAh g-1at a current density of 0.1 A g-1with a high average Coulombic efficiency of 95.8% over five cycles.This demonstrates that the ultrathin Al2O3coating both improve the ICE and Na+storage capacity of NVG.Likewise,the surface coated NVG with different thickness of Al2O3denoted as NVG-ALD3,NVG-ALD10,and NVG-ALD20 electrodes show drastically increased reversible capacity of 819.3,506.9,and 312.3 mAh g-1compared with that of bare NVG electrode(184.4 mAh g-1)after 10 cycles(Figure S5).It is discernible that the Al2O3coated NVG shows deteriorate reversible capacity when the deposited cycle number of Al2O3increased to more than five cycles.This mainly attributes to the decrease of electronic conductivity and degenerated ionic transport for electrodes coated with thick Al2O3.

Figure 4.Cyclic voltammograms of a)NVG and b)NVG-ALD5 at a scan rate of 0.1 mV s-1.c)Galvanostatic charge-discharge profiles of NVG and NVGALD5 at a current density of 0.1 A g-1.d)Charge-discharge profiles of NVG-ALD5 at different current densities.e)Rate capacity of NVG and NVG-ALD5 at different current densities from 0.1 to 3 A g-1.f)Comparation of rate capacity between this work with representative anode materials.g)Cycling performance of NVG-ALD5 at a current density of 2 A g-1.
Galvanostatic charge-discharge tests were performed at various current density from 0.1 to 3 A g-1as shown in Figure 4d,e.As expected,NVG-ALD5 delivers much high average discharge capacities of 849.3,656.6,557.9,391.9,307.8,243.3,and 193.0 mAh g-1at current densities of 0.1,0.25,0.5,1,1.5,2,and 3 A g-1,respectively.When the current density returns to 0.1 A g-1,the discharge capacity recovers to 784.2 mAh g-1,demonstrating the excellent reversibility of NVG-ALD5.In comparison,NVG exhibit quite low average discharge capacities.Notably,the NVG-ALD5 shows impressive rate performance in low current density,which is superior to many reported carbon-based anodes for SIBs,as highlighted in Figure 4f.[5,12-14,16,33,38-41]Although the reversible capacity of NVG-ALD5 decays in high current density,it still exhibits a relatively high capacity of 193.0 mAh g-1in the high current density of 3 A g-1,which is superior to many reported carbon-based anodes.To further evaluate the long-term cycling stability of NVG-ALD5,we performed galvanostatic charge/discharge measurement at 2 A g-1for 5000 cycles(Figure 4g).The capacity is still maintained at 218.4 mAh g-1with a high retention of 92.3% after 5000 cycles and an average Coulombic efficiency exceeds 99.79% during the long-term cycling,suggesting a long cycle life for NVG-ALD5 electrode.Importantly,the excellent electrochemical performance of NVG-ALD5 is superior to many recently reported graphene and carbon anode materials for SIBs(Table S1).
To gain an insight into the high performance of NVG-ALD5,the sodium storage process was evaluated through CV tests at different scan rate of 0.1,0.2,0.5,1,2,and 5 mV s-1.As shown in Figure 5a,the typical rectangular shapes indicates obvious pseudocapacitive behavior in sodium storage process.[35]This capacitive contributed process can be quantitatively evaluated according to the following equation:[12,39]i(V)=k1v+k2v1/2.The current under a given potential(V)in CV curves can be divided into capacitive contribution(k1v)and diffusion-controlled contribution(k2v1/2).Figure 5b shows the dominant capacitive contribution of 66.7% at a scan rate of 2 mV s-1,indicating that the main capacity comes from the capacitive behavior for NVGALD5.Furthermore,the capacitive contributions at other scan rates are also determined by the same process,displayed in Figure 5c.The percentage of capacitive contribution ratio increases from 39.4% to 87.3% at the scan rate from 0.1 to 5 mV s-1,which is much higher than that of NVG(Figure S6).The capacitive dominant behavior of NVG-ALD5 indicates an enhanced reaction kinetics in sodium storage process.[36]This can be confirmed by the electrochemical impedance spectroscopy(EIS)and galvanostatic intermittent titration technique(GITT)measurements.Nyquist plots show that all spectra consist of a semicircle in the high frequency region and a straight line in the low frequency region(Figure 5d).The charge-transfer resistance(Rct)obtained based on the equivalent series resistance circuit inserted in Figure 5d reflects the charge transfer behavior between the electrolyte and electrode.The Rctdrastically decreases from 785.6 Ω (NVG)to 529.6 Ω(NVG-ALD5)and increases to 1306.0 Ω(NVG-ALD20)(detail information in Figure S7),indicating that the proper thickness of Al2O3could contribute to fast charge transfer between electrolyte and electrode in the electrochemical process.Meanwhile,EIS of NVG-ALD5 electrode before cycle and after 10 and 100 cycles also shows a decreased Rctfor the cycled cell,suggesting a beneficial interface formed after cycling(Figure S8).GITT measurements were further performed by discharging/charging the batteries with an interval of 10 min at a galvanostatic current of 0.03 mA,relaxing for 10 min(Figure 5e).Obviously,the discharging/charging time of NVG-ALD5 is longer than that of NVG,indicating a higher capacity of NVG-ALD5.Besides,the diffusion coefficient of Na+is calculated using the equation[42]:DNa+=4/πτ× (mBVm/MBS)2× (ΔEs/ΔEτ)2;where τ represents charging/discharging time;mB,MB,S and Vmdenote the mass weight,molecular weight,total area of contact,and molar volume of the electroactive compound.ΔEsis the difference of the voltage measured before discharging/charging process and after relaxation.ΔEτis the difference of the voltage measured after discharging/charging process and after relaxation.As can be seen in Figure 5f,the diffusion coefficient of NVG-ALD5 is much higher than that of NVG,especially in the initial discharging/charging process,suggesting a faster electrochemical process.Based on above results,the ultrathin coated Al2O3of NVG-ALD5 is constructive for Na+diffusion,resulting in enhanced reaction kinetics and electrochemical performance.
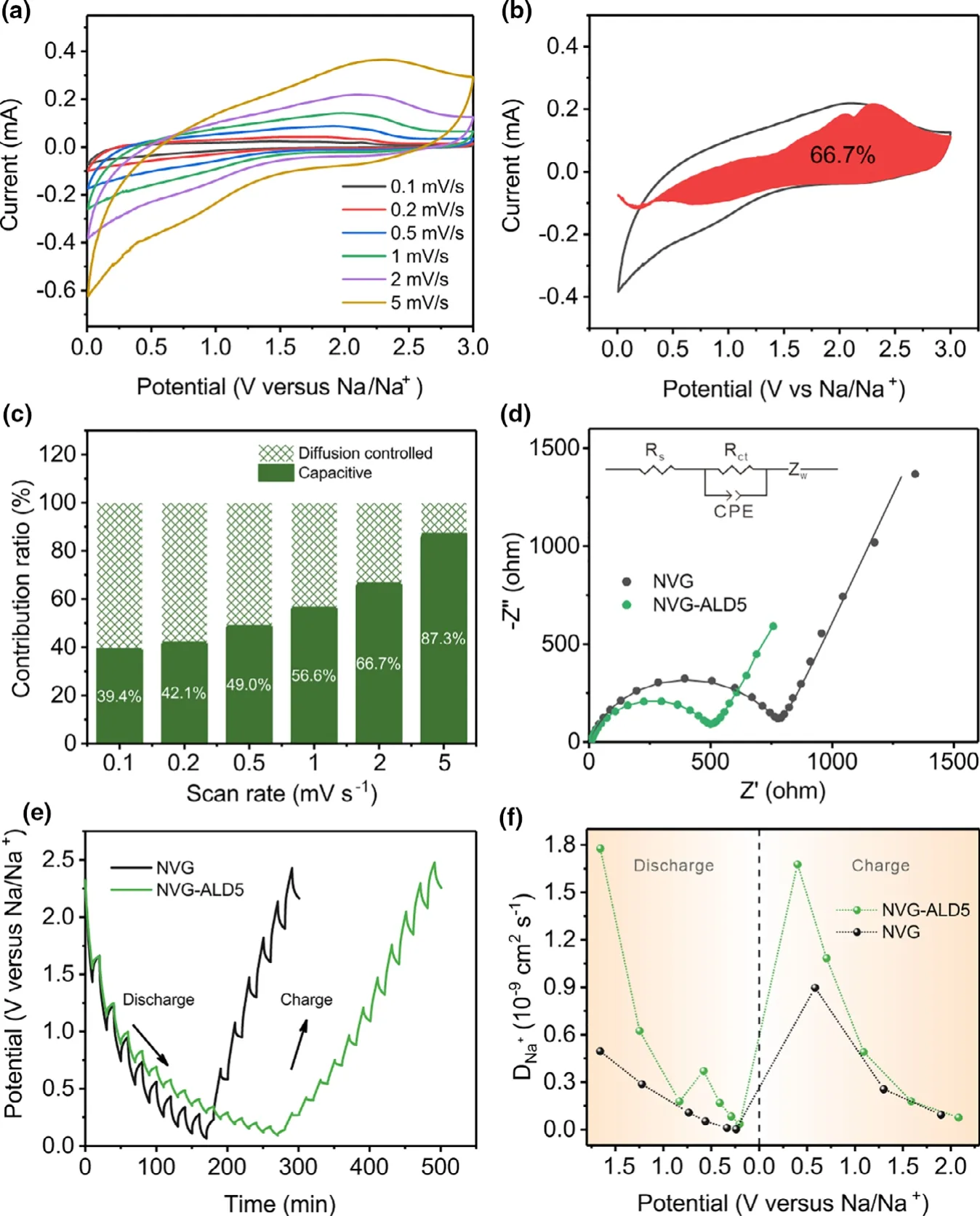
Figure 5.a)Cyclic voltammograms of NVG-ALD5 at different scan rate from 0.1 to 5 mV s-1.b)Capacitive charge storage contribution to capacity of NVG-ALD5 at 2 mV s-1and c)the ratio of capacitive contribution at various scan rates.d)Nyquist plots of NVG and NVG-ALD5 electrodes.e)GITT curves and f)Na+diffusion coefficients for NVG and MVG-ALD5.
Ex situ morphology observation was carried out to monitor the interfacial characteristics of both NVG and NVG-ALD5 after the electrodes were charged.As can be seen in Figure 6a-c,the surface of NVG is covered with sharp tips and dendrites of micrometer scale with large cracks.The SEM image and corresponding EDX elements mapping verify the large particles on the surface of NVG are composed of C,Na,O,F,and Cl(Figure S9).In contrast,the NVGALD5 shows a quite uniform and homogenous glue-like film on the surface of the graphene nanosheets(Figure 6d-f and Figure S10),indicating a quite different interface formed between electrodes and electrolyte after coating Al2O3.Generally,the fluoride and oxygen-rich compounds on the surface of electrode originate from the decomposition from FEC in additives,which are thought to prohibit decomposition of linear carbonates and form a stable SEI layer.[43]However,the defective and porous structure was expected to lead the none-uniform nucleation of decomposed electrolyte.As a result,inert fluoride and oxygen rich components form compound bulks at the surface of electrodes and block the ionic transport between the electrode and electrolyte.After coating of ultrathin Al2O3layer,the oxygen-containing groups of Al2O3are thought to form a uniform interface between electrolyte and electrodes(an artificial SEI),alleviate the electrolyte composition and strengthen the capture capability of Na ions(discussed later).As a result,the preferable interface between electrode and electrolyte could allow more sodium ions interact with the active sites on the electrode both on the horizontal and vertical direction.This assumption was verified by the precise element distribution of Na and O along the cross-sectional direction of NVG-ALD5 via electron probe X-ray microanalysis(EPMA),as shown in Figure 6g.The uniform element distribution of Na along the vertical direction of NVG-ALD5 compared with that of NVG(Figure S11)indicates that the sodium can be fully adsorbed at the Al2O3coated NVG.This result confirms the efficient construction of 3D interface between the electrolyte and electrode,providing accessible pathway for Na ion during electrochemical process.As illustrated in Figure 6h,compared with the poor 2D interface of NVG,the successful construction of beneficial 3D interface through introducing ultrathin Al2O3allows the access of electrolyte from the surface to interior of electrode and enlarge the reactive interface region.Therefore,the introduction of ultrathin Al2O3successfully construct a beneficial 3D interface and effectively suppress the side reactions of electrolyte during sodium storage process,contributing to an excellent sodium storage capability and a long-term cycling stability of SIBs.
To theoretically investigate the effect of Al2O3coating on the adsorption of Na,we performed DFT calculations by theoretical modelling,as seen in Figure 7a-c.The relative adsorption energy(ΔEa)of Na atom on unadopted vertical graphene,NVG,and ALD coated Al2O3were calculated.Obviously,the ALD coated Al2O3gives the largest relative adsorption energy of-5.73 eV,compared with that of undoped vertical graphene(-0.79 eV),NVG of pyrrolic N(-2.87 eV),and other nitrogen bonding configurations(Figure S12a,b).The large relative adsorption energy indicates that the ALD coated Al2O3shows significantly higher adsorption affinity than other structures and helps to form a stable artificial interface between electrode and electrolyte.In addition,the adsorption ability of Na atom can also be reflected in different charge density of Na absorption.As can be seen in Figure 7d-f,the charge density accumulate near the nitrogen defects is heavier than that of undoped vertical graphene after adsorption of Na atom and the distribution of charge density in ALD coated Al2O3is more uniform and denser than that of others.This obvious charge redistribution indicates enhanced interactions with Na ions,being consistent with the relative adsorption energy.Although the ALD coated Al2O3shows quite strong adsorption ability towards Na atom,the Na ions still exhibit sufficient diffusion in amorphous Al2O3structure(Figure S12c).Based on the calculation results,we can conclude that the ultrathin Al2O3significantly enhanced the adsorption ability of Na ions and help construct a robust and beneficial interface.Based on the morphology observation and electrochemical results,this favorable interface is reasonable for the high performance of NVG-ALD5 electrode with high reversible capacity and cycling stability.
3.Conclusion
In conclusion,we have proposed the ultrathin Al2O3coated nitrogen doped vertical graphene nanosheets as binder-free anode for high-performance SIBs.The optimal Al2O3coated NVG sample possesses vertically oriented,defective,and porous structure,guaranteeing sufficient active sites for Na storage and effective diffusion of Na ions.Besides,the deposited Al2O3layer stably bonded with NVG and contributes to the formation of favorable 3D interface between electrode and electrolyte.This beneficial interface suppresses side reactions of electrolyte and provides unblocked ions diffusion pathway for easy access to adsorption sites of Na ions.Consequently,the NVG-ALD5 electrode delivers a high reversible capacity of 835.0 mAh g-1at a current density of 0.1 A g-1and excellent cycling stability with a high-capacity retention of 92.3% after 5000 cycles.This work exhibits an interface optimized nitrogen doped vertical graphene electrode by ultrathin Al2O3coating as anode for SIBs,which may pave a new way for designing energy storage devices with high reversible capacity and cycling stability.
4.Experimental Section
Fabrication of NVG:The nitrogen-doped vertical graphene nanosheets were all fabricated in a laboratory-built high- flux plasma-enhanced chemical vapor deposition system.The commercial copper foam was ultrasonic cleaned in acetone,ethanol,and deionized water before loading on the substrate holder.The vacuum chamber was pumped down to 1.0×10-5mbar,and then,the plasma power was launched of 1700 W with Ar gas at a flow rate of 50 standard cubic centimeter per minute(sccm)diluted with H2gas at a flow rate of 10 sccm.After the pre-treatment of 10 min removing off the surface contamination,H2gas was then substituted by a mixed gas of CH4(10 sccm)and N2(0,2,4,6,8,10 sccm)to grow NVG materials for 90 min.The copper foam was then cooled down to room temperature at vacuum condition and turned upside down for material growth on the other side.
Synthesis of Al2O3Coated NVG:The NVG sample was placed on the sample holder in the atomic layer deposition system(Picosun R-200 Standard).High purity N2gas was used as the carrier gas.Trimethylaluminum(TMA)(Suzhou Fornano Electronic Technology Co.,Ltd.)and deionized water were used as precursors.The substrate was maintained at 300°C and the time for each pulse cycle of 0.1 s and purge cycle of 4 s for TMA and pulse cycle of 0.1 s and purge cycle of 4 s for deionized water.The Al2O3coated NVG with different ALD cycle number(3 cycles,5 cycles,10 cycles,and 20 cycles)were noted as NVG-ALD3,NVG-ALD5,NVG-ALD10,and NVG-ALD20.
Materials Characterization:X-ray diffraction(XRD)using a Rigaku Ultima IV system with Cu-Kα radiation was conducted to identify the crystalline structure.Raman spectroscopy was conducted in a Horiba LabRAM HR Evolution Spectrometer with an excitation laser wavelength of 532 nm calibrated using a highly oriented pyrolytic graphite.The morphology of the samples was characterized by field-emission scanning electron microscopy(SEM,ZEISS SIGMA 500)operating at 3 kV and transmission electron microscopy(TEM,FEI Tecnai G2 F20)operating at 200 kV,attached with an energy dispersive X-ray(EDX)spectrometer.X-ray photoelectron spectroscopy(XPS)analyses were conducted using an AXIS Supra system.EPMA mapping was conducted using an electron probe microanalyzer(Shimadzu EPMA-8050G).
Electrochemical Measurement:NVG and NVG-ALD fabricated on copper foams were directly used as the anode of SIBs without addition of binder or extra additive.Such working electrodes were assembled into CR2032-type coin cells,using sodium metal foil as the counter electrode,glass micro fiber filter(GF/D,Whatman)as the separator,and 1MNaClO4dissolved in a mixture of ethylene carbonate(EC),diethyl carbonate(EDC)solutions(1:1,by volume)containing 5.0% fluoroethylene carbonate(FEC)as the electrolyte in an argon filled glove box.The mass loading of each electrode was about 0.5 mg cm-2(with almost no mass change after ALD process).The electrochemical tests were performed after the coin cell was rested for 24 h.The cyclic voltammetry(CV)measurement and the electrochemical impedance spectroscopy(EIS)were performed using an electrochemical workstation(VMP-300).The galvanostatic charge/discharge test was performed using a battery test station(Land CT2001A)in the voltage range of 0.01-3 V at 30°C.
Computational Methods:The first principles calculations were carried out with the Vienna ab initio simulation package(VASP).In this work,the Perdew-Burke-Ernzerhof(PBE)functional with GGA exchange-correlation(XC)is employed to relax the structural configurations of NVG and NVG-ALD,due to these local-density based XC functionals are reasonably dependable in evaluating structural energies of condensed matter.For the geometric relaxation of the structures,summation over the Brillouin Zone(BZ)is performed with Monkhorst-Pack k-point spacing being smaller than 0.04°A-1to guarantee precision,and we use a 3×3×1 mesh in the irreducible Brillouin Zone for structure relaxation.The adsorption energies of Na atom on the above surfaces were calculated using the equation:ΔE=Etotal-Esurface-ENa,where Etotalis the total energy of the adsorption system,Esurfaceis the optimized clean surface of different structure,ENais the energy of Na.
Acknowledgements
The work is supported by the National Natural Science Foundation of China (Nos.51602290,91233101,11174256),and the Fundamental Research Program from the Ministry of Science and Technology of China(No.2014CB31704),and Project funded by China Postdoctoral Science Foundation (No.2016M592310).
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Introduction of Frontier Outlook
- Sn Alloy and Graphite Addition to Enhance Initial Coulombic Efficiency and Cycling Stability of SiO Anodes for Li-Ion Batteries
- Biomass Template Derived Boron/Oxygen Co-Doped Carbon Particles as Advanced Anodes for Potassium-Ion Batteries
- A Stretchable Ionic Conductive Elastomer for High-Areal-Capacity Lithium-Metal Batteries
- Gas Generation Mechanism in Li-Metal Batteries
- Understanding the Coffee ring Effect on Self-discharge Behavior of Printed micro-Supercapacitors
