Koyamasia and Struchium (Asteraceae, Vernonieae), Two Newly Recorded Genera for China
CHEN Yousheng, WANG Qinglong, LIAO Junjie, CHEN Bin
and(Asteraceae, Vernonieae), Two Newly Recorded Genera for China
CHEN Yousheng1, WANG Qinglong2, LIAO Junjie1, CHEN Bin3
(1.Key Laboratory of Plant Resources Conservation and Sustainable Utilization,South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China; 2. Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agriculture Science,Haikou 5711101, China; 3. Shanghai Chenshan Botanical Garden,Shanghai 201602, China)
Two suspiciousspecies of Asteraceae tribe Vernonieae were discovered in Hainan Province, China. Detailed morphological investigation suggests that they areandMolecular data based on nrDNA ITS sequence provide further support of the status ofThese two species represented two genera that have not been reported from China. Therefore, they are two newly recorded genera for China.
China; Hainan; Asteraceae; New record
In November 2019, Dr. Bin Chen discovered an unusual population of the Asteraceae from Bawang- ling, Changjiang County, Hainan Province of China. We took an expedition in December 2019 to the locality again, but unfortunately flowers had withered at that time. In 2020, we took another expedition to Bawangling in November. Fortunately, we successfully collected some flowering specimens (Fig. 1, 2: B). Also we collected a similar specimen from Mt. Xianan-silin, Maogan Town, Baoting County, Hainan Province of China. Because its capitula homogamous, florets all tubular, phyllaries herbaceous, green, 6–7 series, style branches long, slender, subulate, achenes columnar, pappus of many filiform bristles, this plant obviously belongs to Asteraceae tribe Vernonieae. However, the most unique feature of this species is that its involucre bracts are reversed and apex needlelike, which is not consistent with all the species of tribe Vernonieae in China. In order to determine its phylogenetic status, we carried out morphological and molecular phylo- genetic studies.
In March 2018, Qinglong Wang discovered an unusual population of the Asteraceae at low altitude wetlands from Danzhou County, Hainan Province (Fig. 2: D, 3). Another population of the same species was found in Wenchang County, Hainan Province. This plant also belongs to Asteraceae tribe Vernonieae, but also not consistent with all the species of tribe Vernonieae in China. In order to better observe this species, Mr. Wang introduced some plants and cultivated them in Danzhou City, Hainan province of China.After flowering, we carried out a detailed morphological study of this species.

Fig. 1 Koyamasia curtisii in the wild (China, Hainan Province, Changjiang County, Bawangling, 18 Nov. 2020, Jun-Jie LIAO et al. LL2020014). A: Habitat; B: Habit; C: Leaves; D: Capitula (top view); E: Disc florets; F: Capitula (side view); G: Abaxial surface of phyllaries from outer series (left) to inner series (right); H: Achene. (Photos taken by Jun-jie LIAO)

Fig. 2 Specimens of Koyamasia curtisii and Struchium sparganophorum. A: Lectotype of K. curtisii (Curtis 2127, SING0068747); B: Specimen of K. curtisii in China, Jun-jie LIAOLL2020014; C: Epitype of S. sparganophorum (G. R. Proctor 20182, BM000576316); D: Specimen of S. sparganophorum in China, Qing-Long WANG 18134.
1 Material and methods
1.1 Morphological observation
For the first species from Changjiang and Bao-ting, we collected three collections with both flowering and fruiting plants, and two plants were cultivated in Guangzhou. For the second species from Danzhou and Wenchang, 5 plants were cultivated in Danzhou City, Hainan Province. Two specimens were pressed after flowering. Specimens are deposited in IBSC. Photos of floral elements were took using a stereo- scopic microscope. We carefully examined its morpho- logical characters, searching literatures and comparing with digital specimens from BM, E, K, KYO, L, P, SING, U, WAG (herbaria acronyms following Index Herbariorum).
1.2 Taxonomic sampling, DNA extraction, PCR reaction and ITS sequencing
In order to determine its phylogenetic status of the first doubtful species from Changjiang and Bao- ting, we selected one sequences of the nuclear ribo- somal internal transcribed spacer (nrDNA ITS) regions. 99 collections representing 96 taxa belong to Asteraceae tribe Vernonieae were selected for molecular studies. The ITS sequences of 10 collections repre- senting 7 taxa are newly sequenced (Table 1), among them three collections of the first doubtful species were selected, and the ITS sequences of 89 taxa are downloaded from NCBI (Table 2), mainly from the research results by Keeley et al.[1].
Total genomic DNA of the nine accessions (Table 1) was isolated from silica geldried leaves using a modified cetyltrimethylammonium bromide procedure[2].
Amplification and sequencing were performed using the primers ITS1 and ITS4 for the ITS region[3]. The PCR mixture contained 1L (50–100 ng) of sample DNA, 2×2L of primer (10 pmol), 5L of 10× PCR buffer, 3L of Mg2+(25 mm), 0.8L of deoxyribonucleotide triphosphate (each 25 mm), 0.5L ofDNA polymerase (5 U/L) and sterile water for a final volume of 50L. The PCR parameters were as follows: initial denaturation for 4 min at 95 ℃, followed by 30 cycles of denaturation (95 ℃, 1 min), annealing (56 ℃, 40 s) and extension (72 ℃, 1 min), and a final extension of 10 min at 72 ℃[4].
PhyloSuite[5]was used to conduct, manage and streamline the analyses with the help of several plug- in programs: 1 sequence was aligned with MAFFT[6]using ‘FFT-NS-1 (fast)’ strategy and normal alignment mode. ModelFinder[7]was used to select the best-fit model using BIC criterion. Maximum likelihood phylogenies were inferred using IQ-TREE[8]under the model automatically selected by IQ-TREE (‘Auto’ option in IQ-TREE) for 20 000 ultrafast bootstraps[9], as well as the Shimodaira-Hasegawa-like approximate likelihood-ratio test[10]. Bayesian Inference phylogenies were inferred using MrBayes 3.2.6[11]under SYM+G model (2 parallel runs, 5 000 000 generations), in which the initial 25% of sampled data were discarded as burn-in.
2 Results and discussion
The ITS region has a total length of 637 bp, the best fit model for ITS was SYM+I+G. Character state changes were equally weighted and gaps were treated as missing data. All analyses produced similar topology and only the Maximum Likelihood tree is presented in Fig. 4 with ML bootstrap (LP), and PP values for each clade.
The molecular evidence showed that the three samples of Baoting and Changjiang populations in Hainan province were grouped together with strong support (LP=100%, PP=1.00 in Fig. 4) and nested within the genusclade (LP=100%, PP= 1.00 in Fig. 4).H. Rob. is a genus with only two species distributed in India, Laos, Malaysia, Myanmar, Thailand and Vietnam[11]. It belongs to subtribe Erlangeinae under tribe Vernonieae and is characterized by oblong achenes, reflexed phyllaries, 3-porate pollens, short and caduceus pappus[12–13]. This genus has not been documented to occur in China[14]. We studied the specimens from Hainan, and found the plants also have the characters of oblong achenes, reflexed phyllaries, short and caduceus pappus. So the above plants from Baoting and Chang- jiang are most possibly belong to genusHainan is a natural extension of the genusin Southeast Asia. Thus, our identification of this species based on morphological and geographical distribution is further corroborated by molecular data. Since its involucres campanulate, 7–10 mm long, 8–15 mm in diameter, florets ca. 60, the plant should be(Craib & Hutch.) Bunwong, Chantar. & S. C. Keeley. In fact, this plant (Fig. 1) perfectly match the type specimen of(Fig. 3: A).(Kitam.) H. Rob., another species with larger capitulum in this genus, is closed related to the three populations ofin Hainan Province (LP=100%, PP=1.00 in Fig. 4).

Table 1 Newly sequenced taxa, localities and accession number of ITS

Table 2 Taxa sampled, reference and GenBank accessions
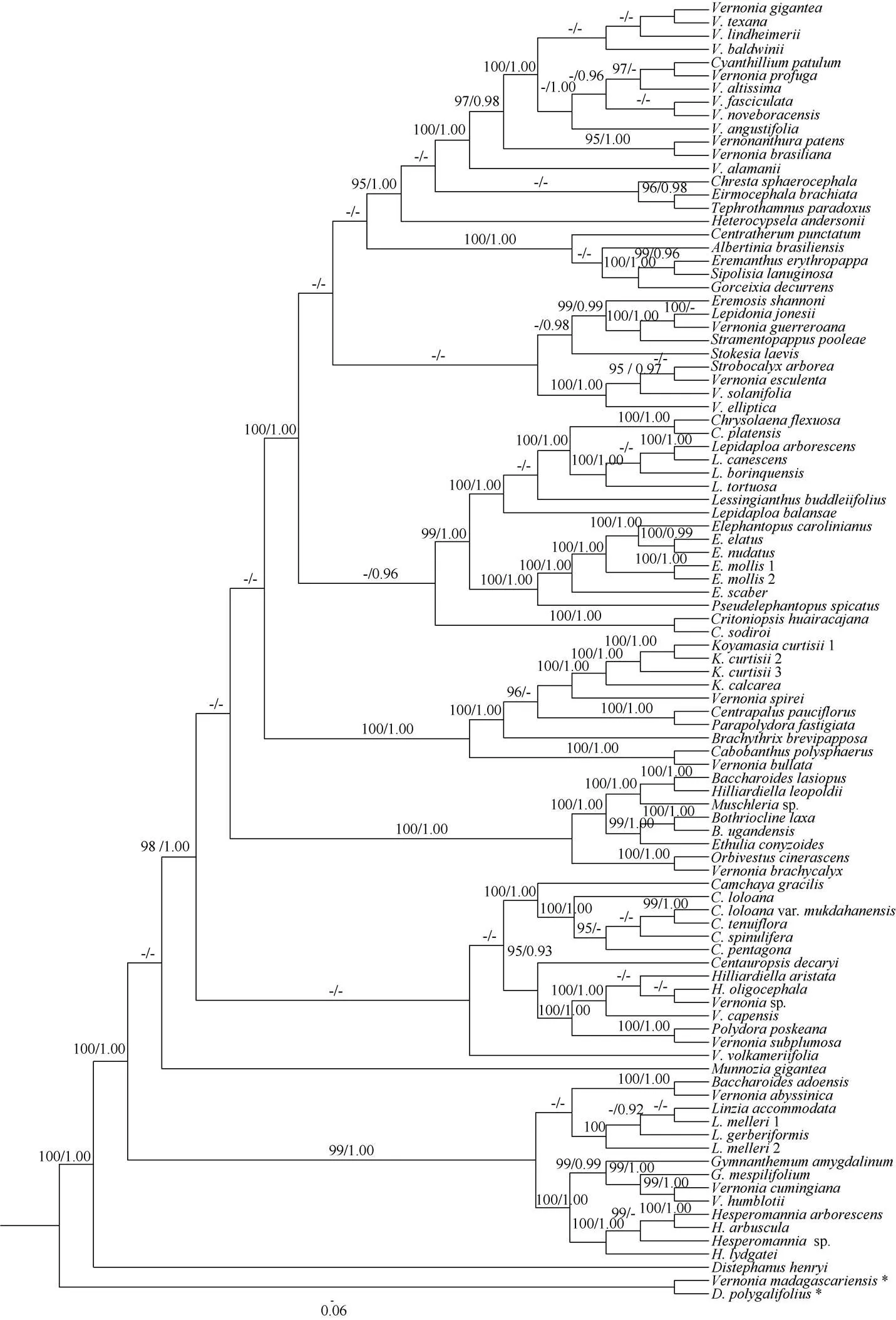
Fig. 4 Cladogram of the maximum likelihood (ML) tree from ITS showing the position of Koyamasia curtisii. Data above branch represent bootstrap values (LP) for maximum likelihood and Bayesian posterior probabilities (PP), respectively; the dash (–) indicates LP<95% or PP<0.90; *: Outgroup taxa.
Genusis poorly known to science, with very few specimens has been collected. Bunwonget al.[12]reportedto be a perennial herb. After our examination in the wild, we found it is in fact a shrub with woody stems (The detailed morpho- logical characteristics are shown on the Fig. 1, 2). After examining specimens from Thailand, we found some collectors also recorded it is a shrub.grows on limestone hills in Hainan province. The discovery ofin Hainan represents the first generic record ofin China.
The plants from Danzhou and Wenchang are annual herbs, leaves simple, alternate, capitula axiallary and sessile, phyllaries imbricate and nearly subequal, florets white, pappus coroniform. After a survey of literature and herbarium specimens, we found that these plants (Fig. 3, 2: D) perfectly match the morphological characteristics of(L.) Kuntze (Fig. 3: C).P. Browne is a monotypic genus distributed widely in the pantropical area[12,15–19], andbelongs to subtribe Verno- niinae under tribe Vernonieae and is characterized by sessile and axillary capitulum, subequal phyllaries, 3–4-lobed corolla, and thick and coroniform pappus. Since this genus is well distin- guished from other genera in tribe Vernonieae, there is no need to carry out molecular systematics to deter- mine the status of this species.also has not been documented to occur in China[13]. The discovery ofin Hainan represents a new generic record for China.
3 Newly recorded species and genera
3.1 Koyamasia curtisii (Craib & Hutch.) Bunwong, Chantar. & S. C. Keeley 距格菊(Fig. 1, 2: A, B)
In PhytoKeys 37: 82, 2014. =Craib & Hutch. Bull. Misc. Inform. Kew 1910: 22. 1910. —— Type: Malay Peninsula, Kedah, Trutow, November 1889, Curtis 2127 (SING–lectotype, K– isolectotype).
Shurbs 20–60 cm tall. Stems erect, conspicuously ribbed, puberulous with stipitate glands. Leaves 5– 15 cm long, 2–7 cm wide, ovate or elliptic, margin serrate, apex acute to acuminate, base attenuate, chartaceous; both surfaces puberulous with whip- shaped hairs and capitate glands; lateral veins 7–12- paired; petioles up to 4 cm long. Capitulescences terminal, solitary or loosely paniculate. Capitula campanulate, 15–20 mm long, pedunculate. Receptacle flat, glabrous. Involucres campanulate, 7–10 mm long, 8–15 mm in diam. Phyllaries imbricate, in 6–7 series, light green or purple apex, margin entire, outer surface puberulous; the outer and the middle ones ovate to lanceolate, apex acuminate with reflexed, the inner ones lanceolate to oblong, apex caudate. Florets ca. 60; corollas funnelform, purple, pubescent with soft hairs and capitate glands; corolla tubes 7–10 mm long; corolla lobes 2–3 mm long. Anthers 2.8–3 mm long, apical appendage acute, base obtuse. Styles purple. Achenes clavate,3–3.5 mm long, 10-ribbed, sparsely glandular. Pappus in one series of bristles, 2–8 mm long, deciduous.
Phenology: Flowering in November in Hainan (possibly also in October).
Habitat: Growing on limestone slopes at 660– 950 m elevation.
Distribution: China (Hainan), India, Laos, Malaysia, Myanmar, Thailand and Vietnam.
Specimens examined:CHINA. Hainan, Chang- jiang County, Bawangling Town, Mt. Exianling, 940 m,25 December 2019, J.J. Liao CJ1912002 (IBSC); Hainan, Changjiang County, Bawangling Town, Mt. Exianling, 940 m, 25 December 2019, J.J. Liao CJ1912003 (IBSC); Hainan, Changjiang County, Bawangling Town, Mt. Exianling, 940 m, 11 Novermber 2020, J.J. Liao CJ2020014 (IBSC); Hainan, Baoting County, Maogan Town, Mt. Xianansilin, Xianrendong, 676 m, 16 Novermber 2020, J.J. Liao et al. LL2020002 (IBSC). MALAY PENINSULA. Langkawi, Palau Chupa, 19 November 1941, E.J.H. Corner 37834 (L, SING); Langkawi, September 1901, Curtis 3690 (K). THAILAND. Banggrajon, Samut Prakan, 2 January 1970, J. F. Maxwell 70-19 (L); Thamphathai Forest Park, Ngao District, 400 m, 3 October 1982, F. Konta et al. T29668 (KYO, L); Chiang Mai, Doi Chiang Dao, 600–1 300 m, 25 September 1971, G. Murata et al. T14893 (L); Lampang, Muang Ngao, 17 January 1931, Put 4019 (BM, K, P); Lampang, Muak Lek, 4 September 1928, Put 1879 (BM, E, K, P); Saraburi, Khao Sawng Phi Nawng, 4 October 1963, T. Smiti- nand & H. Sleumer 1372 (K, L).
Conservation status:is sparsely distributed in China (Hainan), India, Laos, Myanmar, Thailand, Vietnam, Malay Peninsula, and Malay islands. But in China, it is only found from two limestone areas in Hainan, with about 60 plants in total. These two populations are severely fragmented, so maybe easily threaten by habitat lose. Since it is small size of population, this species should be considered Vulnerable (VU: B1, 2a; C2a) based on the IUCN Red List Categories and Criteria[20].
3.2 Struchium sparganophorum (L.) Kuntze 婴带菊 (Fig. 2: C, D; Fig. 3)
In Revis. Gen. Pl. 1: 366. 1891 =L., Sp. Pl.: 1171. 1763. Type: [Guade- loupe], near Grand Etang, 400–425 m, 27 November 1959, G. R. Proctor 20182(BM000576316–epitype designated by Hind)[21].
Annual herbs, 20–50 cm tall. Stems erect, incon- spicuously ribbed, puberulous. Leaves 8–15 cm long, 4–5 cm wide, elliptic, pubescent, margin serrate, apex acute, base attenuate, chartaceous; both surfaces puberulous with cylindrical hairs and capitate glands; lateral veins 7–11-paired; petioles up to 12 mm long. Capitulescences axillary, solitary or clustered. Capitula hemispherical, sessile, 4–6 mm in diam. Receptacle convex, 2–2.5 mm in diameter, glabrous. Involucres 3–4 series, 3–4 mm long, imbricate, hemispherical. Phyllaries light green, margin piliferous, outer surface puberulous without glands; the outer ovate to lanceolate, apex acute to acuminate; the inner ones obovate- lanceolate, apex acuminate. Florets 50–70; corollas funnelform, white, glandular; corolla tubes 1–1.5 mm long; corolla lobes 3–4, 0.5–1 mm long. Anthers ca. 1 mm long, apical appendage acute, base acute. Styles purple, ca. 2 mm long, branches 1–1.5 mm long, inner surface covered with stigmatic papillae. Achenes turbinate, 3–4-angular, 1–1.5 mm long, 3–5-ribbed, glandular. Pappus of 3–4 parts, coroniform, ca. 1 mm long, whitish.
Phenology: Flowering all year round in Hainan (According the cultivation observation in Danzhou City by Qinglong Wang).
Habitat: Growing in wetlands at low elevations.
Distribution: China (Hainan), India, Indonesia, Malaysia, Philippines, Singapore, Sri Lanka, Thailand, Vietnam, also tropical Africa, Madagascar, tropical South America, Central America, Mexico, and West Indies[11,14–18].
Specimens examined: CHINA. Hainan, Danzhou County, Zhonghe, Qili, 4 March 2018, Qing-Long Wang 18134 (IBSC). GABON. Mabounie, 24 m, 15 November 2013, O. Lachenaud et al. 1380 (WAG). GUINEA. Nzerekore, 456 m, 26 April 2011, C.C.H. Jongkind 10371 (WAG). INDONESIA. Kalimantan Timur, northern part of Samarinda, 1 January 1979, G. Murata et al. B-96 (KYO, L); North Sumatra, Gunung Leuser Nature Reserves, Alas River Valley, 50 m, 5 July 1979, W.J.J.O. de Wilde & B.E.E. de Wilde- Duyfjes 18533 (L); Sulawesi Tenggara, 20–250 m, 26 November 1978, S. Prawiroatmodjo & S. Soewoko 1978 (L3706203). Malaysia. Sabah, Beluran, Sapi, 25 June 2007, G. Pius et al. SAN148860 (L); Sarawak, Belarga, 26 August 1954, W.M.A. Brooke 9093 (L1588310); Sarawak, Bau, 15 September 1955, J.W. Purseglove 4446 (L1588311). SURINAM. Sipaliwini, Tapanahoni, 2 July 2013, T.R. van Andel, C. Toika, T.E.Vossen 6142 (U). THAILAND. Lamphun, Muang District, 20 December 1994, P. Palee 265 (L); Bangkok, Klong San, 20 Febuary 1971, J. F. Maxwell 71-107 (L); Ka Pur, 7 December 1979, T. Shimizu, H. Toyo- kuni, H. Koyama, T. Yahama & C. Niyomdham 26302 (L); Ranong, 10 April 1969, C. Chermsirivathana 1266 (L1588636); Trang, Khao Chong, 13 August 1975, J.F. Maxwell 75-824 (L); Trang, Khao Pap Pa, 150 m, 13 March 1974, K. Larsen & S. Larsen 33266 (L1588635); Songkhla, Rattaphume, 3 August 1986, J.F. Maxwell 86-636 (L).
Conservation status:is a weed widely distributed in the pan-tropical area. It is a weed in wetland areas in Hainan, but usually overlooked by previous plant collectors. According to Qinlong Wang, it is also found in Wenchang County in Hainan Province.
Acknowledgments We are grateful the curators of BM, E, IBSC, K, KYO, L, P, SING, U, WAG for allowing us to study their digital specimen collections. We thank Mr. Jun LI or his assistance in field works.
[1] KEELEY S C, FORSMAN Z H, CHAN R. A phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals Old/New World long distance dispersal: Support from separate and combined congruent datasets (L-F,F, ITS) [J]. Mol Phylogenet Evol, 2007, 44: 89–103.
[2] Doyle J J, Doyle J D. A rapid DNA isolation procedure for small quantities of fresh leaf tissue [J]. Phytochem Bull, 1987, 19(1): 11–15.
[3] White T J, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics [M]// Innis M A, Gelfand D H, Sninsky J J, et al. PCR Protocols: A Guide to Methods and Application. San Diego: Academic Press, 1990: 315–322.
[4] LI W P, YANG F S, Jivkova T, et al. Phylogenetic relationships and generic delimitation of Eurasian Aster (Asteraceae: Astereae) inferred from ITS, ETS andL-F sequence data [J]. Ann Bot, 2012, 109(7): 1341–1357. doi: 10.1093/aob/mcs054.
[5] Zhang D, Gao F, Jakovlić I, et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies [J]. Mol Ecol Res, 2020, 20(1): 348–355. doi: 10.1111/1755-0998.13096.
[6] Katoh K, Standley D M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability [J]. Mol Biol Evol, 2013,30(4): 772–780. doi: 10.1093/molbev/mst010.
[7] Kalyaanamoorthy S, Minh B Q, Wong T K F, et al. Model Finder: Fast model selection for accurate phylogenetic estimates [J]. Nat Meth, 2017, 14(6): 587–589. doi: 10.1038/NMETH.4285.
[8] Nguyen L T, Schmidt H A, Haeseler A, et al. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies [J]. Mol Biol Evol, 2015, 32(1): 268–274. doi:10.1093/ molbev/msu300.
[9] Minh B Q, Nguyen M A, Haeseler A. Ultrafast approximation for phylogenetic bootstrap [J]. Mol Biol Evol, 2013, 30(5): 1188–1195. doi: 10.1093/molbev/mst024.
[10] Guindon S, Dufayard J F, Lefort V, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0 [J]. Syst Biol, 2010,59(3): 307–321. doi: 10.1093/sysbio/syq010
[11] Ronquist F, Teslenko M, Mark P, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space [J]. Syst Biol, 2012, 61(3): 539–542. doi: 10.1093/sysbio/ sys029
[12] Bunwong S, Chantaranothai P, Keeley S C. Revisions and key to the Vernonieae (Compositae) of Thailand [J]. PhytoKeys, 2014, 37: 25–101. doi: 10.3897/phytokeys.37.6499.
[13] Robinson H. Tribe Vernonieae Cass. [M]// Kadereit J W, Jeffrey C. The Families and Genera of Vascular Plants, Volume VIII. Berlin, Heidelberg, New York: Springer, 2007, 149–174.
[14] Shi Z, CHEN Y L, CHEN Y S, et al. Asteraceae (Compositae) [M]// Wu Z Y, Raven P H. Flora of China, Vol. 20–21. Beijing: Science Press & St. Louis: Missouri Botanical Garden Press, 2011: 1–891.
[15] Ridley H N. The Flora of the Malay Peninsula, Vol. II [M]. London: L. Reeve & Co. Ltd, 1923: 918.
[16] Bien L K. Asteraceae [M]// Flora of Vietnam, Vol. 7. Ha Noi: Science and Technics Publishing House, 2007: 723.
[17] Vasudevan R.(L.) O. Kuntze, a new record for India [J]. Bull Bot Surv India, 1966, 8: 202–203.
[18] Uniyal B P. Tribe Vernonieae Cass [M]// Hajra P K, Rao R R, Singh D K, et al. Flora of India, Vol. 13. Asteraceae. Culcutta: Botanical Survey of India, 1995: 330–394.
[19] Jeffrey C, Beenntje H J. Vernonieae [M]// Beenntje H J. Flora of Tropical East Africa, Compositae (part 1). London: Royal Botanic Gardens, Kew, 2000: 108–284.
[20] IUCN Standards and Petitions Subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14 [M]// IUCN Standards and Petitions Subcommittee. 2019.
[21] Jarvis C E, Turland N J. Typification of Linnaean specific and varietal names in the Compositae (Asteraceae) [J]. Taxon, 1998, 47(2): 347–370. doi: 10.2307/1224388.
中国菊科二新记录属
陈又生1,王清隆2,廖俊杰1,陈彬3
(1. 中国科学院华南植物园,中国科学院植物资源保护与可持续利用重点实验室, 广州 510650;2. 中国热带农业科学院热带作物品种资源研究所, 海口 571101;3. 上海辰山植物园,上海 201602)
报道了中国菊科2新记录属:距格菊属(H. Rob.)、婴带菊属(P. Browne)和2新记录种:距格菊[(Craib & Hutch.) Bunwong, Chantar. & S. C. Keeley]、婴带菊[(L.) Kuntze]。这2属都来自海南,属于菊科(Asteraceae)斑鸠菊族(Vernonieae)。
中国;海南;菊科;新记录
2021-05-28
2021-08-27
10.11926/jtsb.4453
This work was supported by the National Natural Science Foundation of China (Grant No. 31970209); and the Program of Ministry of Ecology and Environment of China.
CHEN Yousheng (Born in 1972), Male, Associated professor, Studied in plant taxonomy. E-mail: yschen@scbg.ac.cn

