Near-zero thermal expansion in β-CuZnV2O7 in a large temperature range
Yaguang Hao(郝亚光), Hengli Xie(谢恒立), Gaojie Zeng(曾高杰),Huanli Yuan(袁焕丽), Yangming Hu(胡杨明), Juan Guo(郭娟), Qilong Gao(高其龙),Mingju Chao(晁明举), Xiao Ren(任霄), and Er-Jun Liang(梁二军)
Key Laboratory of Materials Physics of Ministry of Education,and School of Physics and Microelectronics,Zhengzhou University,Zhengzhou 450052,China
Keywords: negative thermal expansion materials,β-CuZnV2O7,expansion mechanism,Raman spectrum
1. Introduction
Negative thermal expansion(NTE)materials are a series of abnormal materials with volumes reducing as the temperature rises.[1,2]This abnormal temperature behavior of NTE materials takes great advantages in the applications of precise instruments, especially extending the service time and optimizing the performance, resorting to the restraint of volume changes when temperature varies. NTE materials, especially the near-zero NTE material with a low NTE coefficient which is the best choice but very rare, provide a good chance to overcome the mismatch of thermal expansion coefficients between materials as temperature varies. Many oxide NTE materials have excellent NTE properties such as ZrMnMo3O12, Sc2-xGaxW3O12and Ca2RuO4with oxygen vacancy.[3-5]Nevertheless, many challenges remain[6,7]such as the hydroscopicity[8,9]and the limited NTE temperature range attributed to the structural phase transition.[10,11]Just like ZrW2O8, a well-known NTE material relying on frame structure to induce NTE property,[12]the family of vanadate with the chemical formulae ofAV2O7(A=Zr,Hf)[13-16]andB2V2O7(B=Cu, Zn)[17-21]also shows distinct advantages of NTE characteristics. Apart from vanadate, phosphate, especiallyα-Cu2P2O7(space groupC2/c), also displays high NTE properties along theaandbaxes with the volume expansion coefficientαv=-27.69×10-6K-1.[22]The parent compounds of vanadate display the NTE property in different temperature ranges, but their limited NTE temperature ranges and the uncontrollable NTE coefficients hinder their further applications. A mount of efforts has been made to enrich the kinds of NTE materials in vanadate,in which doping new elements to the A, V, and B sites were proven to be an efficient way to modulate the NTE property. For example,with the individual site substitution of V by P or V by Mo,it was turned out that the structural phase transition temperatures of ZrV2-xPxO7(x= 1) and ZrV2-xMoxO7(x= 0.5)are successfully decreased to 231 K and 225 K, respectively,reducing the low limit of the NTE temperature of ZrV2O7,which is 375 K.[23,24]Moreover, doping new elements to theBsite ofB2V2O7(B=Cu,Zn),such as Cu1.5Mg0.5V2O7andβ-Cu1.8Zn0.2V2O7, could also broaden the NTE temperature range from 153 K-573 K to 100 K-700 K.[25,26]
In addition, doping new elements also plays an important role in regulating the NTE coefficient, especially to obtain the near-zero expansion materials. For theAV2O7(A= Zr, Hf) vanadate, with the partial substitutions of Zr to Hf and V to P in ZrV2O7, the Zr0.5Hf0.5VPO7displays near-zero thermal expansion behavior with the volume expansion coefficient ofαv=-1.77×10-6K-1in the temperature range of 310 K-673 K.[27]Like the Zr0.5Hf0.5VPO7,with the partial substitutions of Zr by Fe and V by Mo in ZrV2O7, the Zr0.1Fe0.9V1.1Mo0.9O7shows near-zero thermal expansion property with the volume expansion coefficient ofαv=-2.53×10-6K-1in the temperature range of 300 K-873 K.[28]
Nevertheless,the doped NTE materials mentioned above are all multi-site substitutions, which is not economical and high-efficiency. Actually, the compounds by individual substitution in the family ofB2V2O7(B=Cu,Zn)have been successfully synthesized. With Cd partially doping to the Zn site in Zn2V2O7,β-Zn1.6Cd0.4V2O7behaves near-zero thermal expansion property with a broad temperature range of about 273 K-873 K.[29]However, the low limit of NTE temperature inβ-Zn1.6Cd0.4V2O7is just at 273 K, which cannot satisfy the further applications in low-temperature environment.Recently, theα-Cu1.8Zn0.2V2O7obtained by single-site substitution through Cu by Zn displays near-zero NTE behavior from 100 K to 475 K,[30]attracting much attention. This temperature range involves room temperature and touches down below 273 K,which reveals thatα-Cu1.8Zn0.2V2O7could be a candidate for wide applications. Based on the fact that the volumetric NTEs ofα-Cu2-xZnxV2O7(x=0,0.1,0.2)is successfully tuned from-10.19×10-1K-1to-1.58×10-1K-1in the temperature range of 100 K-475 K,the content of Zn is considered to be a pivotal factor to modulate the NTE property ofα-Cu2-xZnxV2O7usefully.[30]
Motivated by the fact inα-Cu2V2O7, here we report a new type of near-zero thermal expansion material with a molecular formula ofβ-CuZnV2O7. This new kind of NTE material is acquired by doping the equivalent amount of Zn to the site of Cu in the parent compound ofβ-Cu2V2O7, another stable phase of Cu2V2O7exceptα-Cu2V2O7.The linear NTE coefficients ofβ-CuZnV2O7areαl=-2.15×10-6K-1(173-325 K) andαl=-0.72×10-6K-1(325-673 K). The NTE temperature range ofβ-CuZnV2O7is large enough to be over the 273 K and room temperature,which meets the needs for multiple applications. Apart from the NTE property, we also demonstrate that no structural phase transition exists inβ-CuZnV2O7from 173 K to 673 K by variable-temperature x-ray diffraction and Raman experiment at atmospheric pressure. However, two structural phase transitions are observed by the high-pressure Raman measurement.
2. Experimental
High quality polycrystal powder ofβ-CuZnV2O7was prepared by the solid phase sintering reaction method. According to the chemical stoichiometry of CuZnV2O7,the raw materials CuO (99.0%), ZnO (99.0%), and V2O5(99.0%)were ground in a mortar for about half an hour in advance.Then an appropriate amount of alcohol was added into the mixed powder and the mixture was stirred for 2-3 h. After fully grinding, the mixed powder was pressed into a cylinder shape with a diameter of 10 mm and a height of 8 mm by a tablet press (specification for 769YP-15A & 24B hydraulic press). Then the compressed cylinder was put into a box furnace(AY-TF-100-120)and heated to 873 K with a heating rate of 5 K/min,and sintered at 873 K for 4 h. Finally,the box furnace cooled down naturally and high quality polycrystal powder ofβ-CuZnV2O7was obtained.
A temperature-dependent x-ray diffraction (XRD) data was collected using (Japan, Rigaku Corporation, Cu,Kα,λ=1.5418 °A)x-ray diffractometer to measure the structural transformation ofβ-CuZnV2O7from 80 K to 573 K. The xray diffraction data was analyzed by the Full-Prof software and Rietveld method refinement. A dilatometer (LINSEIS DIL L75) was used to test the thermal expansion property ofβ-CuZnV2O7with heating and cooling rates of 5 K/min. The microstructure of the sample was observed by the scanning microscope(JSM-6700F).The Raman spectrum of the sample was measured by a Horiba Lab RAM HR Evolution Raman spectrometer (λ=633 nm, 143-663 K) with a resolution as high as 0.6 cm-1using 1800 gr/mm. High-pressure Raman measurement were carried out by the laser with a wavelength of 532 nm. The samples used in high-pressure Raman experiment were kept in a diamond anvil cell with a small number of rubies for pressure calibration and silicone oil was used as the pressure transmitting medium
3. Results and discussion
3.1. Structure analysis
Figure 1(a)shows the refined analysis of the XRD pattern at room temperature. The diffraction peaks ofβ-CuZnV2O7could be well indexed according to the standard PDF card ofβ-CuZnV2O7. It belongs to a centrally symmetric monoclinic phase structure with the space group ofC2/c(No. 15). The unit cell parameters refined by the Rietveld method area=7.54671 °A,b=8.18740 °A,c=10.10099 °A,β=110.9198°,andV=582.978 °A3. TheRvalues areRp=15.6,Rwp=14.6,andRexp=8.53, verifying the accuracy of the fitting result.According to our refined XRD result,the schematic of framework structure ofβ-CuZnV2O7could be presented as the model shown in Fig. 1(b). This model is consistent with the schematic structure ofβ-Cu2V2O7,which holds the same structure ofβ-CuZnV2O7.[18]In Fig. 1(b), two VO4tetrahedrons form the V2O7double tetrahedron structure unit by sharing one oxygen atom at the same vertex angle.
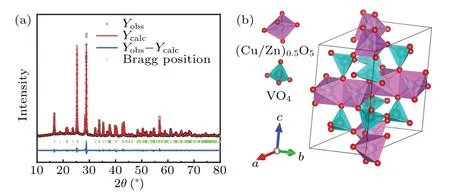
Fig. 1. (a) Rietveld refined analysis of XRD data of β-CuZnV2O7 at room temperature. (b)Rietveld refined structure of β-CuZnV2O7.
Each V2O7block possesses a staggeredCssymmetric structure.[31]The(CuZn)0.5O5quadrangular pyramid is composed of one Cu/Zn and five O, and two quadrangular pyramids share one side. Due to the Jahn-Teller distortion effect,a distorted quadrangular pyramid chain is formed.[31,32]The distorted tetragonal pyramid chain is connected to the V2O7double tetrahedron by sharing the apex oxygen atom to form a chain,forming a layered staggered structure.[31,32]
3.2. Morphology and composition
The SEM image of the sample can be seen from Fig.2(a),and particles in the polycrystal powder ofβ-CuZnV2O7are relatively smooth with the diameter of each particle about 1-2 µm. The population distribution of particles is relatively dense except for fewer tiny voids, holes, and micro-cracks remaining among the particles. To determine the composition of the material elements, the EDS spectrum of the sample was tested [Fig. 2(b)]. The analysis of the EDS energy spectrum composition (atomic fraction at.%, mass fraction wt.%) is shown in Table 1, and the element content ratio of Cu:Zn:V:O meets the ratio of the chemical formula ofβ-CuZnV2O7within the allowable error range.
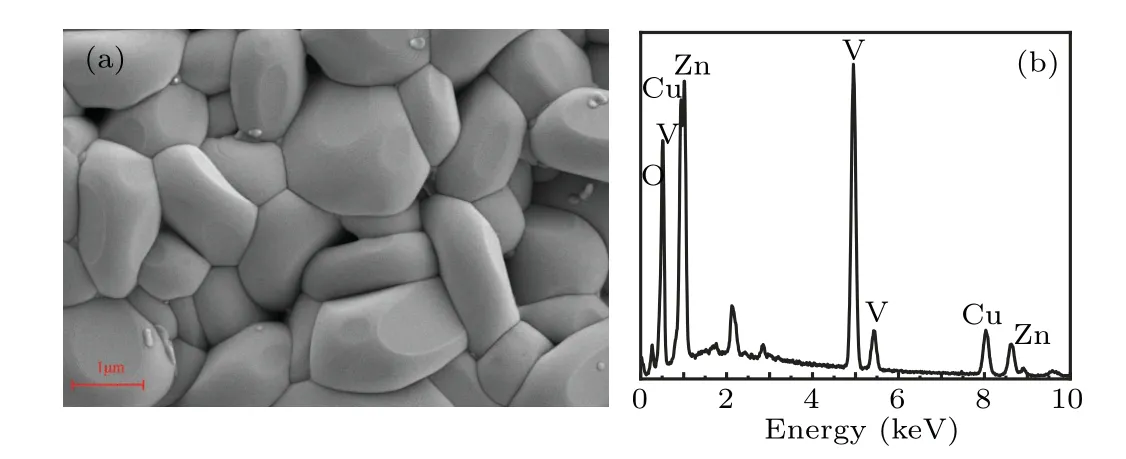
Fig.2. (a)SEM image of β-CuZnV2O7 sample,(b)the EDS spectrum in(a).
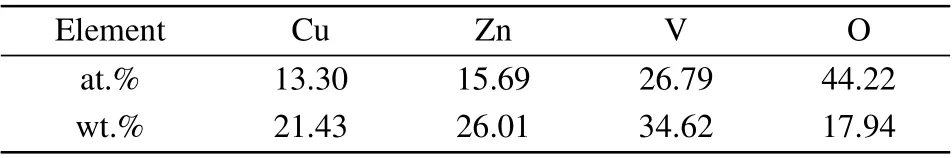
Table 1. Composition analysis of β-CuZnV2O7 EDS(atomic fractionat.%,mass fraction wt.%).
3.3. Thermal expansion performance and no structural phase transition at atmospheric pressure
The temperature-dependent x-ray diffraction pattern ofβ-CuZnV2O7at atmospheric pressure is shown in Fig.3(a). All diffraction peaks show normal temperature-dependent behavior from 80 K to 573 K, with no anomalies being observed,which indicates that the crystal structure ofβ-CuZnV2O7remains unchanged. Figures 3(b)and 3(c)are partially enlarged views of two diffraction peaks indexed by [002] and [200].The shifts to the high angle of these two diffraction peaks demonstrate the contraction of thecandaaxes with temperature rising.
According to the Rietveld refinement result of XRD data obtained by Fullprof software, theaandcaxes gradually decrease upon temperature rising (Fig. 4(a)), which is in sync with the changes as shown in Figs. 3(b) and 3(c). However, thebaxis is just the reverse. According to the data in Fig. 4(a), the thermal expansion coefficients ofa,b, andcaxis areαa=-2.7×10-7K-1,αb=4.4×10-6K-1andαc=-6.3×10-6K-1. Figure 4(b)shows the volume change ofβ-CuZnV2O7varying with temperature. The calculated volume expansion coefficient based on the data in Fig. 4(b)isαv=-2.34×10-6K-1. The average liner expansion coefficient obtained from the volume expansion coefficient isαl=-0.78×10-6K-1. Since the XRD reveals the intrinsic lattice variations, the low thermal expansion coefficients calculated from the XRD data suggest thatβ-CuZnV2O7belongs to a near-zero thermal expansion material.

Fig.3. (a)XRD spectra of β-CuZnV2O7 at different temperatures;(b)and(c)a partially enlarged view of temperature-dependent XRD data.
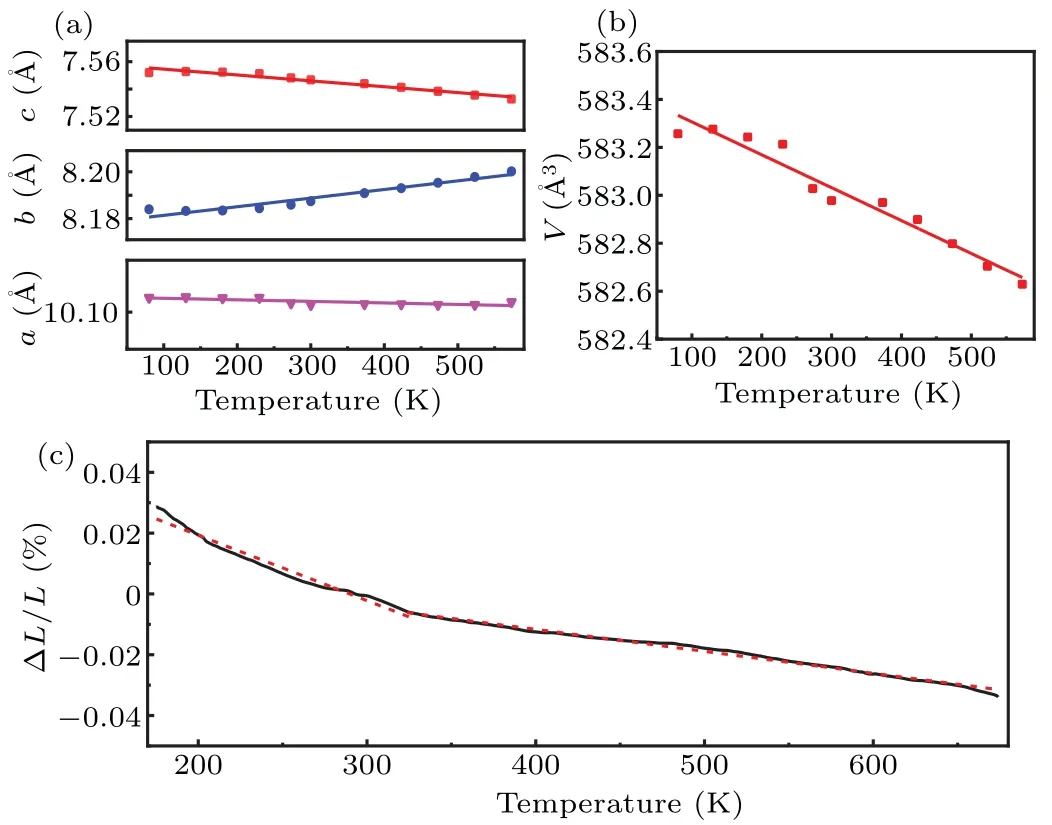
Fig.4. (a)and(b) Changes in the lattice constants and cell volume of β-CuZnV2O7 of low-temperature XRD;(c)relative length(%)changes of the sample at temperatures from 173 K to 673 K.
The relative length change ofβ-CuZnV2O7is depicted in Fig.4(c)from 173 K to 673 K.This thermal expansion curve could be fitted well by two dotted polylines in the temperature ranges of 173-325 K and 325-673 K,resulting in two different linear thermal expansion coefficients,which areαl=-2.15×10-6K-1(173-325 K) andαl=-0.72×10-6K-1(325-673 K). The ZTE behavior ofβ-CuZnV2O7is comparable with the zero expansion material of Ta2Mo2O11(αl=0.37×10-6K-1, 200-600 K), MgZrF6(αv=-2.38×10-6K-1,300-675 K) and TiCo(CN)6·2H2O (αl=-2.2×10-8K-1,100-300 K),[33-35]but with a larger ZTE temperature range.These two linear thermal expansion coefficients are different from the value(αl=-0.78×10-6K-1)obtained by the Rietveld refinement of XRD data. This deviation could be attributed to the tiny voids, holes, and micro-cracks shown in Fig. 2(a). As the thermal expansion curve measured by dilatometer represents the macroscopic properties of materials, the minor segmented linear thermal expansion coefficients also point out the near-zero expansion property ofβ-CuZnV2O7,which brings into correspondence with the intrinsic XRD result.
Figure 5(a)exhibits the Raman spectrum ofβ-CuZnV2O7at 303 K. Our Raman results are consistent with those of Cu1.5Mg0.5V2O7andβ-Cu2V2O7above 200 cm-1,[25,31]which verifies the fact thatβ-CuZnV2O7possesses the same structure with Cu1.5Mg0.5V2O7andβ-Cu2V2O7.[18,25]This consistency supports the high quality of our sample. More detailed peaks of the spectra under 200 cm-1could be detected resorting to the high resolution(0.6 cm-1)and ultra-low filter of our Raman spectroscopy. The temperature-dependent Raman spectrum ofβ-CuZnV2O7is shown in Fig.5(b). The fitted peak positions of each peak in Fig.5(b)at different temperatures are shown in Fig.5(c). According to Fig.5(c),all peak positions show monotonous red shifts with temperature rising and no additional peaks appear,except the peaks of 847 cm-1,796 cm-1, 463 cm-1, and 349 cm-1, which are too broad to be fitted at high temperatures resulting from the anharmonicity of phonon. Our Raman data supports that no structural phase transition exists from 143 K to 663 K at atmospheric pressure,which is in sync with our XRD result.
As the element of Zn is adjacent with Cu in the periodic table, inβ-CuZnV2O7andβ-Cu2V2O7, the phonons with similar energy could be attributed to the similar atoms vibration modes in the Raman spectrum. The vibration modes in different energy ranges ofβ-Cu2V2O7have been identified by de Waal and Hutter. For the Raman spectra of theβ-Cu2V2O7, the Raman peaks in the regions of 350-400 cm-1and 800-1200 cm-1correspond to the bending vibration mode and the stretching vibration mode of the VO4tetrahedron, respectively; the Raman peaks in the regions of 400-550 cm-1and 660-800 cm-1correspond to the bridge V-O-V symmetric and anti-symmetric stretching vibration mode,respectively.[31]
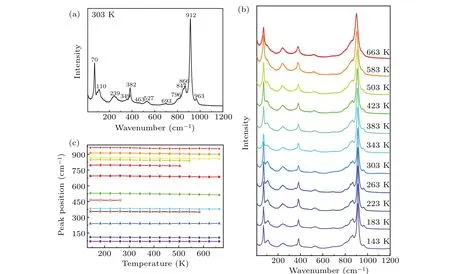
Fig.5. (a) Raman spectra of β-CuZnV2O7 at room temperature; (b) the Raman spectrum of β-CuZnV2O7 at different temperatures; (c) the fitted phonon energies of β-CuZnV2O7 varying with temperature.
According to the identification of the Raman peaks ofβ-Cu2V2O7above,in the Raman spectrum ofβ-CuZnV2O7,the 349 cm-1and 382 cm-1peaks belong to the bending vibration of the VO4tetrahedron. The 847 cm-1,866 cm-1,912 cm-1,and 963 cm-1peaks indicate the stretching vibration mode of the VO4tetrahedron. The 463 cm-1and 527 cm-1peaks represent the V-O-V symmetrical stretching vibration. The 693 cm-1and 796 cm-1peaks stand for the V-O-V antisymmetric stretching vibration. Furthermore, though the peaks 70 cm-1, 110 cm-1, and 239 cm-1were not indexed to any atomic vibrations previously,we infer them to be translational and librational movements of the VO4tetrahedron empirically. Because in many other NTE compounds with open framework structures,Raman modes at below 280 cm-1can be identified as translational and librational vibrations of tetrahedra,such as Zr0.3Sc1.7Mo2.7V0.3O12,HfMgW3O12,and Zn2V1.7P0.3O7.[36-38]
3.4. Two structural phase transitions under high-pressure and the mechanism of NTE
To improve the understanding of the NTE mechanism inβ-CuZnV2O7, a high-pressure Raman experiment has been carried out to acquire the contribution of phonon anharmonicity to NTE properties. Figure 6(a) shows the pressuredependent Raman spectra. The peaks labeled by the diamond marker are not the Raman signals ofβ-CuZnV2O7but silicon oil which is the pressure transmitting medium. As is well known, the discontinuous changes in Raman peaks demonstrate the structure changing. Apparently,two structural phase transitions exist as pressure varying from 0 to 7.84 GPa. The first phase transition appears at 0.94 GPa, symbolized by the distinct differences of the Raman peaks marked by the inverted triangle from 800-1000 cm-1, where the number and the shape of peaks have changed none monotonously. The second phase transition happens at 6.35 GPa,which could be revealed by the appearance of the peak at about 850 cm-1denoted by the circle.
To get insight into the mechanisms of NTE inβ-CuZnV2O7, the mode Gr¨uneisen parameterγiis a powerful medium to know more about the thermal property of materials,[39,40]
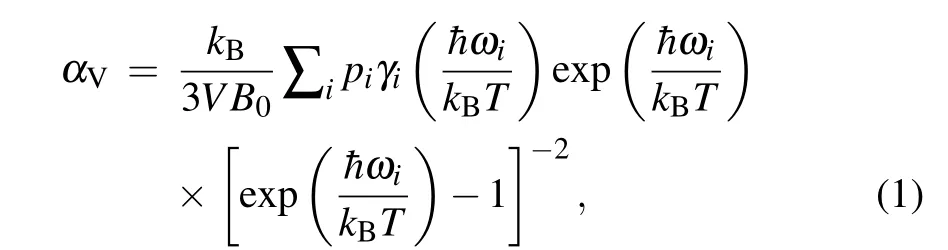
whereωiis the frequency of theith mode,Vis the molar volume,B0is the bulk modulus,kBis the Boltzmann constant,andpiis the degeneracy of the phonon mode with frequencyωiat the Brillouin zone center.
In Eq. (1), the sign ofαVis totally determined byγi.That is to say, if the material behaves NTE property, whereαVis negative, theγishould be minus, and vice versa. The Gr¨uneisen parameterγicould be obtained through formula
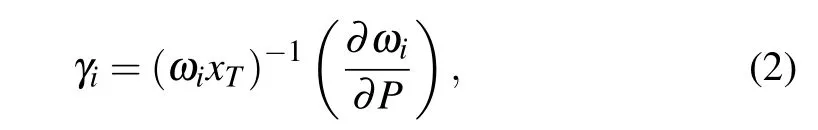
whereωiis Raman frequency of the modei,Pis the pressure,andxTis the isothermal volume compressibility. The sign ofγiis completely decided by the sign of∂ωi/∂P. If the phonon energy decreases with increasing pressure,the sign of∂ωi/∂Pwould be negative.
In our case, from 0 to 0.57 GPa, most of the Raman peaks exhibit blue shifts, which is a regular behavior resulting from the shortening of bond length as pressure increasing,except for the 69 cm-1,352 cm-1,and 526 cm-1peaks. Each∂ωi/∂Pof the 69 cm-1,352 cm-1,and 526 cm-1peaks is minus according to the data in Fig.6(b),where the energy of each Raman peak tends to decrease with pressure rising. Thus,the corresponding Gr¨uneisen parameters of 69 cm-1, 352 cm-1,and 527 cm-1peaks are all negative,indicating that these three Raman modes give a major contribution to the NTE characteristic ofβ-CuZnV2O7.
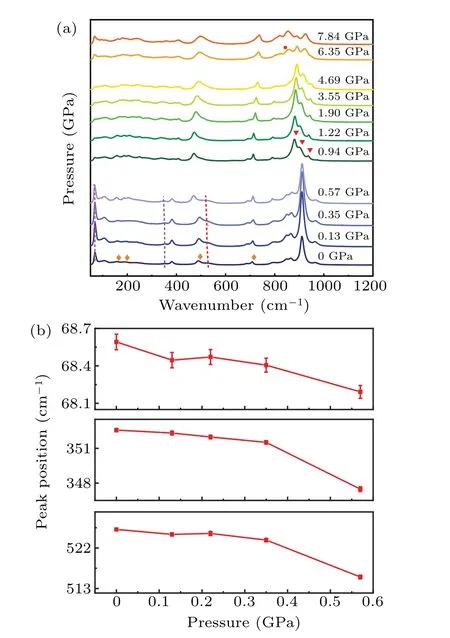
Fig. 6. (a) Raman spectra of β-CuZnV2O7 at different pressures; (b)Raman mode frequency shifts as a function of pressure.
Compared to the phonon anharmonicity, the variation of bond angle varying with temperature could provide a more direct approach to uncover the NTE mechanism inβ-CuZnV2O7. Figure 7 shows the temperature dependence of the angles between Cu(Zn)-O2-V, Cu(Zn)-O3-V, and V-OV,respectively, obtained by the Rietveld refinement based on the low-temperature XRD data in Fig.3(a).As the temperature rises, the lateral vibration of the bridge oxygen atoms shared by the VO4tetrahedron and the(CuZn)0.5O5pentahedron increases, resulting in a gradual decrease in the angle between Cu(Zn)-O3-V and Cu(Zn)-O2-V, which makes a great contribution to the negative thermal expansion of the crystal axesaandc. The insets in Figs.7(a)and 7(b)give a more intuitive manner to observe the reducing of theaandcaxes. Unlike the decreasing of angle above,the angle between V-O-V gains as pressure increasing, which is counterintuitive. According to our high-pressure Raman result given above,theηpeak contributes greatly to the NTE property ofβ-CuZnV2O7. Based on our analysis of the atoms vibrations in Raman experiment mentioned above, this peak represents the V-O-V symmetrical stretching vibration,which would lead to rising of the angle between V-O-V as shown by the vibration sketch of atoms in Fig.7(c). Therefore,this increase of the angle between VO-V could also give a significant contribution to the NTE ofβ-CuZnV2O7.
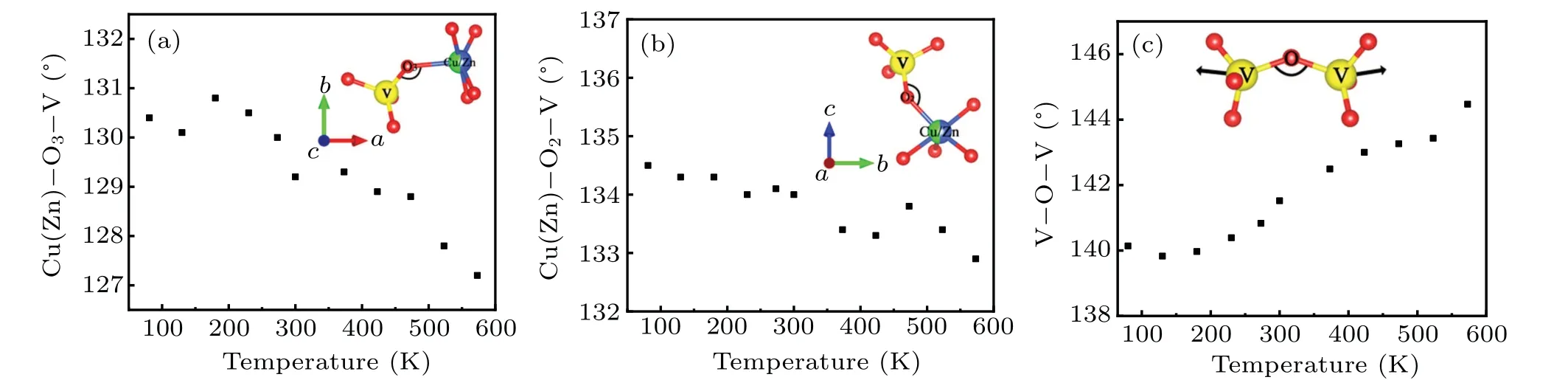
Fig. 7. Angles of (a) Cu(Zn)-O3-V, (b) Cu(Zn)-O2-V, and (c) V-O-V varies with temperature. The insets are schematics of (a) angle of Cu(Zn)-O3-V in a direction, (b) angle of Cu(Zn)-O2-V in c direction, and (c) probable schematic diagram of the V-O-V symmetrical stretching vibration,which is just a qualitative representation of the vibration mode.
4. Conclusion and perspectives
In our experiment,a solid phase reaction method is used to synthesize the polycrystal powder ofβ-CuZnV2O7. Both of the temperature-dependent XRD and Raman experiments reveal that no structural phase transition exists at atmospheric pressure. However, detailed high-pressure Raman data suggests that two structural phase transitions happen at 0.94 GPa and 6.35 GPa,respectively,manifested as anomalous changes in the position and quantity in phonon peaks.The macroscopic linear thermal expansion coefficient obtained by the dilatometer isαl=-2.15×10-6K-1(173-325 K)andαl=-0.72×10-6K-1(325-673 K),which is almost consistent with the intrinsic linear thermal expansionαl=-0.78×10-6K-1. This consistency declares that theβ-CuZnV2O7belongs to a nearzero NTE material with a large NTE temperature range from 173 K to 673 K. The XRD data supports the decrease ofaandcaxes with the increasing temperature, which could be demonstrated expressly by the schematic diagrams. The negative Gr¨uneisen parameters indicate that the anharmonicity of 69 cm-1,352 cm-1,and 527 cm-1peaks gives a great contribution to the NTE characteristics inβ-CuZnV2O7.The atomic vibration of the 527 cm-1peak explains why the increase of the angle between V-O-V could lead to the shortening of volume inβ-CuZnV2O7upon temperature increasing. This work provides not only detailed insights into the structure and the NTE mechanism inβ-CuZnV2O7but also important significance for understanding the NTE in other framework materials.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11874328, 12004339,11574276, and 21905252), the China Postdoctoral Science Foundation (Grant Nos. 2018M640679, 2019T120629, and 2019M652558),and the Zhongyuan Academician Foundation(Grant No.ZYQR201810163).
- Chinese Physics B的其它文章
- Quantum walk search algorithm for multi-objective searching with iteration auto-controlling on hypercube
- Protecting geometric quantum discord via partially collapsing measurements of two qubits in multiple bosonic reservoirs
- Manipulating vortices in F =2 Bose-Einstein condensates through magnetic field and spin-orbit coupling
- Beating standard quantum limit via two-axis magnetic susceptibility measurement
- Neural-mechanism-driven image block encryption algorithm incorporating a hyperchaotic system and cloud model
- Anti-function solution of uniaxial anisotropic Stoner-Wohlfarth model

