Corticospinal activity during a single-leg stance in people with chronic ankle instability
Masafumi Teraa*,Kyle B.Kosik,Ryan S.MCann,Colin Drinkar,Phillip A.Grile
a College of Sport and Health Science,Ritsumeikan University,Kusatsu,Shiga 525-8577,Japan
b Department of Athletic Training&Clinical Nutrition,University of Kentucky,Lexington,KY 40536-0200,USA
c School of Rehabilitation Sciences,Old Dominion University,Norfolk,VA 23529,USA
d Westlake High School Athletics,Austin,TX 78746,USA
Abstract
Keywords: Ankle sprain;Joint instability;Motor cortex;Postural control
1. Introduction
The most prevalent musculoskeletal injury is the lateral ankle sprain.1-3 Up to 75% of lateral ankle sprainers will suffer from recurrent injury and lifelong residual symptoms(i.e., perceived instability),4-6 commonly referred to as chronic ankle instability (CAI).7The long-term negative consequences of CAI are evident because patients with CAI continue to experience persistent disability for more than 7 years after an initial lateral ankle sprain,4limiting their physical activity8and leading to decreased healthrelated quality of life.4,9,10Furthermore, recent evidence has shown that CAI subsequently accelerates ankle joint cartilage degeneration.11-13 Therefore, CAI is a pathological condition that could negatively impact longterm joint and general health.
Sensorimotor maladaptations associated with CAI can manifest into self-reported functional disability and threaten quality of life.14-16 Optimal neuromuscular control is achieved with proper integration of the sensory inputs from somatosensory structures and motor outputs from the central nervous system.17-19 Although sensory dysfunction following an initial lateral ankle sprain may contribute to functional limitations associated with CAI,20it has been proposed that alterations in supraspinal motor control may also compromise physical and self-reported function,21-25 as evidenced by decreased corticospinal excitability of the fibularis longus14,26and tibialis anterior,26as well as compensatory muscle activation patterns of proximal musculature in patients with CAI.27Furthermore, previous investigations have shown the inefficiency of the central nervous system in the controlling reflexive response of the ankle stabilizers in those with CAI.28
Proper efferent signaling from the corticospinal system is important for proper muscle recruitment and movement corrections,29,30and the primary motor cortex acts in an executive role for neuromuscular control.31Although motor outputs from corticospinal pathways are most often measured during a seated position using transcranial magnetic stimulation(TMS),14,32it lacks a sense of functionality and limits our understanding as to how altered corticospinal excitability and inhibition are associated with CAI during a postural task.TMS measures may depend on change in body position and the type of task;33therefore,the findings of altered corticospinal excitability in previous studies14may not adequately reflect neuromuscular function during a balance task.Previous studies have attempted to examine the descending corticospinal pathways during a double-leg stance task,29,30but only in healthy control participants. Therefore, there is a lack of information on how the corticospinal excitability and inhibition of the ankle stabilizers are affected in individuals with CAI during a challenging postural task. Better understanding of the link between CAI and corticospinal excitability during a balance task will aid in the understanding of how corticospinal deficits are associated with CAI,leading to improved clinical interventions for CAI.
There is a known cohort of individuals with histories of lateral ankle sprain, but there is an absence of CAI-related characteristics, such ankle dysfunction, complaints of perceived instability, and episodes of giving-way. The populations who do not develop CAI after a lateral ankle sprain have been categorized as lateral ankle sprain copers.34-40 Lateral ankle sprain copers restore sensorimotor control and functional levels similar to a population with no history of lateral ankle sprains.41Therefore, a study41has focused on identifying differences in characteristics between lateral ankle sprain copers and patients with CAI in order to understand the underlying mechanism that elucidates why some individuals develop CAI while others do not. Understanding the coping mechanism may guide future research focused on interventions to convert patients with CAI into lateral ankle sprain copers, with the long-term goal of reducing disability and maintaining joint health.
Therefore, the purpose of this study was to determine whether corticospinal excitability and inhibition of the tibialis anterior during single-leg standing differs among individuals with CAI, lateral ankle sprain copers, and healthy controls.We hypothesized that individuals with CAI would have decreased corticospinal excitability and increased corticospinal inhibition of the tibialis anterior during single-leg standing compared to lateral ankle sprain copers and healthy controls;however, there would be no difference in our selected variables between the lateral ankle sprain copers and healthy control groups. The tibialis anterior muscle was chosen and evaluated for this study because it has a significant role in maintaining stability during a single-leg stance,42,43and altered motor control patterns of the tibialis anterior during standing tasks have been documented in individuals with CAI.27,44-47 It is believed that deficits in tibialis anterior function can impair one’s ability to accelerate the center of mass in the direction of the support limb,which may increase a risk for the contralateral ankle sprain.48-50 Additionally, the tibialis anterior muscle is one of the dynamic stabilizers in the ankle and protects the ankle joint against injury because it eccentrically controls ankle plantar flexion that is a common mechanism of ankle injury.
2. Methods
2.1. Study design
This study was conducted with a single-blinded case-control design. A single investigator screened participants for inclusion criteria,while 2 investigators responsible for measuring corticospinal excitability and inhibition of the tibialis anterior were blinded to group membership.Participants reported to the research laboratory for a single testing session. Specifically, the second investigator(KBK)was responsible for recording and analyzing all of the primary outcome measures,whereas the primary author (MT) was responsible for coil placement during the TMS testing. We estimated a sample size of 20 participants in each group(60 total)from corticospinal excitability and inhibition data from previous studies,14,25,32,51a predetermined a level of 0.05,and an estimated power of 0.80.
2.2. Participants
Seventy physically active participants from the university community volunteered for the current study(Table 1).Participants’ physical activity levels were determined by using the Godin Leisure-Time Exercise Questionnaire,and being physically active was defined as having a Godin Leisure-Time score of 24 or above.52All participants read and signed an informed written consent approved by the University of Kentucky Institutional Review Board prior to the study. All participants had(1)no diagnosed balance or vestibular disorders,(2)no history of self-reported low back pain,(3)no history of surgery in the lower extremity, (4) no history of a concussion in the past 6 months, and(5) no history of any self-reported musculoskeletal or neurovascular injuries or disorders in the lower extremities in the previous 2 years other than lateral ankle sprains.All participants met additional inclusion criteria for TMS in accordance with the TMS safety guidelines outlined by the National Institutes of Neurological Disorders and Stroke.53
After enrolling the participants and screening selfreported questionnaires, the participants were initially separated by previous history of lateral ankle sprain. Participants without histories of lateral ankle sprain were placed in the healthy control group. Participants reporting a previous history of lateral ankle sprain were screened and included in the CAI group based on the guidelines endorsed by the International Ankle Consortium.54The specific inclusion criteria for participants with CAI included: (1) a previous history of at least 1 significant lateral ankle sprain resulting in swelling, pain, and temporary loss of function; (2) a history of feelings of giving-way at least twice in the past 6 months; (3) ongoing perceived ankle instability and dysfunction during daily activities; and (4) a score of -5 on the Ankle Instability Instrument (AII), -11 on theIdentification of Functional Ankle Instability, and -24 on the Cumberland Ankle Instability Tool.54The CAI group included 23 participants. No participant with CAI had acutely sprained an ankle in the previous 3 months before testing. If a participant had a history of bilateral ankle injury, we measured the limb with the greatest amount of self-reported instability. A test limb for the control group was randomly selected.
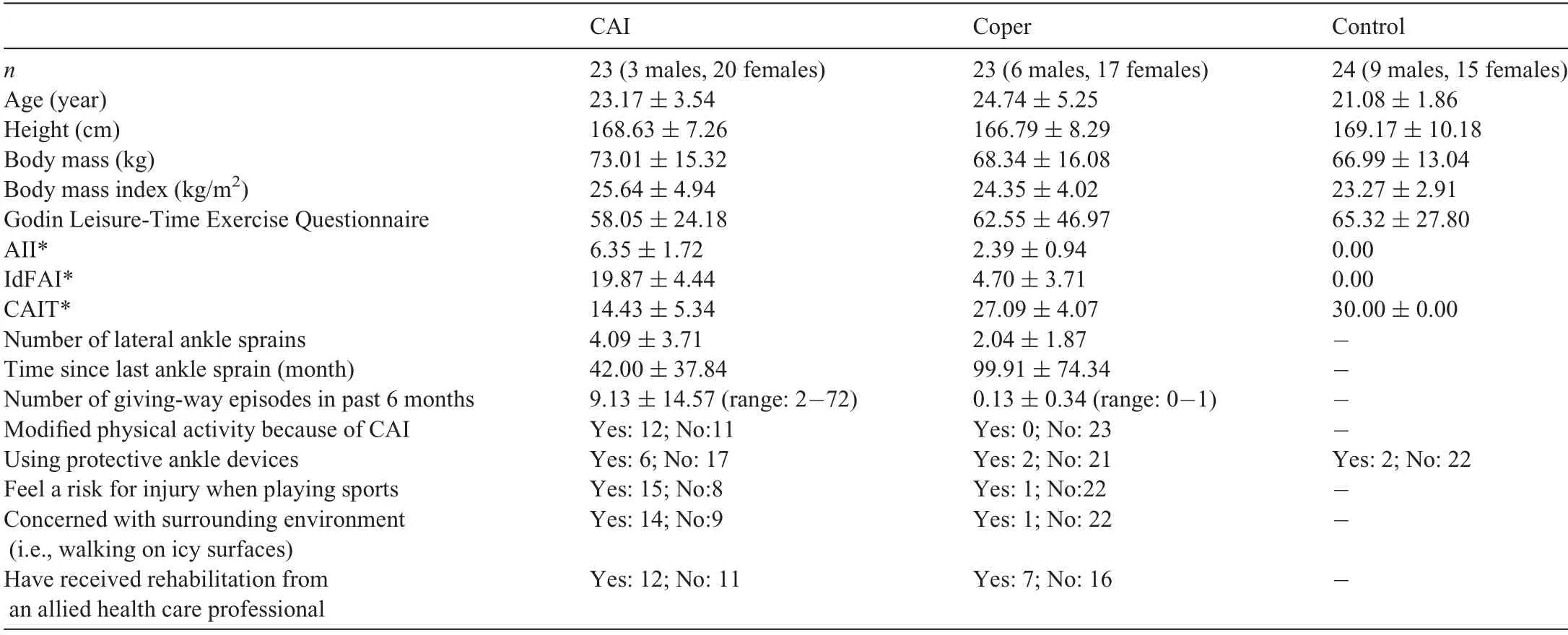
Table 1 Demographic and ankle injury characteristics for CAI,Coper,and control groups(mean§SD).
Participants who did not meet the CAI inclusion criteria were then screened according to the lateral ankle sprain coper inclusion criteria. Twenty-six participants were included in the lateral ankle sprain coper group, defined as participants with a history of lateral ankle sprains but no reported episodes of giving-way, perceived instability, loss of function, or modification of physical activity. Lateral ankle sprain copers had to (1) have no history of feelings of giving-way fewer than twice in the past 12 months; (2)have no ongoing perceived ankle instability and dysfunction during daily and physical activities; and (3) score <5 on the AII and <11 on the Identification of Functional Ankle Instability. We did not use the Cumberland Ankle Instability Tool scores to classify participants as lateral ankle sprain copers because it has been reported that the cutoff score of -25 resulted in a high rate of false-positives in the lateral ankle sprain coper group.55No participant in the lateral ankle sprain coper group had acutely sprained an ankle in the previous 12 months before testing.
The control group included 24 participants. The specific inclusion criteria for participants in the control group were(1)no history of lateral ankle sprain; (2) a score of 0 on both the AII and Identification of Functional Ankle Instability; and (3)a score of 30 on CAIT.Means and standard deviations for participant demographics and ankle injury information are found in Table 1.
2.3. Experimental procedures
Participants reported to the research laboratory for the single TMS testing session. Two 10 mm pre-gelled Ag/AgCl electromyography (EMG) electrodes (EL503; BIOPAC Systems, Goleta, CA, USA) were placed 1.75 mm apart over the midpoint of the tibialis anterior muscle belly, and a ground electrode was placed over the contralateral medial malleolus.56The areas were shaved, abraded with fine sandpaper, and cleaned with isopropyl alcohol wipes prior to electrode placement. EMG signals were recorded at a sampling rate of 2000 Hz (gain set at 1000, common mode rejection ratio=110 dB, input impedance=1 MOhms) (EMG100C;BIOPAC Systems).A 16-bit converter(MP150;BIOPAC Systems)was used to process analog-to-digital signal conversion.EMG signals were filtered with a high pass of 10 Hz and low pass of 500 Hz. EMG and stimulation signals were visualized through AcqKnowledge 4.1 Software(BIOPAC Systems).
Participants wore a Lycra swim cap (Sprint Aquatics, Rothhammer International,San Luis Obispo,CA,USA)that helps to mark the approximate primary motor cortex location so that the location of a double-cone coil did not change during the experiment.On the cap,a standard 1 cm2dot grid was marked,and 2 straight lines were drawn vertically in the sagittal and frontal planes. These lines intersected over the vertex of the skull: one line separating the hemispheres sagittally and the other connecting the apexes of the ears bisecting the other line. The doublecone coil was positioned over the intersected lines and dot grids.
A Magstim 200(Magstim,Whitland,Wales,UK)was used to produce a magnetic stimulus (max 2.0 Tesla) over the primary motor cortex contralateral to the test limb. During the TMS testing,participants stood in a barefoot single-leg stance on the middle of a force platform (AccuSway Plus, AMTI,Watertown, MA, USA) integrated with Balance Clinic software(AMTI),kept their hands on the waist,and stared at an X in front of them while keeping their foot flat on the force platform (Fig. 1). An investigator held the TMS coil to secure it on the participant’s head while providing the magnetic stimuli.We used the force platform to visually monitor center-of-pressure displacement to ensure that participants remained still while in position and that the investigator holding the coil did not influence participant’s performance during the TMS testing.The magnetic stimulation was provided approximately 3 s after participants assumed the single-leg balance position. To minimize the potential effect of fatigue, a minimum of 30 s rest was provided between each trial. The trial was discarded and repeated if (1) the nontested limb made contact with the force platform; (2) the nontested limb made contact with the stance limb; (3) the participant took a step with the stance limb;(4)the participant removed the hands from the chest;or(5)the participant abducted the hip or laterally flexed the trunk into>30 degrees.To determine the optimal stimulating point location, a series of magnetic stimuli at 50% of the maximal stimulator output was delivered at a number of locations on the grid until the largest and most consistent motor-evoked potentials(MEPs)were observed.57Once the optimal stimulating location was detected, an investigator stabilized the TMS coil at this location for all subsequent tests.
The active motor threshold (AMT) was assessed by using the method previously described.57Briefly,the MEP threshold was calculated by determining the average peak amplitude of the background EMG signal collected while participants performed the single-leg balance without magnetic stimulus.57The cut-off threshold was set 2 SDs above this EMG amplitude.57The AMT was determined as the lowest stimulator intensity required to elicit at least 4 of 8 MEPs whose peak-topeak amplitudes exceeded this MEP threshold.57,58A higher AMT indicates decreased corticospinal excitability because a greater stimulator intensity is required to excite the neurons within the corticospinal pathway.59Once AMT was established,8 stimuli were delivered at intensities of 100%of AMT and 120% of AMT, and peak-to-peak MEP amplitudes were recorded for each trial. The amplitude of the 8 MEPs at the intensities of 100%of AMT and 120%of AMT were averaged and normalized to mean amplitudes of background EMG activity that was recorded 50 ms prior to a stimulus in order to minimize the effect of background EMG on the MEP amplitude.60The MEP amplitude provides an estimate of the overall excitability of the corticospinal tract.59
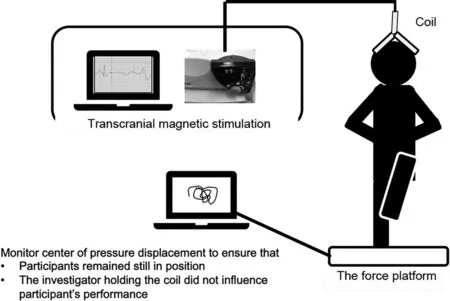
Fig.1. Experiment set-up.
Cortical silent period(CSP)can be used to evaluate inhibition within the corticospinal pathway,which may be mediated through g-aminobutyric acid-B receptors.61The CSP period of the tibialis anterior was obtained at intensities of 100% and 120% of AMT and was measured as the time from the end of the MEP to a return of the mean EMG signal plus 2 times the standard deviation of the baseline (pre-stimulus) EMG signal.62The CSP was reported in ms. A longer CSP indicates greater corticospinal inhibition to the tibialis anterior.
2.4. Statistical analysis
All statistical analyses were performed using SPSS software (Version 23.0; IBM Corp., Armonk, NY, USA). Significance was set at p < 0.05 for all analyses. We could not collect the CSP data from a few of participants(9 participants at 100%intensities of AMT and 10 participants at 120%intensities of AMT) because the EMG signal during the expected CSP time window was obscured, resulting in the landmark to define CSP being absent. Therefore, 13% of CSP at 100%intensities of AMT(9/70)and 14%of CSP at 120%intensities of AMT (10/70) in the data set were imputed using the multiple imputation method with 5 repetitions.63
Based on an analysis of the data using a Kolmogorov-Smirnov Z test for normality,64we found that MEP measures were not normally distributed (p <0.05). Therefore, a separate independent-samples Kruskal-Wallis test was performed to compare MEP measures among the CAI, lateral ankle sprain coper, and control groups. A Mann-Whitney U test was conducted as post hoc analysis in the case of statistical significance. Last, r effect sizes (Z/x-n) were calculated to determine the magnitude of significant group differences.Effect sizes were interpreted as small(r=0.10-0.29),moderate (r=0.30-0.49), large (r=0.50-0.69), or very large(r>0.70).65
Other outcome measures (AMT, CSP at 100% and 120%intensities of AMT, and background EMG) were compared among the CAI,lateral ankle sprain coper,and control groups using separate one-way analysis of variance models. Bonferroni post hoc testing was conducted as needed. Last, Cohen d effect sizes using the pooled standard deviations were calculated for outcome measures that were normally distributed,66along with 95% confidence intervals, to determine the magnitude of difference in outcome variables between groups. The strength of effect sizes was interpreted as weak (d < 0.40),moderate(d=0.40-0.79),or large(d -0.80).66
3. Results
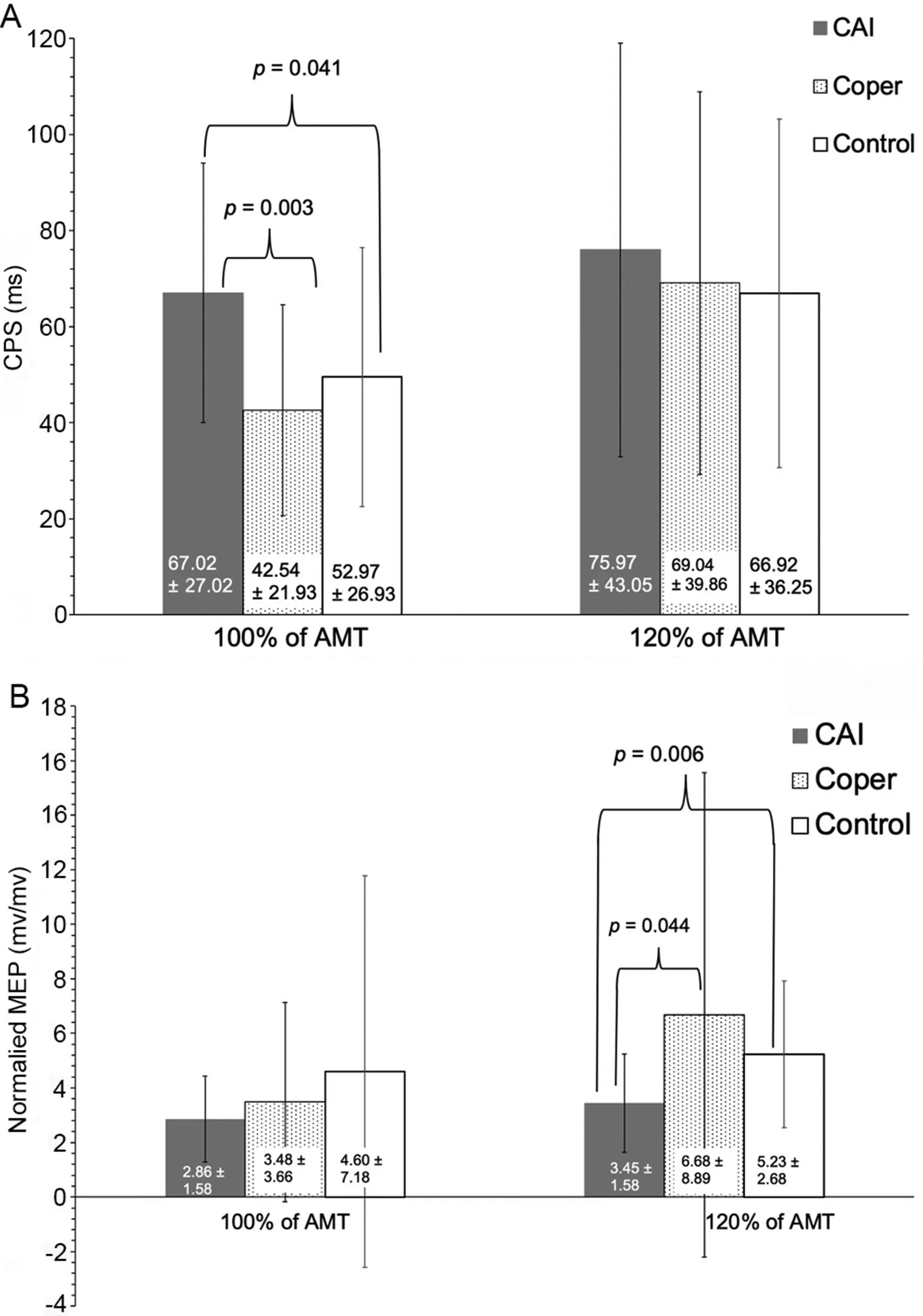
Fig.2. Corticspinal excitability and inhibition results(mean§SD).(A)Quantified at 100% of active motor threshold; (B) Quantified at 120% of active motor threshold.AMT=active motor threshold;CAI=chronic ankle instability;CSP=cortical silent period;MEP=motor evoked potential.TagedEnd
Between-group differences were observed for CSP at 100%intensities of AMT (p=0.003) (Fig. 2A) and MEP at 120%intensities of AMT(p=0.021)(Fig.2B)of the tibialis anterior.Post hoc analysis revealed that participants with CAI had significantly longer CSP at 100% intensities of AMT and lower normalized MEP at 120% intensities of AMT compared to lateral ankle sprain copers (CSP at 100% intensities of AMT:p=0.003; MEP at 120% intensities of AMT: p=0.044) and controls(CSP at 100%intensities of AMT:p=0.041;MEP at 120% intensities of AMT: p=0.006), which were supported by moderate to strong effect sizes (Table 2) (Figs. 2 and 3).There were no differences between the lateral ankle sprain coper and control groups in CSP at 100% intensities of AMT(p=1.000)and MEP at 120%intensities of AMT(p=0.774).
AMT was not significantly different among the groups(CAI=35.57% § 9.52%, lateral ankle sprain coper=36.70%§ 11.59%, control=35.88% § 11.54%; p=0.936). There were no differences in CSP at 120% intensities of AMT(p=0.975) and MEP at 100% intensities of AMT (p=0.425)between the CAI, lateral ankle sprain coper, and control groups(Fig.2A and 2B).For background EMG,no differences were observed between groups(CAI=0.28§0.20 mV,lateral ankle sprain coper=0.27§ 0.20 mV,control=0.17§ 0.13 mV;p=0.07). All effect size comparisons for AMT, CSP at 120%intensities of AMT, and MEP at 100% intensities of AMT were weak, with 95% confidence intervals that crossed 0 (Table 2)(Fig.3).

Table 2 Pairwise comparisons and effect sizes for outcome measures that were not normally distributed.
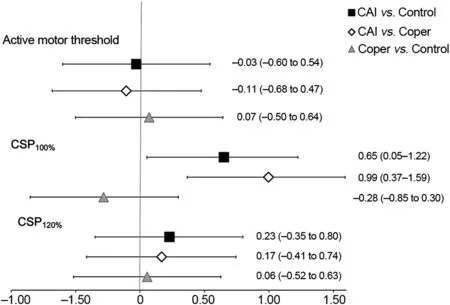
Fig. 3. Cohen d effect sizes and associated 95% confidence intervals.CAI=chronic ankle instability;CSP=cortical silent period.TagedEnd
4. Discussion
The association between CAI and altered neuromuscular control of the tibialis anterior during sophisticated tasks incorporating postural control and gait has been well documented;27,44,45however, our understanding of the neurophysiological mechanism underlying this alteration is undiscovered. Researchers have hypothesized that altered supraspinal organization of the central nervous system may influence motor control in individuals with CAI.21Our study examined differences in corticospinal excitability and inhibition during a single-leg stance between individuals with and without CAI.We observed that participants with CAI demonstrated longer CSP at 100% of AMT and lower MEP at 120%of AMT in the tibialis anterior during the single-leg standing compared to lateral ankle sprain copers and controls, supporting the previous speculation of centrally mediated changes within corticospinal pathways.14These results indicate that participants with CAI may have an increase in the sensitivity of inhibitory intermediate neurons and a decrease in the sensitivity of excitatory neurons in the corticospinal pathway. To our knowledge, we are the first to investigate corticospinal excitability and inhibition during a balance task in a CAI population.
4.1. Potential corticospinal maladaptations
Declines in sensorimotor control during a single-leg stance have been consistently observed in individuals with CAI.41,67Van Deun et al.45observed altered muscle activation patterns of the tibialis anterior during the transition from double-leg to single-leg standing in individuals with CAI.Furthermore,individuals with CAI have demonstrated the inability to control spinal reflex excitability of the ankle stabilizers during postural transitions,28potentially suggesting an altered supraspinal mechanism within the central nervous system.Impairments in the somatosensory system at the damaged ankle joint due to the presence of CAI may decrease sensory inputs to the central nervous system,which could create a chronic central-mediated alteration in neuromuscular function.21Altered corticospinal adaptations in the motor control of the tibialis anterior observed in our study indicate that individuals with CAI may encounter more difficulty in controlling the tibialis anterior muscle during single-leg stance,indicating that movement patterns are altered during a balance task in those with CAI.
4.2. Corticospinal inhibition
Increased CSP in the tibialis anterior muscle of participants with CAI at the intensity of 100% of AMT indicates greater corticospinal inhibition to the corresponding muscle.61However, there were no differences in CSP at 120% of AMT among the CAI,lateral ankle sprain coper,and control groups.This may be attributable to the effect of TMS intensity on CSP duration. CSP duration is prolonged with higher TMS intensity.68At lower TMS intensity, inhibitory intermediate neurons in the corticospinal pathway may already be activated in participants with CAI. As the intensity increases, inhibitory neurons in the pathway are more activated in lateral ankle sprain copers and control participants;thus,they become more similar to those with CAI. The higher intensities likely have less effect on CSP in those with CAI because there may not be many additional inhibitory neurons left to activate.
4.3. Corticospinal excitability
Similar to our results,Needle et al.32reported that AMT of the tibialis anterior was not different between the CAI and control groups.AMT is considered to be an outcome measure that estimates the membrane excitability of pyramidal neurons.59Based on findings from Needle et al.32and our current study,individuals with CAI do not require higher magnetic stimulation in order to excite the pyramidal cells in the primary motor cortex, possibly indicating no effects of CAI on corticomotor excitability. However, we observed less normalized MEP amplitude at 120%AMT in the tibialis anterior for participants with CAI than for lateral ankle sprain copers and controls.Decreased MEPs at 120% of AMT may indicate that the degree of motor responses in the tibialis anterior with high TMS intensity may be small during a single-leg stance in participants with CAI.69MEPs represent the magnitude of motor outputs induced by magnetic stimulus that is relayed to the muscles via the entire corticospinal system.32This decrease in the percentage of outgoing motor information being delivered to the tibialis anterior through the corticospinal system is,therefore, interpreted as a decrease in the corticospinal excitability of this muscle in the CAI group.
Although normalized MEP amplitude at 120% AMT was decreased in the CAI group relative to the lateral ankle sprain coper and control groups,the amplitude of responses at 100%AMT was not different among the groups. These results indicate that the excitation of the number of a central core of tibialis anterior motor neurons that arise from the primary motor cortex may not be different at 100% of AMT among the groups. However, the higher normalized MEPs observed at 120% of AMT in the lateral ankle sprain coper and control groups may be attributed to additional recruitment of inherently less excitable neurons in the primary motor cortex.Recording MEP amplitudes at multiple intensities is considered a stimulus response, which takes into account both the response of neurons with lower and higher thresholds and the potentials increase in the MEP amplitude of the signal as the intensity of TMS increases.70,71The higher TMS intensity can recruit more motor neurons that are less excitable or located farther from the center of activation by the magnetic stimulus,thereby increasing the MEP amplitude.70Therefore,decreased MEPs at 120% of AMT in the CAI group may indicate that a smaller portion of motor neurons in the corticospinal system could be excited by a high level of excitation to the primary cortex.
4.4. Potential coping mechanism and clinical implications
Corticospinal excitability and inhibition of the tibialis anterior in lateral ankle sprain copers more closely resembled that of control participants compared to participants with CAI.This provides a potentially significant explanation for the differences between lateral ankle sprain copers and CAI patients.Corticospinal outcomes observed during the single-leg stance in lateral ankle sprain copers is suggestive of adequate motor outputs delivered to the tibialis anterior through the corticospinal pathway following lateral ankle sprain.This may help lateral ankle sprain copers to make postural control adjustments and maintain ankle stability. Optimal postural control requires proper transfer of motor information to the postural muscles from the corticospinal system in order to achieve proper muscle recruitment during postural control.17Researchers have shown better postural control performance in lateral ankle sprain copers than in participants with CAI.41It is possible that lateral ankle sprain copers could successfully reorganize corticospinal activity following the ankle injury, leading to appropriate postural-control strategies. Our findings suggest that treatment and rehabilitation aimed at improving postural control in patients with CAI might include manipulation of corticospinal excitability and inhibition. However,because of our cross-sectional,retrospective study design,it remains unknown exactly how lateral ankle sprain copers have adopted neuromuscular strategies without perceived instability following lateral ankle sprains. Thus, future prospective studies should explore the time-course of the corticospinal changes after an initial lateral ankle sprain and the response to interventions during the rehabilitation process.
Understanding the effect of ankle joint injury on the corticospinal pathway may be critical in the advancement of our knowledge of neural mechanisms causing function limitations. However, we did not include spatiotemporal measures of a single-leg stance performance in the current investigation. We do not know whether the corticospinal alterations observed in the tibialis anterior actually influence postural control and movement patterns during the single-leg stance. Therefore, future studies should incorporate these measures to further determine the level of CAI’s influence on corticospinal pathway alterations and how they influence functional measures.
4.5. Limitations
We acknowledge that more specific neurophysiological mechanisms responsible for the observed corticospinal outcomes cannot be determined using only the single-pulse TMS paradigm. The MEP and CSP can be modulated at both the cortical and spinal levels,59,70and we did not quantify the Hoffmann reflex (spinal reflex excitability) of the tibialis anterior during the task. Thus, it is difficult to identify more specific local modulation in the cortical or spinal regions that contribute to CAI. A paired-pulse TMS paradigm provides additional information related to intracortical excitability and inhibition during a postural control task.Therefore,in order to parse out specifically where a change has occurred, further research is needed to investigate the association between the CAI and intracortical measures using paired-pulse TMS paradigms.
We carefully and visually monitored center-of-pressure displacement using the force platform to ensure that participants remained still, but we did not truly control postural sway direction in the single-leg stance when delivering magnetic stimulation. Previous studies have shown that postural sway direction influenced the size of MEP amplitude due to a change in the length of muscle fibers and/or spinal or subcortical modulation.30,72Specifically, the tibialis anterior fascicles become shortened when swaying in the forward direction,42and this shortening of the muscle alters proprioceptive feedback which, in turn, influences corticospinal excitability.30,72It is possible that lateral ankle sprain copers and control participants were swaying backward at the time of stimulation, whereas those with CAI were swaying in the forward direction. However, the background EMG that was recorded 50 ms prior was not different among groups (p=0.07). Therefore, the betweengroup differences in our selected corticospinal measures were likely to be attributed to the presence of CAI.
Although we did not acquire corticospinal variables during sitting, it is important to note that results from our study may not be representation of corticospinal characteristics assessed during sitting. Because posture and standing tasks influence TMS measures of corticospinal excitability and inhibition,33TMS measures obtained in the single-leg stance may be more relevant and sensitive indices of neuromuscular characteristics during functionally relevant posture compared to measurements during sitting.73If background activation levels are matched between standing and sitting positions, corticospinal characteristics may not be different between standing and sitting.33,73However,it is not clear how the presence of CAI alters posturerelated effects on corticospinal excitability and inhibition of the tibialis anterior muscle.Therefore,future studies are needed to compare corticospinal excitability and inhibition between sitting and standing in a population with CAI.
Evaluation of corticospinal excitability and inhibition of the tibialis anterior muscle using TMS has been demonstrated to be reliable (intraclass correlation coefficient=0.89-0.98).74However, we did not assess intra-and inter-rater reliability of the selected TMS variables during the standing condition.Furthermore, our study included only the tibialis anterior muscle because it is an important ankle and postural stabilizer. The fibularis longus muscles and soleus also have vital roles for ankle stabilization and maintenance of upright stance.75Further investigation is needed to include quantification of corticospinal excitability and inhibition of other ankle joint stabilizers during a balance task.Last,in our study we did not assess the presence of mechanical instability in the CAI group,and mechanical instability following an initial ankle sprain remains an important factor in understanding the CAI paradigm.
5. Conclusion
Our study examined differences in corticospinal excitability and inhibition during single-leg stance between individuals with and without CAI. Participants with CAI demonstrated longer CSP at 100% of AMT and lower MEP at 120% of AMT in the tibialis anterior during the single-leg balance compared to lateral ankle sprain copers and controls. Our investigation found that corticospinal excitability and inhibition in the tibialis anterior muscle was altered in CAI participants during the single-leg stance, illustrating centrally mediated changes within corticospinal pathways. Altered corticospinal adaptations to motor control of the tibialis anterior indicate that those with CAI may encounter more difficulty in controlling the tibialis anterior muscle during a single-leg stance,potentially influencing postural control performance in those with CAI.Future studies should incorporate alternative analyses to further determine the level of influence of the motor control via the corticospinal pathway on postural control performance in CAI populations.
Acknowledgment
The authors thank Dr.Abbey Thomas,PhD,ATC,for assistance with aspects of the study design.
Authors’contributions
MT,KBK,and PAG participated in the design of the study and contributed to data collection and reduction/analysis and to the interpretation of results;RSM participated in the design of the study and interpretation of results; CD participated in the design of the study and contributed to data collection. All authors contributed to the manuscript writing.All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.08.008.
 Journal of Sport and Health Science2022年1期
Journal of Sport and Health Science2022年1期
- Journal of Sport and Health Science的其它文章
- Does eccentric exercise stimulate sarcomerogenesis?
- The Beijing 2022 Winter Olympics:An opportunity to promote physical activity and winter sports in Chinese youth
- Beijing 2022 Winter Olympic Games:Commitments to science and public health
- Knee biomechanics of patients with total knee replacement during downhill walking on different slopes
- Biceps femoris long head sarcomere and fascicle length adaptations after 3 weeks of eccentric exercise training
- Estimates of voluntary activation in individuals with anterior cruciate ligament reconstruction:Effects of type of stimulator,number of stimuli,and quantification technique
