Biceps femoris long head sarcomere and fascicle length adaptations after 3 weeks of eccentric exercise training
Ptriio A.Pinheir*,Meliss A.Boswell,Mrtino V.Frnhi,Sott L.Delp,Glen A.Lihtwrk
a School of Human Movement and Nutrition Sciences,The University of Queensland,Brisbane,QLD 4072,Australia
b Department of Bioengineering and Mechanical Engineering,Stanford University,Stanford,CA 94305,USA
c Department of Biomedical Sciences,University of Padova,Padova 35122,Italy
Abstract
Keywords: Hamstrings;Nordic hamstring exercise;Sarcomerogenesis
1. Introduction
Eccentric training is beneficial for enhancing muscle strength,power,and speed.1,2It can improve mechanical muscle function more than other exercise modalities can(e.g.,concentric and isometric).3Longer fascicle lengths have been measured in training programs that sufficiently loaded the eccentric phase of a movement,4,5-7 which is attributable to either longer fiber resting lengths or longitudinal fiber growth.Although this adaptation has been linked to muscle protection during subsequent exercise-induced muscle damage8as well as to reduced strain-related injury risk,7,9,10the mechanisms underlying these fascicle length changes are still unclear.
Nordic hamstring exercise (NHE) is a such eccentric exercise that elicits changes in fascicle length.5,7However,the macroscopic and microscopic changes contributing to the increase in hamstring fascicle lengths remain poorly understood. The hypothesis put forward is that repetitive eccentric contractions cause an addition of sarcomeres in series (sarcomerogenesis)within muscle fibers,11thus reducing the likelihood of overstretching the muscle in subsequent eccentric loading.However,this hypothesis has not been tested due to the lack of simultaneous in vivo measurements of fascicle and sarcomere changes in human subjects. Additionally, methodological limitations,such as extrapolation when measuring fascicle length (e.g.,using linear trigonometry to extrapolate fascicle length to regions where the fascicle is not visible)12may hamper the interpretation of previous findings.5-7 It is necessary to establish whether the increases in resting fascicle length observed after eccentric training result from sarcomerogenesis or from a change in resting sarcomere length in order to provide a scientific basis for training program design.
Muscle remodeling processes are related to the stress and strain applied to the muscle,13,14and muscle strain may be heterogeneous across different regions of the same muscle.15,16This heterogeneity suggests that morphological,structural,and molecular adaptations to eccentric training may be nonuniform within the muscle.17,18For example,in vivo measures of sarcomere length in the human tibialis anterior muscle have shown that sarcomeres are longer in the distal compared to the proximal region, despite fibers being of a similar length.19Animal studies also suggest that different muscle architectures and fiber lengths may influence whether and how serial sarcomere adaptations occur.20This highlights the need to use multiscale measurements to understand the nature of region-specific adaptations due to eccentric exercise.
The in vivo measurement of sarcomere and fascicle length has historically been unattainable,but promising new methods have emerged to examine both fascicle length and individual sarcomere numbers in live subjects. Freehand 3D ultrasound (3DUS) combines 2D ultrasound scanning and 3D motion analysis to provide direct in vivo measurements of fascicle length within a wide area of tissue structure.21As such,it is possible to measure fascicles that are much longer than the field of view of conventional B-mode imaging.12Minimally invasive optical microendoscopy visualizes individual sarcomeres and their dynamical length variations in vivo.22,23The technology can be used to sample sarcomere lengths from multiple fibers and,hence,provide an indication of mean sarcomere lengths in various regions of muscle.19,22,23Combining freehand 3DUS with optical microendoscopy may provide evidence of whether eccentric exercise induces sarcomerogenesis in live human muscle.
The purpose of the present study was to measure in vivo changes in both full fascicle lengths and sarcomere lengths of the biceps femoris long head (BFlh) in response to eccentric training in order to determine whether changes in fascicle length are associated with changes in sarcomere number measured.We provide the first concurrent in vivo measurement of both fascicle length and median sarcomere length in multiple human BFlh muscle regions before and after an eccentric training intervention. We sought to investigate adaptations in the hamstring muscle because of the large increases in fascicle length that are reported to occur after relatively short intervention periods.5-7 Also, the hamstrings are important muscle groups to study,considering that BFlh strains are the most prevalent noncontact injury in sports involving running.24We hypothesized that in BFlh, fascicle length would increase in response to NHE and that such adaptations would be accompanied by an addition of sarcomeres in series within fascicles.
2. Methods
2.1. Participants
Ten recreationally active volunteers(3 females:age=25§1 years,mass=55§3 kg,height=164§4 cm;7 males:age=29§ 4 years, mass=77 § 11 kg, height=181 § 9 cm; mean §SD) with no prior experience with NHE training and no active training for an athletic competition participated in the study.Participants were excluded if they had any lower-limb,trunk,or wrist injuries or any surgeries within the past 18 months, or if they had performed NHE within the past 6 months.Participants provided written informed consent to participate. The protocol was approved by the Stanford University Institutional Review Board,ratified by the University of Queensland ethics committee,and conducted according to the Declaration of Helsinki.
2.2. Study design
The NHE training lasted 3 weeks.Previous studies have shown that short periods of eccentric training are sufficient to elicit lengthening of passively measured BFlh fascicles in untrained individuals.5-7 On Day 1, baseline measurements of BFlh fascicle and sarcomere lengths were conducted. Then participants were familiarized with the NHE device and underwent a baseline assessment of eccentric knee flexor strength. All measurements were performed on the right leg,which was the dominant leg for all participants(i.e.,the leg that participants used to kick a ball).On Day 2,fascicle length was measured again prior to the first NHE training session.Participants underwent a total of 9 training sessions in the 3 weeks that followed(Table 1).During the training period,participants were allowed to maintain their normal levels of physical activity(» 3-5 h/week),but were asked to avoid any hamstringspecific strength training. Post-training BFlh fascicle and sarcomere lengths were measured 1 day after the last training session.
2.3. NHE training
Participants performed the NHE training using a custom-made device, similar to previous studies.5,25Participants kneeled on a padded board with their ankles secured by braces located superior to the lateral malleolus.The ankle braces were attached to uniaxial load cells(HT Sensor Technology,Shaanxi,China)secured to the board via a rod and swivel.From this position,participants leaned forward slowly with control(»30˚/s)as they followed along with a metronome.To minimize concentric loading,once participants’hands touched the floor, they would push themselves back to the starting position with their arms.Strong verbal encouragement was provided to the participants during all testing and training sessions to ensure maximal effort in each repetition.
2.4. Eccentric knee flexor strength measurement
The hamstrings’ eccentric strength was measured on the same custom device used for the training. Before testing, participants completed a warm-up set of 4 repetitions at 75%of their perceivedmaximum effort.After a 3-min rest period,participants then completed 1 set of 4 maximal NHE repetitions.The peak knee flexion moment was computed from the load cell measurement at the ankle.The average of the 3 largest strength measurements of the 4 trials was analyzed. This measure produces consistent results between consecutive days(Pearson correlation coefficient r=0.97,confidence interval: 0.65-0.99; r2=0.95; mean absolute error(MAE)=34§25 N).
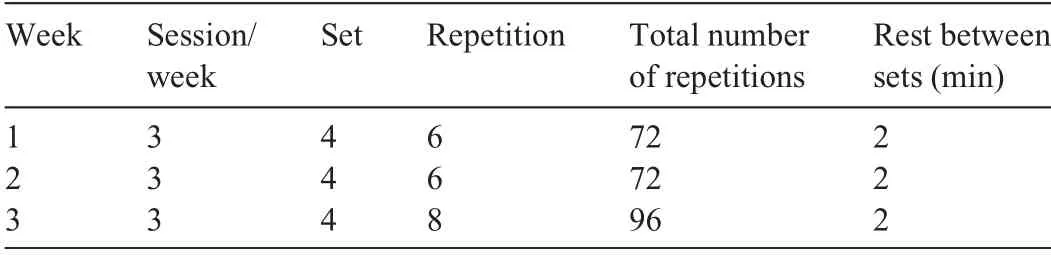
Table 1 Nordic hamstring exercise training intervention variables.
2.5. Fascicle length
Fascicle lengths were measured using freehand 3DUS, as described elsewhere.21The 3DUS scan involved B-mode ultrasound imaging (ArtUS EXT-1H beamformer and L15-7H40-A5 transducer (40 mm); Telemed, Vilnius, Lithuania) with synchronous optical tracking (Optitrack; NaturalPoint, Corvallis, OR,USA) of the position and orientation of the transducer. The scan was collected and analyzed using open-source software(Stradwin software,Version 5.4;Engineering department of Cambridge University,Cambridge,UK).Before testing,the system was calibrated temporally and spatially as previously described.21
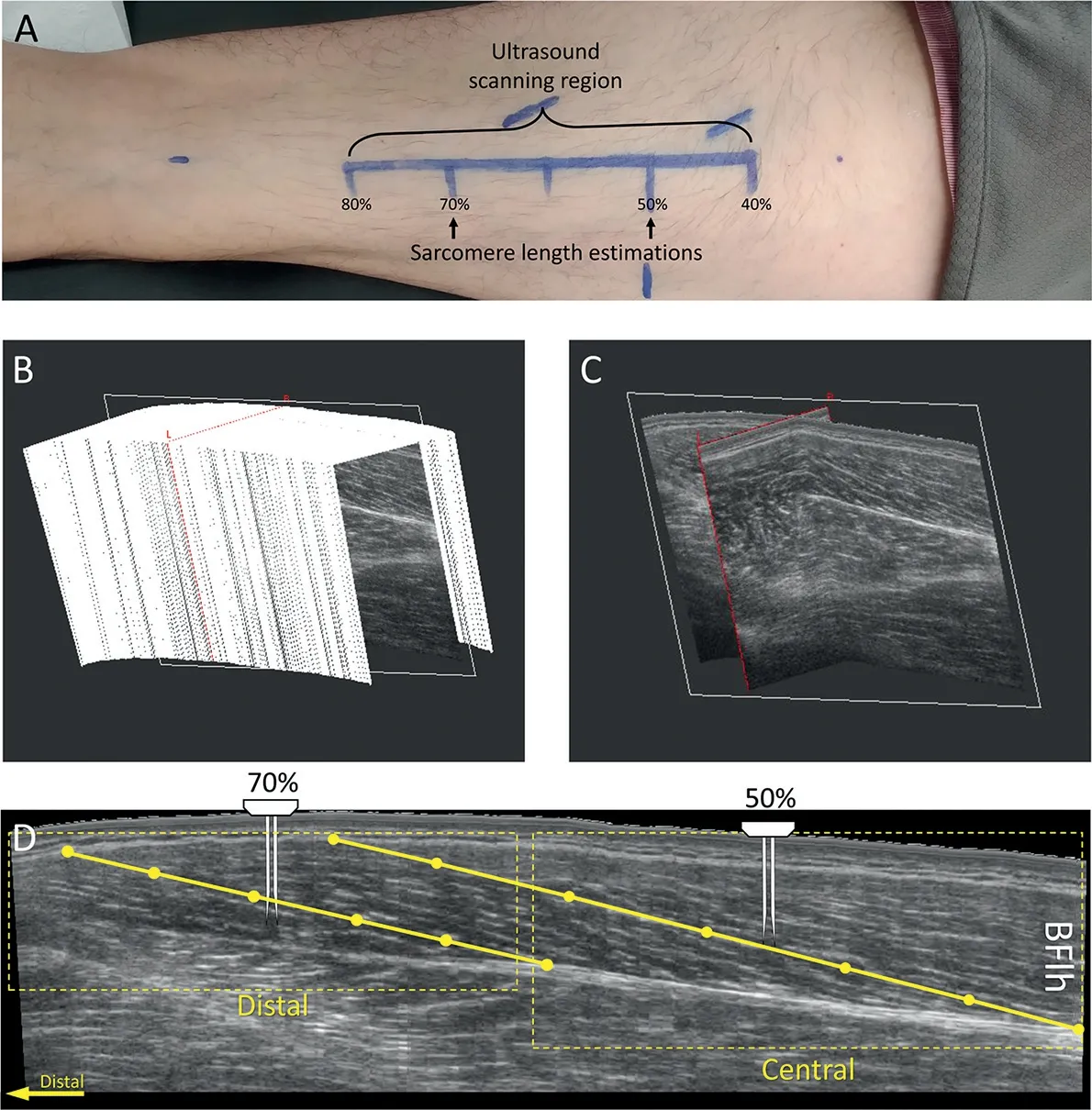
Fig. 1. Experimental set-up for biceps femoris long head (BFlh) fascicle and sarcomere measurement.(A)The ultrasound scanning region defined between 40% and 80% of the distance measured from the ischial tuberosity to the top of the patella. Sarcomere length estimations were made at 50% and 70% of the same distance; (B) A stack of 2D B-mode ultrasound images from the BFlh from 3D ultrasonography; (C) A 2D plane extracted from the stack of images;(D)An image of the BFlh extracted from the 2D plane with the distal and central regions indicated by the dotted box.A muscle fascicle is shown by the yellow solid line,which we defined with 6 points.The needle probe for sarcomere imaging is shown at the locations of insertion.
During the 3DUS scans, participants lay prone, with their hips and knees fully extended and their feet in a neutral position off the end of the bed. The BFlh scanning regions(Fig. 1A) were quantitatively determined. The position halfway between the ischial tuberosity and the lateral side of the top of the patella was marked, followed by distances 10%proximal and 30% distal to the midway point. Then a line for the path of the BFlh between the proximal and distal end of this scanning region was drawn. At a near constant speed and pressure(subjectively judged by the operator(PAP),the ultrasound transducer was moved over the scanning region in a transverse orientation that allowed identification of the collagenous tissue running along the length of the muscle.Six freehand 3DUS scans were performed in each session.The 2 scans with the best image quality (e.g., clear and visible fascicles and aponeuroses)were selected for 2D image extraction.
From the stack of 2D B-mode ultrasound images collected(slice thickness: » 0.36 mm; Fig. 1B), a 2D plane covering the entire length of the BFlh fascicles(Fig.1C)was collected and positioned on the thickest part of the proximal cross-sectional area of the muscle.From this image,the central and distal regions for fascicle length estimation were determined(Fig.1D).Fascicle orientation may change between regions of interest,12,26so the location of the 2D plane was adjusted separately for each region by rotating the image along the 3 axes of the muscle volume recorded, optimizing for fascicle continuity. In each image and region, 3 entire fascicles were visually digitalized using the 3D coordinates of 6 points per fascicle.Then the coordinates were imported into Matlab(Version 2018b;The MathWorks,Natick,MA,US),and fascicle length was calculated as the length of a parametric spline approximation fitted to the 3D coordinates. This method allowed the consideration of fascicle curvature in the length estimations.Finally,6 fascicle length estimations were averaged in each region.
One researcher (PAP) completed all fascicle-length measurements while blinded to participant and time.Between-day fascicle length variability due to measurement error was found to be low(Pearson correlation coefficient r=0.74,95%confidence interval:0.22-0.94;r2=0.55;MAE=0.49§0.25 mm).
2.6. Sarcomere length and number
Sarcomere lengths were measured using second harmonic generation microendoscopy(Zebrascope,Zebra Medical Technology, Enspectra Health, Mountain View, CA, USA).22,23The needle probe consisted of a pair of 2 cm needles with side-mounted lenses and 1 mm separation. One needle emits the laser light, while the other receives the second harmonic generation signals from the muscle fibers. The signal appears as repeating dark(myosin)and light bands.To reduce participant discomfort, the needle probe was inserted using a rapid,spring-loaded device. Because in vivo sarcomere lengths are not uniform across intact muscles,19,272 different insertion sites were defined:the central insertion site at 50%of the distance between the ischial tuberosity and the lateral side of the top of the patella and the distal insertion site at 70% of the same distance from the ischial tuberosity. Thus, the needle insertion sites were consistent with the central region of the fascicle length estimation sites (Fig. 1A and 1D). To ensure the fibers remained uncompromised, the probe was inserted such that the needles were approximately perpendicular to the fascicle plane(as determined by ultrasound).
Sarcomere lengths were analyzed as described by Lichtwark et al.19A sequence of images (image depth: 5-15 mm;sampling rate=1 Hz) was collected in real time while slowly moving the microscope and needle through the muscle in order to sample from different fibers and different positions along the length of those fibers. During postprocessing, a Gaussian filter was used to subtract white noise,and a Fourier transform was used to calculate the strongest frequency spectrum between 1.5 mm and 5 mm. A region on the sharpest fiber image was selected for analysis.Across that region(»100 mm in length), the Fourier transform was repeated to estimate the average sarcomere length. One measurement per individual fiber was taken, totaling between 6 and 22 separate muscle fiber estimations (mean=9.2 estimations) for each region per imaging session, to encompass the variability in sarcomere length measurements across different locations.19,27The number of sarcomere-length estimations varied among subjects,so the median sarcomere length was used as a measure of central tendency for each participant and each session.
The number of sarcomeres in series for each fiber was estimated by dividing the fascicle length(used as a proxy for fiber length) by the median sarcomere length. One researcher(MAB) completed all sarcomere length calculations while blinded to participants’names and times.
2.7. Statistical analysis
A paired t test was used to evaluate changes in eccentric knee flexor strength,fascicle length,sarcomere length,and the number of sarcomeres in series from baseline until the end of the training.All statistical tests were conducted in Prism (Version 8.4;Graph-Pad Software, San Diego, CA, USA) with an a level set at p <0.05.All values are presented as mean§ SD.In the results,each participant is individualized with the same color across figures.Effect sizes are presented as partial eta squared(hp2).Effects at the 0.2, 0.5, and 0.8 were considered as small, medium, and large,respectively,as described by Cohen.28
3. Results
All participants completed all of the allocated training sessions.Eccentric knee flexion strength was greater at the end of the 3-week NHE training than at baseline(Baseline:3.27§0.85 N¢m/kg;End: 3.76 § 0.67 N¢m/kg; p < 0.001; hp2=0.75) (Fig. 2). Fascicle length increased in the distal region (Baseline: 7.0 § 0.9 cm,End: 8.5 § 0.8 cm; p < 0.001; hp2=0.81) but not in the central region of the BFlh muscle (Baseline: 9.9 § 0.7 cm, End: 10.1 §0.9 cm; p=0.21; hp2=0.07) (Fig. 3A). Similarly, median sarcomere length increased in the distal region (Baseline: 2.9 § 0.28 mm;End: 3.4 § 0.23 mm; p < 0.001; hp2=0.90) but not in the central region (Baseline: 3.1 § 0.17 mm; End: 3.2 § 0.27 mm; p=0.28;hp2=0.03) (Fig. 3B). The estimated number of sarcomeres in series did not change in the distal region(Baseline:24,611§ 4461 sarcomeres;End:25,382§ 2799 sarcomeres;p=0.22;hp2=0.07)nor in the central region(Baseline:32,212§2778 sarcomeres;End:32,092§ 4271 sarcomeres; p=0.46; hp2< 0.01) (Fig. 3C). In Fig. 4, we present images displaying architectural adaptations for a representative subject.In Fig.5,we present all participants’sarcomere length measurements for all individual measures in each session and for each region of muscle.This provides an indication of how the distribution of sarcomere length measures changed between sessions.
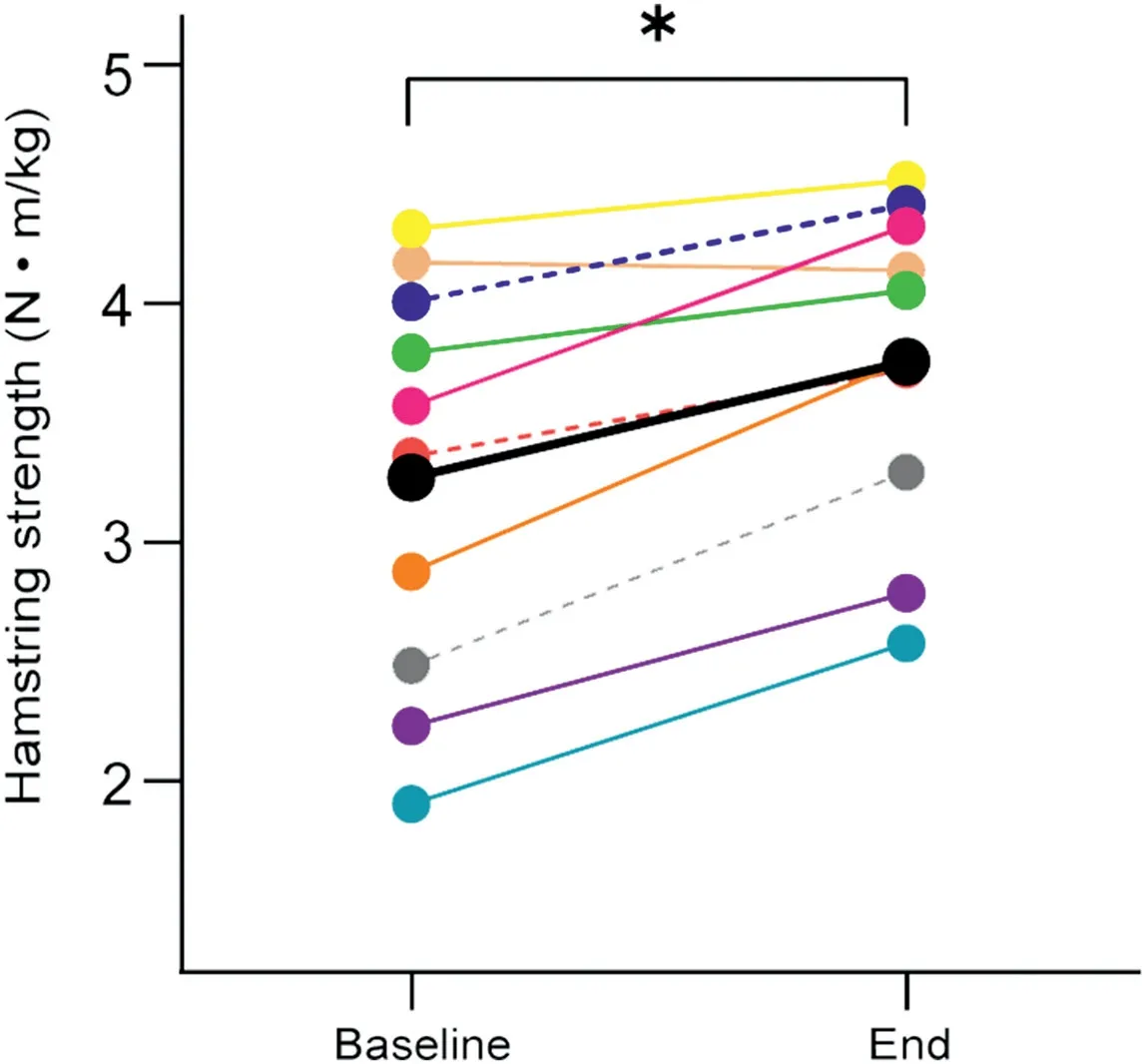
Fig.2. Eccentric knee flexion strength changes at group level.Values for each participant(different colors)are presented at baseline and at the end of the 3-week Nordic hamstring exercise training. Dotted lines are used to denote female participants. The average across all participants is presented in black.* p < 0.05, significant difference when comparing baseline and end time points.
4. Discussion
In this study, we measured in vivo fascicle and sarcomere lengths in the human BFlh before and after an eccentric training intervention.Three weeks of NHE led to increased resting fascicle and median sarcomere lengths by 21% and 17%, respectively, in the distal portion of the BFlh,which suggests that a large portion of the changes in fascicle length can be accounted for by increases in sarcomere length. However, we found no changes in the estimated number of sarcomeres in series.These findings provide new information about the early adaptations of the BFlh muscle in response to eccentric training.
4.1. Fascicle and sarcomere adaptations to eccentric training
BFlh fascicle length increased after a short period of eccentric training (9 training sessions distributed over 3 weeks),which is similar to previous observations in the midportion of the muscle.5-7 However, we found heterogeneous fascicle length adaptations across the BFlh;fascicle length increased in the distal but not in the central region of the muscle.The average change in fascicle length that we measured (»1.5 cm) is smaller than the change observed previously (»2 cm in the mid-BFlh).5-7 These differences may be related to the differences between the 3D full-field measures used in this study and previous measures made using limited field of view ultrasound imaging5-7 as well as to subtle differences in training load,5scanning site,and/or instruction to the participants.
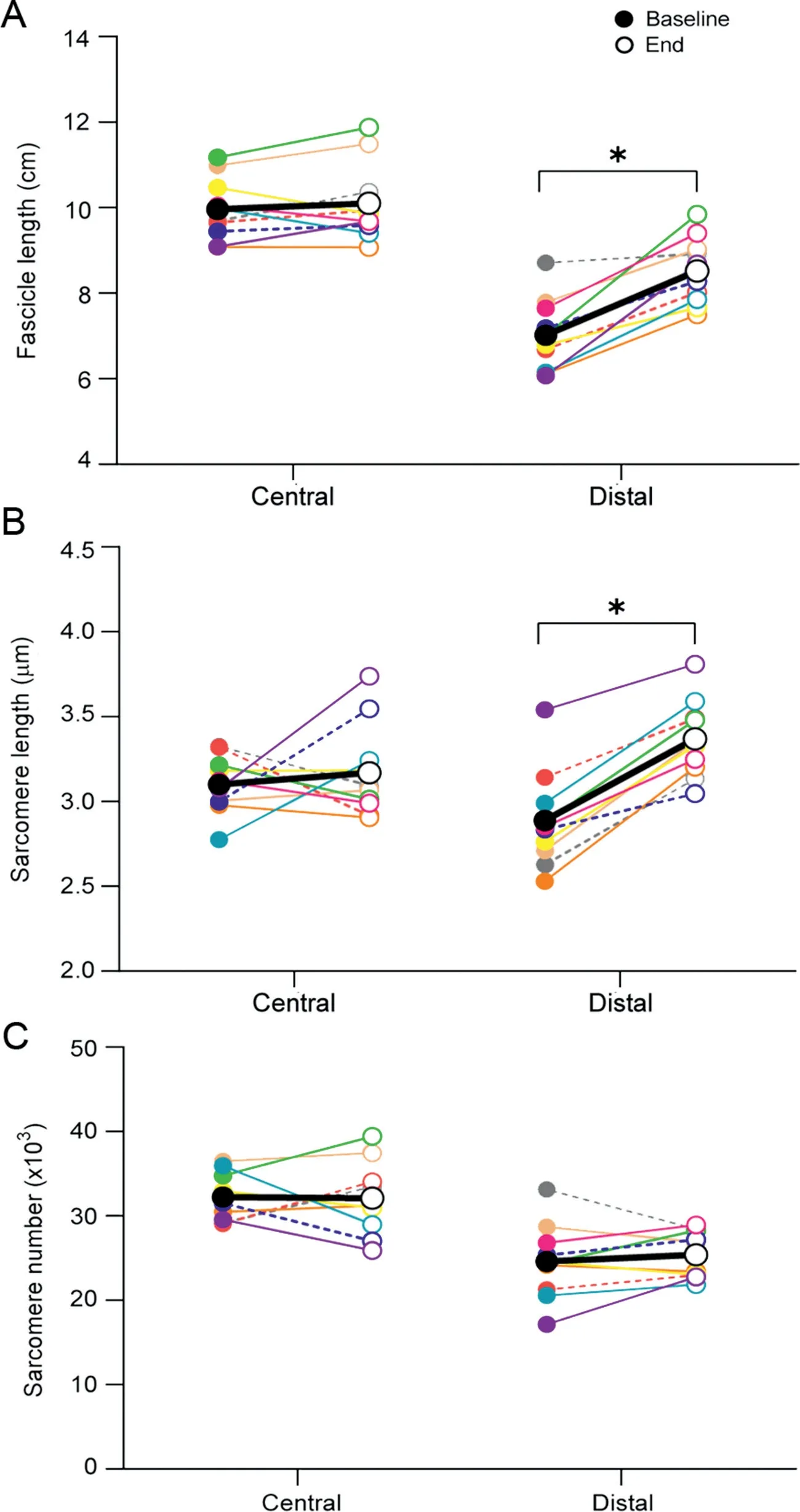
Fig. 3. Biceps femoris long head architectural adaptations at the group level.(A) Fascicle lengths, (B) sarcomere lengths, and (C) sarcomere numbers are presented for the central and distal portions of the muscle at baseline (filled circles) and after 9 sessions of Nordic hamstring exercise training (end, open circles).Each color represents an individual participant.Dotted lines are used to denote female participants. The average across all participants is presented in black. *p < 0.05, significant difference when comparing baseline and end time points.
We found that sarcomere length increased only in the distal portion of the muscle. In vivo measurement of both fascicle and median sarcomere lengths from the same region of muscle allowed us to estimate the number of sarcomeres in series per fascicle. These estimates revealed no addition of sarcomeres in series in either region of the muscle;length changes of fascicles were proportional to changes in median sarcomere length.Previous studies have suggested that sarcomerogenesis underlies the in vivo changes in hamstrings muscle fascicle lengths after eccentric training.5-7 However, these assertions were supported only indirectly, based on changes in fascicle lengths estimated with 2D ultrasonography or on changes in the angle/torque relationship.29Thus,to our knowledge,this is the first study to show that eccentric training(such as 3 weeks of NHE) does not induce the addition of sarcomeres in series in humans, despite site-specific increases in fascicle length.Although this result may be unexpected, animal studies show that some muscles experience sarcomerogenesis in response to eccentric exercise,30,31but others do not.20Further, serial sarcomere number adaptations appear to differ between muscles of the same limb31and within regions of the same muscle.32,33Overall,reported sarcomere adaptation is not consistent in animal models after eccentric exercise, calling into question the predominant theory that sarcomerogenesis underpins the changes in BFlh fascicle lengths after eccentric training.

Fig.4. Images of the biceps femoris long head architectural adaptations for a representative subject. Sarcomere imaging (A) at baseline and (B) at the end of the training are presented for the distal portion of the muscle. The boxes represent the region where sarcomere length was estimated for each muscle fiber (baseline, blue box=2.9 mm; end, orange box=3.6 mm). 2D sagittalplane images(C)at baseline and(D)at the end created from 3D ultrasound are presented for the biceps femoris long head muscle.The overlayed lines represent a fascicle length estimation,with dots separating 5 segments of equal length.The last segment of the fascicle estimation line is drawn with a dotted line to highlight the change in fascicle length(Baseline:93 mm and End:101 mm).
4.2. Interpretation of findings:Potential mechanisms of adaptation
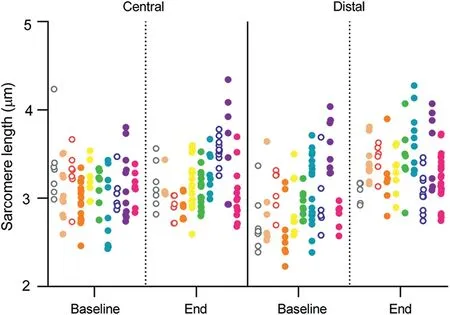
Fig. 5. Sarcomere length measurements at baseline and at the end of training for the central and distal regions. Individual measurements are represented by 1 dot,and individual participants(n=10)are represented by different colors in a column of dots.Measurements from female participants are presented as open dots.
We did not see an addition of serial sarcomeres in the muscle,but the increase in fascicle length seen here and in previous studies5-7 may represent adaptations to noncontractile tissue. Although the current study did not investigate noncontractile tissue adaptation, the change in length measured (in resting muscle)may represent changes in passive tension governed by adaptations in connective tissue, titin function, or both. Extracellular matrix components related to muscle fiber compliance(Tenascin-C)and force transmission to sarcomeres(Collagen XII) adapt quickly in response to eccentric exercise34and may account for our observations. Adaptations in titin stiffness or titin-actin interaction35,36seen after eccentric exercise may also play a role in increasing sarcomere and fascicle length.Furthermore,changes in tendon stiffness37cannot be disregarded as a potential source of adaptation.Overall,the mechanisms underpinning BFlh architectural adaptations are still not well understood and warrant further investigation.
The observed heterogeneous adaptation to fascicle length at different muscle regions may be important for understanding how a training stimulus relates to muscle adaptation.Previous studies suggest that when muscles are placed under significant strain,muscle adaptations tend to predominate distally.38Thus, a heterogeneous distribution of fiber strain39and muscle activity40toward the distal portion of the BFlh may have influenced this region’s adaptations.Because the hip joint angle remains constant during the NHE,changes in fascicle excursion and tissue strain could be larger in the distal(shorter)portion of the BFlh muscle.41Such differences in strain could provide stimulus (e.g., mechanotransduction17) for differential adaptations in fascicle lengths across different muscle regions.Further studies are required to better understand the stress and strain distribution across the muscle during eccentric exercises like the NHE and to explain how this relates to muscle adaptation.
4.3. Practical implications
Clinical interventions that employ NHE can expect increasing fascicle lengths in as short a time as 3 weeks;however,this may occur only in the distal region of BFlh. This finding presents a unique insight into the immediate (within 3 weeks) adaptations that occur in response to NHE,which may be necessary for tailoring injury-prevention training regimes and for identifying muscles at higher risk for injury.Previous studies suggest that,for the BFlh muscle,longer fascicles may help protect athletes from a hamstring injury.7,9,10However,this study questions the proposed mechanism for such protection. Although “long and strong”7,10is considered an effective muscle adaptation to eccentric hamstring exercise,the goal of inducing these changes needs further consideration. The addition of sarcomeres in series may play a role in longer-term training,but we did not detect these changes early in the adaptive process,when most of the change in fascicle length occurs.6There is likely to be little muscle-injury protection due to sarcomerogenesis in early training.Analysis of NHE interventions of longer durations using sarcomere and fascicle imaging could provide essential evidence to further evaluate this commonly used training method.
4.4. Strengths and limitations
Combining measurements in macro(fascicle)and micro(sarcomere) adaptations allowed us to overcome some of the methodological limitations of past studies. In particular, it allowed us to assess the full length of the BFlh fascicles(i.e.,to consider fascicle curvature) in various muscle regions and to visualize human in vivo sarcomere-length adaptations.Nevertheless,we recognize several limitations of our study. Although our intervention successfully increased BFlh fascicle lengths, this was a relatively shortterm training study; hence, further studies are needed to explore whether sarcomerogenesis arises in response to more prolonged training periods (of various intensities). For instance, region-specific adaptations in mechanotransductor pathways related to muscle growth seem to peak at 4 weeks and 8 weeks after eccentric resistance training;17thus,sarcomerogenesis may still occur at later stages in NHE training.Additionally,we did not include a control group. Another limitation is that our postintervention measurements were made 1 day after the end of the training,at which point a forced elongation in the sarcomeres may have remained;but further study is needed to test this speculation. Our study was not designed to detect differences in adaptations between genders,and it included a smaller proportion of female participants, so caution must be exercised when extrapolating the results of this study to other populations. For instance, females have shorter optimal lengths of hamstring muscle compared to males.42Thus, the mechanical stress imposed by the training intervention may elicit differential adaptations between genders.43Muscle activation within the hamstrings is greater in the semitendinosus during the NHE,40so adaptations may have predominated in this muscle rather than in the BFlh.However,as mentioned before,the NHE has effectively increased fascicle lengths in the BFlh.5,7Hence, we believe our exercise selection was appropriate for our research question.As an additional limitation,we estimated only passive sarcomere and fascicle lengths,27and the estimates of sarcomere length represent only those from the sampled area. However, because we sampled at different depths of pennate BFlh muscle(due to the microendoscopy sampling technique), it is likely that we sampled sarcome lengths from different regions of each fiber imaged,giving a better estimate of the central tendency of sarcomere length.
5. Conclusion
NHE training(9 sessions in 3 weeks)increased resting fascicle and sarcomere lengths in the distal, but not the central, region of the BFlh muscle. These results suggest a heterogeneous distribution of strain and adaptation within the muscle.The addition of sarcomeres in series did not accompany the adaptations observed in either region. The mechanisms behind these findings remain unclear,but adaptations in connective tissue,titin,and the extracellular matrix may play an important role.
Acknowledgments
PAP’s travel to Stanford University,Stanford,CA,USA for data collection was partially funded by a Company of Biologists Travelling Fellowship. This project was partially subsidized by a grant funded by the Australian Research Council(Linkage program) in partnership with the Australian Sports Commission(LP140100260).
Authors’contributions
PAP and MAB collected and analyzed the data and drafted the paper.All authors contributed to the study conception and design.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
 Journal of Sport and Health Science2022年1期
Journal of Sport and Health Science2022年1期
- Journal of Sport and Health Science的其它文章
- Does eccentric exercise stimulate sarcomerogenesis?
- The Beijing 2022 Winter Olympics:An opportunity to promote physical activity and winter sports in Chinese youth
- Beijing 2022 Winter Olympic Games:Commitments to science and public health
- Corticospinal activity during a single-leg stance in people with chronic ankle instability
- Knee biomechanics of patients with total knee replacement during downhill walking on different slopes
- Estimates of voluntary activation in individuals with anterior cruciate ligament reconstruction:Effects of type of stimulator,number of stimuli,and quantification technique
