Yangxin Dawayimixike honey paste inhibits atherosclerosis in ApoE-/- mice by attenuating blood lipids and exerting anti-inflammatory activity
Yu Ding,Jin-Rong He,Jie Liu,Shan-Shan Chen,Jin Huang,Hai-Long Yin,Tong Yu,Xin-Hai Jiang,Jin-Que Luo,Yan-Ni Xu,Qiang Yin*
1Key Laboratory for Molecular Diagnosis of Hubei Province,Wuhan 430014,China.2Central Laboratory,The Central Hospital of Wuhan,Tongji Medical College,Huazhong University of Science and Technology, Wuhan 430014, China.3Department of Pathology, The Central Hospital of Wuhan, Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430014,China.4Xinjiang Uighur Pharmaceutical Limited Company,Urumq 830001,China.5Department of Traditional Chinese Medicine,Humanwell Healthcare(Group)Co.,Ltd.,Wuhan 430075,China.6NHC Key Laboratory of Biotechnology of Antibiotics,National Center for New Microbial Drug Screening, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College(CAMS&PUMC),Beijing 100050,China.
#Yu Ding and Jin-Rong He contributed equally to this work.
Abstract Background: Yangxin Dawayimixike honey paste (YDHP) is a representative traditional Chinese medicine, and its main function is curing angina pectoris, palpitation and neurasthenia.However, it is unclear whether YDHP can suppress the development of atherosclerosis.The aim of this study was to validate the potential application of YDHP in atherosclerosis therapy and search for its potential mechanisms.Methods: Seven-week-old ApoE-/- mice were randomly divided into a normal group fed a normal diet, an atherosclerosis model group fed a high-fat diet, YDHP groups fed a high-fat diet mixed with different doses of YDHP and positive control groups fed a high-fat diet mixed with atorvastatin or rosuvastatin.After feeding for 10 weeks, body weight, blood lipids, liver and kidney function indexes, serum inflammatory cytokines and atherosclerotic plaque areas were measured.Serum metabolic profiles were detected by an automatic biochemical analyser.Serum inflammatory cytokines were quantified by enzyme-linked immunosorbent assay kits.Atherosclerotic plaque areas were analysed using Oil Red O staining.Results: The YDHP (200, 400 and 800 mg/kg/d) treated groups showed reduced serum levels of low density lipoprotein-cholesterol (P <0.05, when 200 or 400 mg/kg/d of YDHP group compared with the atherosclerosis model group; P <0.01, when 800 mg/kg/d of YDHP group compared with the atherosclerosis model group), total cholesterol (P<0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group; P<0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group) and triglyceride (P <0.01), however, elevated serum levels of high-density lipoprotein cholesterol (P <0.01) compared to the atherosclerosis model group.YDHP inhibited the area of atherosclerotic lesions.In addition, YDHP suppressed the levels of serum inflammatory cytokines such as interleukin 6 (P <0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group; P < 0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group) and tumor necrosis factor α (P <0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group; P <0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group).Conclusion: Our study demonstrated that YDHP showed considerable activity in alleviating the formation of atherosclerotic plaques in ApoE-/- mice by reducing blood lipids and exerting anti-inflammatory activity.
Keywords: Yangxin Dawayimixike honey paste; atherosclerosis; blood lipid; anti-inflammation;safety;side effects
Background
Atherosclerosis is considered an advanced chronic inflammatory response in the arterial wall that leads to oxidative stress, lipid deposition, recruitment of monocytes, macrophage foam cell formation, endothelial dysfunction, vascular smooth muscle cell differentiation and inflammation [1-3].It is regarded as the most common underlying cause of cardiovascular disease, and cardiovascular disease accounts for 31% of deaths worldwide [4].During the progression of atherosclerosis, the secretion of various proinflammatory factors is increased.The induction of proinflammatory cytokines such as interleukin (IL)-1β, IL- 6 and tumor necrosis factor α (TNF-α) participates in the accumulation of atherosclerotic plaques [5].Although the exact mechanisms involved in atherosclerosis have not been clarified, it is generally considered that lipid disorders and inflammatory responses play vital roles [6, 7].Currently, modern drugs have been applied for the conventional treatment of atherosclerosis such as statins.Nevertheless, more than 20% patients are unable to adapt statins treatment for the reason of the side effects and the residual risk of detrimental cardiovascular events [8, 9].Therefore, available and safe agents targeting the atherogenic processes are urgently needed.
Abundant studies have demonstrated that traditional Chinese medicine can prevent the development of atherosclerosis through antioxidation and anti-inflammation [10, 11].Yangxin Dawayimixike honey paste (YDHP) is a traditional Chinese medicine classical preparation for human to treat heart, brain and liver diseases,originated from the classical Uyghur medical literature “Kharibadin Kader”, the book is written by Mohammed · Ekbel Elzani, it has a history of 1,500 years.In Uyghur medicine, YDHP is called Suxiaojiuxin pill.It has been reported that YDHP has remarkable therapeutic effect on cardiovascular diseases, such as chest pain and tightness caused by overwork, these symptoms are common clinical manifestations of coronary heart disease, angina [12].In 1998, YDHP was included in thePharmaceutical Standard of Ministry of Health -Uyghur Medicine Volume(standard No.Ws3-BW-0171-98) [13].At present, the preparation (the approved No.of FDA in China:Z65020175) is a secondary protection variety of traditional Chinese medicine, and is produced by Xinjiang Uygur Pharmaceutical Co.,LTD.YDHP consists of twenty-six costly medicinal materials,includingMoschus,Santali Albi Lignum,Aquilariae Lignum Resinatum,Croci Stigma, andAmbrum, the component and dosage of YDHP were showed in Supplementary Table S1.It has been widely evaluated for its pharmacological activities, including hypnotic, antinociceptive,and antidepressant activities, and its effects promoting blood circulation.The major function of YDHP is curing angina pectoris,palpitation and neurasthenia [12].
Studies have reported that saffron extracts such as safranal, crocin and crocetin possess cardioprotective and neuroprotective effects[14-16].They could reduce lipid peroxidation in renal [17],hippocampal [18] and muscle skeletal [19] tissues.Accumulating studies have confirmed that saffron extract has antinociceptive and anti-inflammatory effects because of its flavonoid, anthocyanin,alkaloid and saponin contents [20].It has been reported that sandalwood also has anti-inflammatory, antioxidative and antihypertensive biological activities [21, 22].The component content of YDHP have been reported that prssessing antiatherosclerotic potential, such as saffron, musk, agilawood, cinnamon,usnea, coriander, amber.The mechanisms of anti-atherosclerosis are included in regulating lipid metabolism, anti-inflammatory and enhancing plaque stability [23-28].These studies suggest that YDHP may be used as a new agent in atherosclerosis therapy, serving as a new adaptative disease treatment.
Although there is not enough evidence to support the therapeutic effects of YDHP on atherosclerosis, we performed an experiment to demonstrate the effect of YDHP on the progression of atherosclerosis induced by a high-fat diet in ApoE-/-mice and elucidate the underlying mechanisms.Meanwhile, we demonstrated that there has no significant difference on body weight, liver and kidney function between the YDHP-treated group and the atherosclerosis model group.
Methods
Ethics approval and consent to participate
Animal studies were following internationally recognized guidelines with the approval of the local council of ethics committee(Institutional Animal Care and Use Committee of the Institute of Medicinal Biotechnology Institute), according to institutional and government guidelines (certificate No.IMB-20180320-D1).
Materials
YDHP was purchased from Xinjiang Uighur Pharmaceutical Limited Company (Urumqi, China); atorvastatin was purchased from Pfizer Inc.(New York, NY, USA); rosuvastatin was obtained from AstraZeneca Inc.(London, UK); enzyme-linked immunosorbent assay(ELISA) kits for IL-6 and TNF-α were purchased from Sbjbio Biotechnology Co., Ltd.(Nanjing, China); and Oil Red O staining solution was obtained from Sigma-Aldrich.(St.Louis, MO, USA).
Mice and diet
Seven-week-old ApoE-/-mice (weight, 16-18 g) purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China)were housed under specific pathogen-free conditions.Mice were randomly divided into seven groups: normal (0.5% carboxymethyl cellulose sodium, n = 10), atherosclerosis model (0.5%carboxymethyl cellulose sodium, n = 10), positive (atorvastatin, 5 mg/kg/d [29], n = 6), positive (rosuvastatin, 10 mg/kg/d [30], n =6), low dose (YDHP 200 mg/kg/d, n = 10), middle dose (YDHP 400 mg/kg/d, n = 10) and high dose (YDHP 800 mg/kg/d, n = 10)groups.ApoE-/-mice were fed a high-fat diet consisting of 15% cocoa butter and 0.5% cholesterol.After 8 weeks of high-fat diet feeding,all groups were treated by intragastric administration of normal saline or different interventions for 10 weeks.Concentration of animal dose was calculated from the concentration of human dose.Calculation formula: concentration of animal dose (mg/kg/d) =concentration of human dose (mg/d)/60 × 6.25.The dose given on human body is 6,000 mg/d according to the drug instruction of YDHP, thus, the dose for animal is 625 mg/kg/d converted from the calculation formula.Therefore, 200, 400, 800 mg/kg/d was selected.
Sacrifice
Mice were under fasting for more than 12 h before death.Intraperitoneal injection of sodium pentobarbital (75 mg/kg body weight) was used to anethesia mice, all mice were sacrificed by cervical dislocation while anesthetized.
Analysis of serum metabolic profile
Blood was collected from the orbital sinuses of mice after 10 weeks of treatment.Mice were fasted for 8 h before blood collection.Serum was obtained by centrifuging blood samples at 3,000 rpm for 10 min at 4 °C.Serum levels of low density lipoprotein-cholesterol (LDL-C),high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC),triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea and creatinine were evaluated by an automatic biochemical analyser (Hitachi Ltd.,Tokyo, Japan).
ELISA detection for cytokines
Blood samples were collected from the orbital sinuses of mice and then centrifuged at 3,000 rpm for 10 min at 4 °C to obtain serum samples and stored at -80 °C.Serum concentrations of inflammatory cytokines such as IL-6 and TNF-α were quantified by ELISA kits according to the manufacturer’s instructions.50 μL standards with different concentrations (0, 0.5, 1, 2, 4, and 8 mmol/L) were added to standard well.10 μL samples and 40 μL sample diluent were added to sample wells.Then, 100 μL detection antibody labeled with HRP was added to each well and incubated at 37 °C for 1 h.The plate was washed with washing buffer for five times.50 μL of substrate A and substrate B were added to each well and incubated for 15 min at 37 °C in the dark.50 μL of stop solution was added to each well and incubated for 15 min.Absorbance was detected using an EnSpire Multimode plate reader (PerkinElmer Inc., MA, USA) at a wavelength of 450 nm.The standard curve was established according to the concentrations of the standards.The levels of IL-6 and TNF-α were calculated according to the standard curve.
Oil Red O staining
To measure the atherosclerotic lesion area, whole aortas from ApoE-/-mice were dissected and opened, fixed in 10% formalin for 15 min and stained using 0.5% Oil Red O for 15 min at room temperature.Images were photographed by a stereomicroscope (Leica, Frankfurt,Germany).The amount of atherosclerotic plaque was measured as the ratio of the atherosclerotic plaque area to the whole aorta area by NIH ImageJ software.
Statistical analysis
Data were analysed by Student’s t test for comparisons between two groups or one-way analysis of variance for multiple groups.All statistical analyses were performed using SPSS 19.0 software.P<0.05 andP< 0.01 were considered to indicate a statistically significant difference.
Results
Effects of YDHP on body weight, liver and kidney function index
After 10 weeks of treatment, the body weights of the mice were recorded and analysed.As shown in Figure 1A, when fed a high-fat diet for 10 weeks, the body weights of mice in the atherosclerosis model group significantly increased compared with those of mice in the normal group (P<0.01).However, there was no significant difference between the atherosclerosis model group and the YDHPtreated (200, 400 and 800 mg/kg/d) groups (P>0.05).Compared with the atherosclerosis model group, body weight significantly decreased in the positive (rosuvastatin) group (P<0.05).As shown in Figure 1B-E, serum index levels that reflected liver and kidney function, such as ALT, AST, urea and creatinine, in the YDHP-treated groups were not significantly different from those in the atherosclerosis model group (P>0.05).These data showed that there is no side effect on body weight, liver and kidney function between the YDHP-treated group and the atherosclerosis model group.
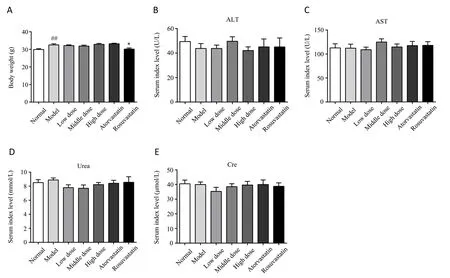
Figure 1 Effects of YDHP on body weight, liver and kidney function index in ApoE-/- mice.
Detection of LDL-C, HDL-C,TC and TG levels
Atherosclerosis generally leads to dyslipidaemia.For the in vivo assay,the potential anti-atherosclerotic effect of YDHP was evaluated by detecting serum lipid levels.Blood samples were collected from ApoE-/-mice in seven groups after the interventions for 10 weeks, and then blood lipid levels were detected.Compared to those in the normal group, LDL-C, TC, and TG levels were elevated (P<0.01),and HDL-C levels (P<0.01) were reduced in the atherosclerosis model group.Compared to those in the atherosclerosis model group,reduced LDL-C, TC, and TG levels and elevated HDL-C levels were observed in the positive group (P<0.01).It is interesting to find that treatment with YDHP at different doses (200, 400 and 800 mg/kg/d) generated an effect similar to that of the positive group(Figure 2).The YDHP treated groups showed reduced serum levels of LDL-C (P< 0.05, when 200 or 400 mg/kg/d of YDHP group compared with the atherosclerosis model group;P<0.01, when 800 mg/kg/d of YDHP group compared with the atherosclerosis model group) , TC (P<0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group;P<0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group), and TG (P<0.01), but elevated serum levels of HDL-C (P<0.01) compared to the atherosclerosis model group.These data showed that YDHP reduced lipid level of atherosclerosis model group.
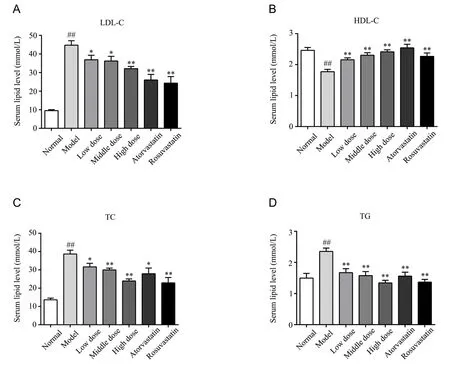
Figure 2 Effects of YDHP on blood lipid levels in ApoE-/- mice.
YDHP reduced the expression level of serum inflammatory cytokines in ApoE-/-mice
Inflammation plays a crucial role in the development of atherosclerosis.Serum inflammatory cytokines IL-6 and TNF-α were detected at the end of the study.Serum concentrations of IL-6 and TNF-α were higher in the atherosclerosis model group than in the normal group (P<0.05).Administration of YDHP at 200, 400, 800 mg/kg/d decreased the level of IL-6 (P<0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group;P<0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group) and TNF-α (P< 0.05, when 200 mg/kg/d of YDHP group compared with the atherosclerosis model group;P< 0.01, when 400 or 800 mg/kg/d of YDHP group compared with the atherosclerosis model group), atorvastatin at 5 mg/kg/d or rosuvastatin at 10 mg/kg/d also obviously reduced the concentrations of IL-6 (P<0.01,when atorvastatin or rosuvastatin group compared with the atherosclerosis model group ) and TNF-α (P<0.05, when atorvastatin group compared with the atherosclerosis model group;P<0.01, when rosuvastatin group compared with the atherosclerosis model group) (Figure 3A, B).These data showed that YDHP inhibited level of IL-6 and TNF-α in atherosclerosis model group.
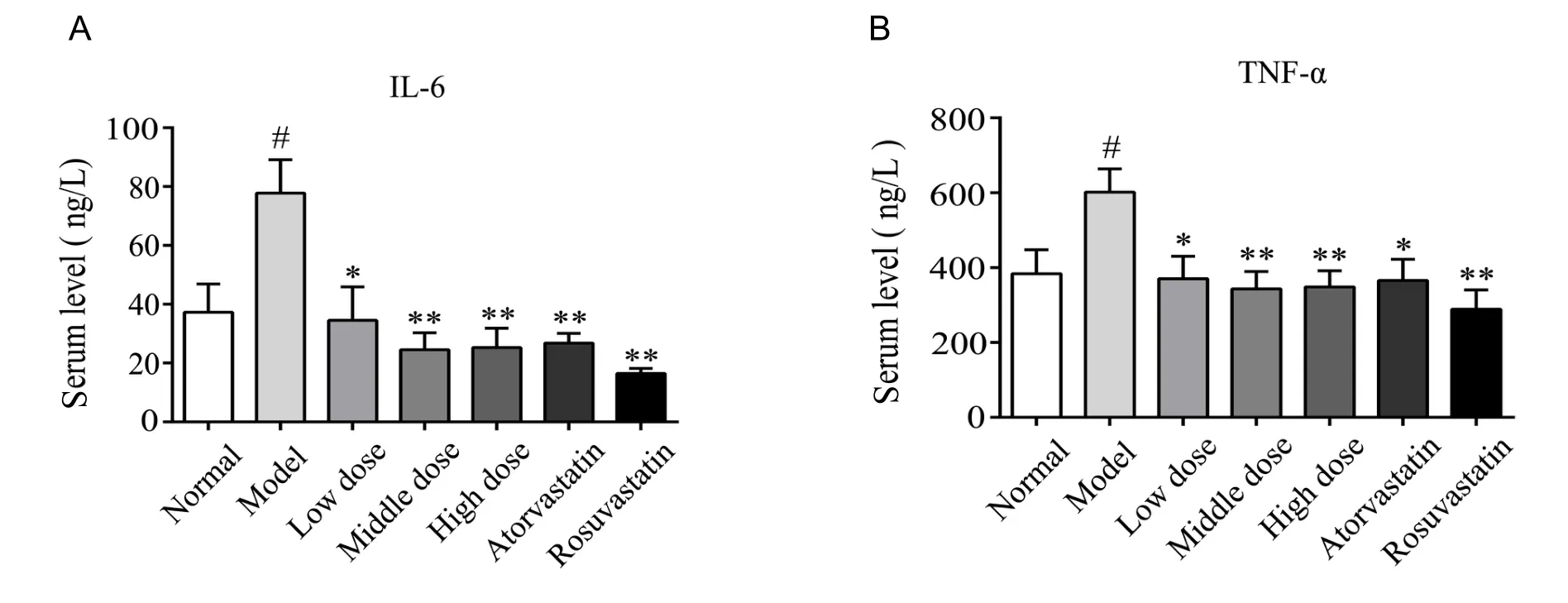
Figure 3 YDHP reduced serum concentrations of inflammatory cytokines in ApoE-/- mice.
YDHP inhibited the area of atherosclerotic lesions in ApoE-/-model mice
To confirm the severity of the atherosclerotic lesion and quantify the plaque area in each whole aorta, Oil Red O staining was performed.An atherosclerotic plaque is shown in Figure 4A.More atherosclerotic plaques were observed in whole aortas from the atherosclerosis model group than in those from the normal group (P<0.01).The 200, 400, 800 mg/kg/d YDHP groups and the atorvastatin and rosuvastatin groups had fewer atherosclerotic plaques than the atherosclerosis model group (P< 0.05) (Figure 4B).These data showed that YDHP supressed the area of atherosclerotic lesions in ApoE-/-model mice.
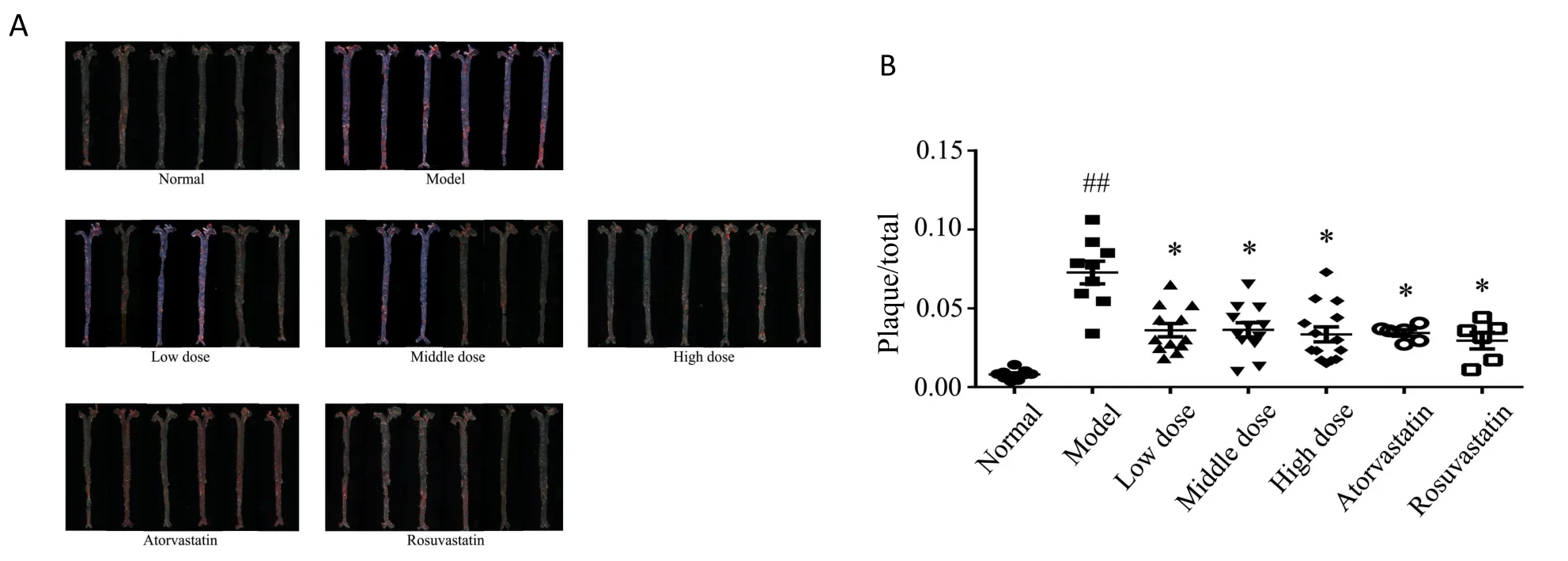
Figure 4 YDHP decreased the atherosclerotic plaque area in whole aortas from ApoE-/- mice.
Discussion
Atherosclerosis is conceived as a chronic inflammatory process that has become a serious threat to human health.It involves intimal thickening, endothelial dysfunction and atheromatous plaque formation [31].Atheromatous plaque formation contains four stages:fatty streak, atheromatous plaque, complicated atheromatous plaque and clinical complications [32].YDHP is a traditional Chinese medicine that has been used for heart palpitations, stomach distention, amblyopia and neurasthenia.However, the antiatherosclerotic effect of YDHP has never been previously studied.
ApoE-/-mice, which can spontaneously develop atherosclerosis with high total cholesterol levels, are frequently used as animal models for atherosclerosis research [33, 34].In this study, ApoE-/-mice fed a high-fat diet for 10 weeks exhibited obvious hyperlipidaemia compared with the normal group, and ApoE-/-mice given rosuvastatin or atorvastatin perfusion showed a significant reduction in blood lipid levels compared with those in the atherosclerosis model group.These results indicated that the atherosclerosis model and antiatherosclerosis positive model were successfully established.
In the present study, the anti-atherosclerotic activity of YDHP was demonstrated using ApoE-/-mice in vivo.Here, the effects of YDHP on blood lipid levels and the formation of atherosclerotic lesions were first studied.The process of atherosclerosis is complicated, and the accumulation of LDL-C and TC could lead to excessive fibrosis of the intima and fatty plaque formation [35, 36].TG is a marker for atherogenic lipoprotein and an independent predictor of endothelial function that has shown a strong association with atherosclerosis[37-40].We observed that YDHP, even at doses as low as 200 mg/kg,could obviously reduce serum levels of LDL-C, TC, and TG while increasing HDL levels compared to those of the atherosclerosis model group.Moreover, YDHP inhibited the area of atherosclerotic lesions in ApoE-/-mice.Furthermore, in the process of atherosclerosis,proinflammatory molecules such as TNF-α, IL-6, IL-18, and C-reactive protein may further accelerate oxidative stress and microinflammation [41, 42].We detected the serum levels of inflammatory cytokines such as IL-6 and TNF-α and found that YDHP could reduce the levels of serum inflammatory cytokines.Therefore,our results indicated that YDHP inhibited atherosclerosis in ApoE-/-mice, possibly by decreasing blood lipid levels and exerting antiinflammatory activity.To observe the liver and kidney toxicity of YDHP, we detected serum levels of ALT, AST, urea and creatinine.The data showed no significant difference between the YDHP-treated group and the atherosclerosis model group.In addition, the body weights of mice in the atherosclerosis model group and YDHP-treated group were not significantly different.
Conclusion
In summary, the present study demonstrated that YDHP showed considerable activity in alleviating the formation of atherosclerotic plaques in ApoE-/-mice by reducing blood lipids and exerting antiinflammatory activity.These findings suggest that YDHP might serve as a new application for preventing the progression of atherosclerosis in cardiovascular diseases in the future.
 Traditional Medicine Research2022年2期
Traditional Medicine Research2022年2期
- Traditional Medicine Research的其它文章
- A comprehensive review of research progress in Chinese medicines for primary liver cancer treatment
- Preparation and characterization of resistant starch type 3 from yam and its effect on the gut microbiota
- Study on technical efficiency of traditional Chinese medicine industry of the Belt and Road Initiative based on environmental complexity
- Virtual screening of flavonoids from Jatropha gossypiifolia L.as potential drugs for diabetic complications
- Traditional herbal medicine as adjunctive therapy for colorectal cancer: a scoping review
- Acupuncture:a new method to treat tic disorders in children
