Recurrent postmenopausal bleeding - just endometrial disease or ovarian sex cord-stromal tumor? A case report
INTRODUCTION
Postmenopausal bleeding (PMB) is a common gynecologic complaint encountered in the clinical setting. Inflammation and benign or malignant tumors of the reproductive organs can cause PMB. The keys to diagnosis and treatment are accurately determining the etiology and preventing and treating malignant diseases. Correct identification of the etiology improves the treatment plan and facilitates early patient recovery. We report the case of a postmenopausal woman who had previously undergone repeated curettage and hysteroscopy due to recurrent PMB and endometrial hyperplasia. Sex-hormone testing revealed her estrogen levels were consistently higher than normal. However, imaging performed as part of her first four operations did not reveal an ovarian mass. An ovarian cellular fibroma with endocrine function was eventually found during the fifth operation, and the relevant literature was reviewed. This case is significant because it demonstrates the necessity of considering the rare possibility of ovarian cellular fibromas as a precursor for PMB.
god said: when i made the woman she had to be special. i made her shoulders strong enough to carry the weight of the world; yet, gentle enough to give comfort
CASE PRESENTATION
Chief complaints
A 64-year-old female patient presented in May 2020 for “postmenopausal vaginal bleeding for one year”.
History of present illness
The vaginal bleeding was intermittent, less than the amount of previous menstruation. The patient had no feeling of dizziness and fatigue, but was accompanied by slight abdominal pain and occasional abdominal distension.
History of past illness
In June 2013, 4 years after menopause, the patient began to experience irregular vaginal bleeding without obvious cause. Transvaginal ultrasonography (TVUS) showed the endometrium was thickened (approximately 1.5 cm in total), and diagnostic curettage was performed. The pathological results suggested simple endometrial hyperplasia.
In May 2014, diagnostic curettage was performed again due to irregular vaginal bleeding, and the pathological results suggested endometrial hyperplasia disorder. The first two curettage procedures were performed at a different hospital.
In June 2016, irregular vaginal bleeding occurred again. Gynecological examination showed that the cervix was normally sized, the uterus was enlarged and the shape was irregular, and there were no abnormalities in the adnexa area of either side. TVUS showed the size of the uterus was about 9.0 cm × 6.1 cm × 6.0 cm, the endometrium was thickened (approximately 1.9 cm), and the echo was uneven. Several hypoechoic masses were observed in the uterine area; the largest one was in the lower part of the anterior wall, and approximately 2.2 cm × 2.1 cm × 1.9 cm in size with a clear boundary. The size of the left ovary was 2.1 cm × 1.4 cm, and the size of the right ovary was 2.0 cm × 1.2 cm. No obvious mass was present in both adnexa. Further hysteroscopy showed that the endometrium of the posterior wall of the uterus was focally thickened, and multiple polypoid lesions were observed in the uterine cavity; the larger one was approximately 1.5 cm × 1.0 cm in size and soft and pink with a smooth surface. The cervical mucosa was smooth. The sex hormone test revealed the following: estradiol (E2), 63 pg/mL (normal range: < 20-40 pg/mL); human follicle stimulating hormone (hFSH), 29.01 mIU/mL (normal range: 16.24-113.59 mIU/mL); human luteinizing hormone (hLH), 26.54 mIU/mL (normal range: 10.87-58.64 mIU/mL); progesterone (Prog), 0.33 ng/mL (normal range: 0.01-0.78 ng/mL); and testosterone (Testo), 0.46 ng/mL (normal range: < 0.1-0.75 ng/mL). Hysteroscopic endometrial polypectomy was performed on June 27, 2016, and the pathological results revealed endometrial polyps with secretory changes. The bleeding disappeared after the operation.
Personal and family history
Sex-hormone test: E2: 124 pg/mL, hFSH: 33.31 mIU/mL, hLH: 26.97 mIU/mL, Prog: 1.04 ng/mL, Testo: 1.10 ng/mL. The serum carbohydrate antigen (CA)-199, carcinoembryonic antigen (CEA), CA-125, and CA-724 levels were normal.
Physical examination
The endometrium gradually escalates from simple hyperplasia to complex hyperplasia and atypical hyperplasia due to long-term estrogen stimulation, which may develop into EC if not treated in time. The present case demonstrates the rare possibility of ovarian cellular fibroma as a precursor for estrogen excess leading to endometrial hyperplasia and PMB.
Laboratory examinations
The patient had experienced menopause at the age of 53; her body mass index was 18.75 kg/m, she reported no risk factors for endometrial cancer such as obesity, diabetes, hypertension, or genetic diseases; she had no history of hormonal drug use; and her adrenal glands were normal in size. She had one pregnancy and one previous delivery (G1P1). Her family history was unremarkable.
Imaging examinations
The woman, who was always on the look-out for something to grumble5 at, grew very jealous of her husband s affection for the bird, and would gladly have done it some harm had she dared
Before the fifth operation: TVUS (Figure 2A and B) showed the endometrium was approximately 0.6 cm thick, and there were several hypoechoic masses in the uterine area. The right ovary was approximately 2.2 cm × 1.7 cm × 1.5 cm in size, with a 1.6 cm × 1.2 cm × 1.2 cm mass being observed inside. It had clear borders and was hypoechoic. Blood-flow signals were detected with color Doppler flow imaging (CDFI). The left ovary was poorly visualized. Additional abdominal computed tomography (Figure 2C) showed a low-density nodule of approximately 1.5 cm × 1.0 cm in the right ovary with clear borders. During the operation, we observed that the uterus was obviously large and irregular, with multiple fibroid nodules. A yellow protruding lesion of approximately 1 cm (Figure 2D) was observed on the surface of the right ovary.

Pathological findings
Histopathological examination of the surgical specimens with immunohistochemistry confirmed the diagnosis of right ovarian cellular fibroma with hilar cell hyperplasia, focal complex endometrial hyperplasia, multiple leiomyomas of the uterus, and chronic cervicitis. The following observations were made:
The tumor cells of the right ovarian lesion were observed to be spindle-shaped and bundle-like with sheet-like arrangement under a microscope, and the cells were densely arranged without obvious atypia. Eosinophilic cell nests with rich cytoplasm were visible in the focal area (Figure 3A). The densely arranged endometrial glands were hyperplastic, and some glandular lumens were irregular (Figure 3B). Immunohistochemistry revealed the following: cytokeratin (focal+, Figure 3C); inhibin (part+, Figure 3D); Wilm’s tumor protein (WT1, part+, Figure 3E); calretinin (+, Figure 3F); Ki-67 (approximately 10% +, Figure 3G); and net staining showed mostly surrounding single cells (Figure 3H).

FINAL DIAGNOSIS
15.You do not understand: The father does not seem overly concerned about his third son. His response conveys that since the smarter ones failed so to must the younger one.Return to place in story.
The patient was diagnosed with right ovarian cellular fibroma with hilar cell hyperplasia, focal complex endometrial hyperplasia, multiple leiomyomas of the uterus, and chronic cervicitis after undergoing laparoscopic hysterectomy and bilateral salpingo-oophorectomy (performed on July 10, 2020).
The patient recovered well after the fifth operation. The sex hormone levels on the first day after the fifth operation were as follows: E2, 22 pg/mL and Testo, 0.73 ng/mL; on the second day, they were E2, < 20 pg/mL and Testo, 0.30 ng/mL. The patient was discharged on the 5th day postoperatively, 2 and 6 mo after the operation, no abnormalities were observed in the outpatient review.
TREATMENT
Full-scale curettage using hysteroscopy was performed on June 23, 2020. Since atypical hyperplasia of the endometrium was not excluded and considering the patient’s age and operation history, we decided to perform laparoscopic hysterectomy and bilateral salpingo-oophorectomy after the fourth hysteroscopic curettage. TVUS was performed again before the operation and a 1.6 cm × 1.2 cm × 1.2 cm mass was observed in the right ovary. Laparoscopic hysterectomy and bilateral salpingo-oophorectomy were performed on July 10, 2020.
The patient was diagnosed with endometrial complex hyperplasia, but the possibility of mild atypical hyperplasia could not be excluded after full-scale curettage using hysteroscopy was performed (performed on June 23, 2020).
OUTCOME AND FOLLOW-UP
Well, I ll tell you, I can t bear the thought of an unplanted corm, bulb, seed plug, you name it. I always start too many seeds in March and by June I m tucking them everywhere I can. I just can t bear the thought of a plant not getting a chance to grow. In other words, she was in luck.
DISCUSSION
PMB, defined as uterine bleeding occurring after at least 12 mo of amenorrhea, is mainly caused by intrauterine sources, and a few cases are related to ovarian tumors. Clarke[1] found that, among 593 women with PMB, 18 (3.0%) had endometrial intraepithelial neoplasia (EIN), and 47 (7.9%) had endometrial cancer (EC). Women with recurrent PMB had higher risks of EIN and EC (4.5% and 10.1%, respectively) than those with an initial episode of PMB (0.5% and 5.1%, respectively;= 0.002)[1]. Endometrial hyperplasia is usually caused by the continuous action of estrogen on the endometrium and is regarded as a precursor to EC. Hysteroscopy is now considered the gold standard for diagnosing and managing intrauterine lesions[2]. TVUS of postmenopausal women with endometrial thickness > 4 mm requires additional endometrial sampling for evaluation. For women with PMB, a negative tissue biopsy after a “blind” sampling of the endometrium is not considered the end point[3].
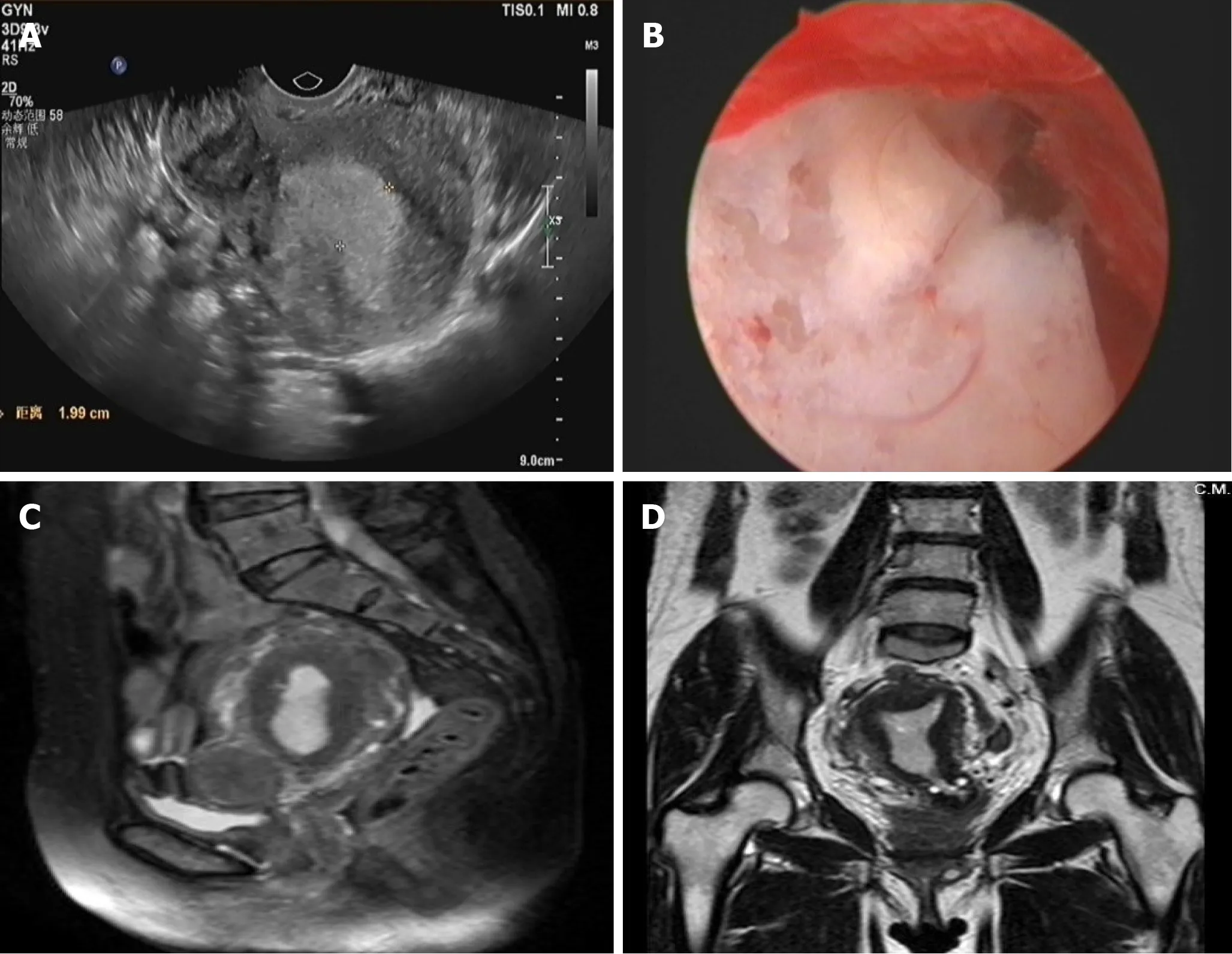
Before the fourth operation: TVUS (Figure 1A) showed the uterus to be approximately 8.2 cm × 6.3 cm × 5.7 cm in size, and the endometrium was approximately 2.0-cm thick. Several hypoechoic masses were observed in the uterine area. The largest one (3.6 cm × 3.4 cm × 2.7 cm) was located in the isthmus of the anterior wall and had a clear boundary. The size of the left ovary was 2.1 cm × 1.7 cm × 1.0 cm, and the size of the right ovary was 1.9 cm × 1.3 cm × 1.2 cm. There was no obvious mass in bilateral adnexa. Hysteroscopy (Figure 1B) revealed that the endometrium was extensively thickened, and the cervical mucosa was smooth. Pelvic magnetic resonance imaging (Figures 1C, D) showed the uterus was enlarged, the endometrium was thickened (approximately 1.6 cm in total), and the signal in the right corner of the uterine cavity was not uniform.
Gynecologic examination: the cervix was normal in size with a smooth surface and no cervical atrophy, the uterus was enlarged and irregular, and no abnormalities were found in bilateral adnexa.
Many were the quarrels which the old rat and his wife had upon the subject, and sometimes they bore on their faces certain marks which looked as if they had not kept to words only
Ovarian fibromas are the most common mesenchymal tumors of the ovary, accounting for 4% of all ovarian tumors[4]. They occur mainly in perimenopausal and postmenopausal women. The most common clinical manifestation of ovarian fibroma is an ovarian mass, sometimes accompanied by pleural effusion and ascites. Approximately 10% of ovarian fibromas are cellular fibromas, with an average age at onset of 51 years[5]; clinical manifestations tend to be benign with occasional local recurrence and low malignant potential. It usually presents as an ovarian mass, which may be accompanied by hemorrhage, edema, and cystic degeneration. Cystic degeneration usually forms a single cyst; in rare cases, it may be polycystic[6].
Unlike granular or theca cell tumors, ovarian fibromas rarely exhibit estrogenic activity. Chechia[4] retrospectively analyzed 24 cases of ovarian fibroma and fibrothecomas, which have no endocrine activity. Haroon[7] retrospectively analyzed 480 cases of ovarian sex cord-stromal tumor (SCST), including 98 cases of ovarian fibroma; of which one was associated with EC and another with endometrial hyperplasia, proving that these tumors have hormonal activity. Identifying whether ovarian fibromas have other concomitant components and/or hormonal activity may explain various clinical characteristics and histopathological findings. Our case presented with hilar cell hyperplasia alongside her other presentations, which increases the rate of androgen secretion. The levels of estrogen and androgen were significantly higher than normal and dropped to the normal range on the second day after surgery; estrogen may be directly secreted by tumors and/or transformed by androgens.
53. Pearls and precious stones: Hansel and Gretel feel no guilt73 for taking the witch s treasure, similar to Jack with the Giant s treasure in Jack and the Beanstalk. The witch s attempt to kill them and subsequent death is implied as justification for taking the jewels.Return to place in story.
So the merchant set out and reached the town as quickly as possible, but only to find that his former companions, believing him to be dead, had divided between them the goods which the ship had brought; and after six months of trouble and expense he found himself as poor as when he started, having been able to recover only just enough to pay the cost of his journey
Ovarian fibromas are easily misdiagnosed as uterine fibroids since they are generally solid tumors. In the present case, TVUS eventually revealed an ovarian hypoechoic mass before the fifth operation. The ovarian mass should have existed at the time of the fourth operation, but it was not reported by ultrasound and imaging doctors. It was likely mistaken for a uterine fibroid, as the patient had several of those. Ovarian cellular fibroma is diagnosed using postoperative pathology. Its pathological characteristics tend to be observed microscopically, and the tumor cells are fusiform and dense, have 0-3 mitoses/10 high-power fields, do not exhibit obvious nuclear atypia, and are arranged in bundles, which may contain a small amount of sex cord components (partly with luteinized cells). It is surrounded by reticular fibers. Immunohistochemistry can show inhibin-α, calretinin, estrogen receptor, Prog receptor,to varying degrees. The tumor immunohistochemistry result in our case is consistent with those reported in the literature. Ovarian cellular fibroma is also easily misdiagnosed as ovarian malignant tumor, especially when it occurs in both ovaries or is accompanied by pleural effusion, ascites, or elevated CA125 Levels. At the same time, ovarian fibroma should also be distinguished from ovarian granulosa cell tumor (GCT) and thecoma, which usually have estrogen activity. GCT usually secretes estrogens and inhibin, especially inhibin B[8]. Therefore, elevated serum inhibin B is regarded as a classic marker of GCT. However, Carballo[9] demonstrated a case of benign ovarian thecoma with markedly elevated serum inhibin B levels, suggesting that inhibin B is not entirely specific to GCT. Patients with endocrine disorders need their inhibin B levels to be evaluated. Unfortunately, we did not consider testing the inhibin B levels in the patient.
Ovarian fibromas are treated with surgery. Laparoscopy facilitated shorter operation times than laparotomy, with no significant differences in perioperative complications occurring[10]. Perimenopausal or postmenopausal patients with ovarian cellular fibromas are recommended to undergo total hysterectomy and bilateral salpingo-oophorectomy with long-term follow-up. In our case, the patient had recurrent vaginal bleeding 4 years ago, and estrogen level was higher than normal, although auxiliary examinations did not indicate ovarian mass, should we have recommended that the patient undergo prophylactic removal of the whole uterus and both fallopian tubes and ovaries to avoid subsequent vaginal bleeding and surgery?
CONCLUSION
PMB should never be ignored, however, the ideal sequence of investigation for a patient with PMB remains controversial[11]. Ovarian cellular fibroma occurs insidiously and may exhibit hormonal activity. Excessive or long-term serum estrogen stimulation of the endometrium may cause endometrial hyperplasia or even cancer, resulting in symptoms such as PMB. Therefore, early diagnosis, determination of a clear cause, and timely treatment are essential. Sex hormone testing is recommended for patients with recurrent PMB and endometrial thickening. If the estrogen level remains elevated even if imaging does not indicate an ovarian tumor, clinicians should first consider whether it may be associated with ovarian SCST. Once ovarian masses are found, they should be removed as soon as possible to prevent endometrial lesions formations, regardless of size. Further study is required to determine whether preventive removal of the whole uterus and bilateral fallopian tubes and ovaries should be recommended to women with elevated estrogen levels, repeated bleeding after menopause, and auxiliary examinations that do not reveal ovarian masses; it may avoid multiple subsequent curettage procedures, which are associated with physical and psychological burdens.
World Journal of Clinical Cases2022年1期
- World Journal of Clinical Cases的其它文章
- Hepatitis B virus reactivation in rheumatoid arthritis
- Paradoxical role of interleukin-33/suppressor of tumorigenicity 2 in colorectal carcinogenesis: Progress and therapeutic potential
- Changes in rheumatoid arthritis under ultrasound before and after sinomenine injection
- Benefits of multidisciplinary collaborative care team-based nursing services in treating pressure injury wounds in cerebral infarction patients
- Outcomes and complications of open, laparoscopic, and hybrid giant ventral hernia repair
- Surgical resection of intradural extramedullary tumors in the atlantoaxial spine via a posterior approach
