Hepatitis B virus reactivation in rheumatoid arthritis
INTRODUCTION
Chronic hepatitis B (CHB) is a prevalent disease worldwide, and it is also the main cause of cirrhosis and liver cancer[1,2]. The application of pegylated interferon (IFN), entecavir, tenofovir disoproxil fumarate (TDF), and other highly effective anti-viral drugs has significantly improved the prognosis of patients[3,4]. Some patients can even obtain hepatitis B surface antigen (HBsAg) clearance to achieve a “functional cure”[5]. However, for some special populations, such as cancer patients undergoing chemotherapy, biological agents are used to treat autoimmune diseases or for organ or tissue transplantation and so on. When combined with hepatitis B virus (HBV) infection, if there is no anti-viral prophylaxis, the use of immunosuppressive agents or chemotherapeutic agents may lead to liver injury or even death[6]. The cause of this condition is now known as HBV reactivation (HBVr). HBVr was first reported in 1975 in patients with lymphoproliferative and myeloproliferative disorders[7,8]. Subsequently, it was observed in various diseases[9,10]. However, there is currently no uniform definition of HBVr.
Rheumatoid arthritis (RA) is an autoimmune disease characterized by erosive arthritis as its main clinical manifestation. The incidence of disability and functional limitation increases with the course of disease[11]. Disease-modifying anti-rheumatic drugs (DMARDs) have significantly improved the clinical and radiographic outcomes in RA patients[12]. Epidemiological studies show that the distribution of RA is global, and the average incidence rate is 1%. The incidence rate of RA in China is 0.2% to 0.4% and in Japan, the prevalence is estimated at 0.6%-1.0%[13]. The Asia Pacific region has a high incidence of HBV infection. The relatively high prevalence of both HBV infection and various forms of RA will result in the coexistent diagnoses of both diseases in a substantial number of patients. For example, a study in Japan showed that approximately 20% or more of patients with rheumatic diseases are infected with HBV[14]. Insufficient HBV screening has been reported in various countries[15-17]. We know that most anti-rheumatic drugs work by downregulating the overactivated immune system. Several studies have reported that people with RA combined with HBV infection, especially those with occult HBV infection or resolved carriers, may reactivate HBV during treatment with anti-rheumatic drugs[18-21]. Due to different research populations, drugs, and definitions of HBVr, the reported rates of HBVr are significantly different, ranging from 0 to 100%[22,23]. The outcome of HBVr varies from liver inflammation to liver failure. Therefore, more attention should be given to the reactivation of HBV in RA patients.
MECHANISMS OF HBV REACTIVATION
The steps for HBV to achieve virus replication and cell infection include cell entry, relaxed circular DNA repair, covalently closed circular DNA (cccDNA) transcription and translation, pre-genomic RNA reverse transcription, and secretion of virus particles[24]. cccDNA is the template for replication. Nucleos(t)ide analogues (NAs) and IFN are the two main types of drugs for CHB anti-viral therapy. NAs can effectively reduce the HBV DNA load by inhibiting virus replication by acting on the retroviral process. IFN has the dual functions of direct anti-viral and immune regulation. However, neither drug has an effect on HBV replication template cccDNA. Therefore, the continuous existence of cccDNA in the hepatocyte nucleus is an important source of persistent HBV replication and infection. Studies have shown that even if HBV DNA in the peripheral blood of patients is lower than the detection threshold or even if HBsAg is cleared, the presence of cccDNA can still be detected in liver cells[25,26]. From the above information, it is very difficult to eradicate HBV, and the long-term existence of cccDNA is an important cause of HBVr.
It was eight o clock when he took up his post, and for the first hour he was quite proud of his courage; during the second hour he was well pleased with the large reward that he would get, but in the third hour, when it was getting near eleven, the effects of the wine passed off, and he began to get uncomfortable, for he had heard about this post; that no one had ever escapeed alive from it, so far as was known
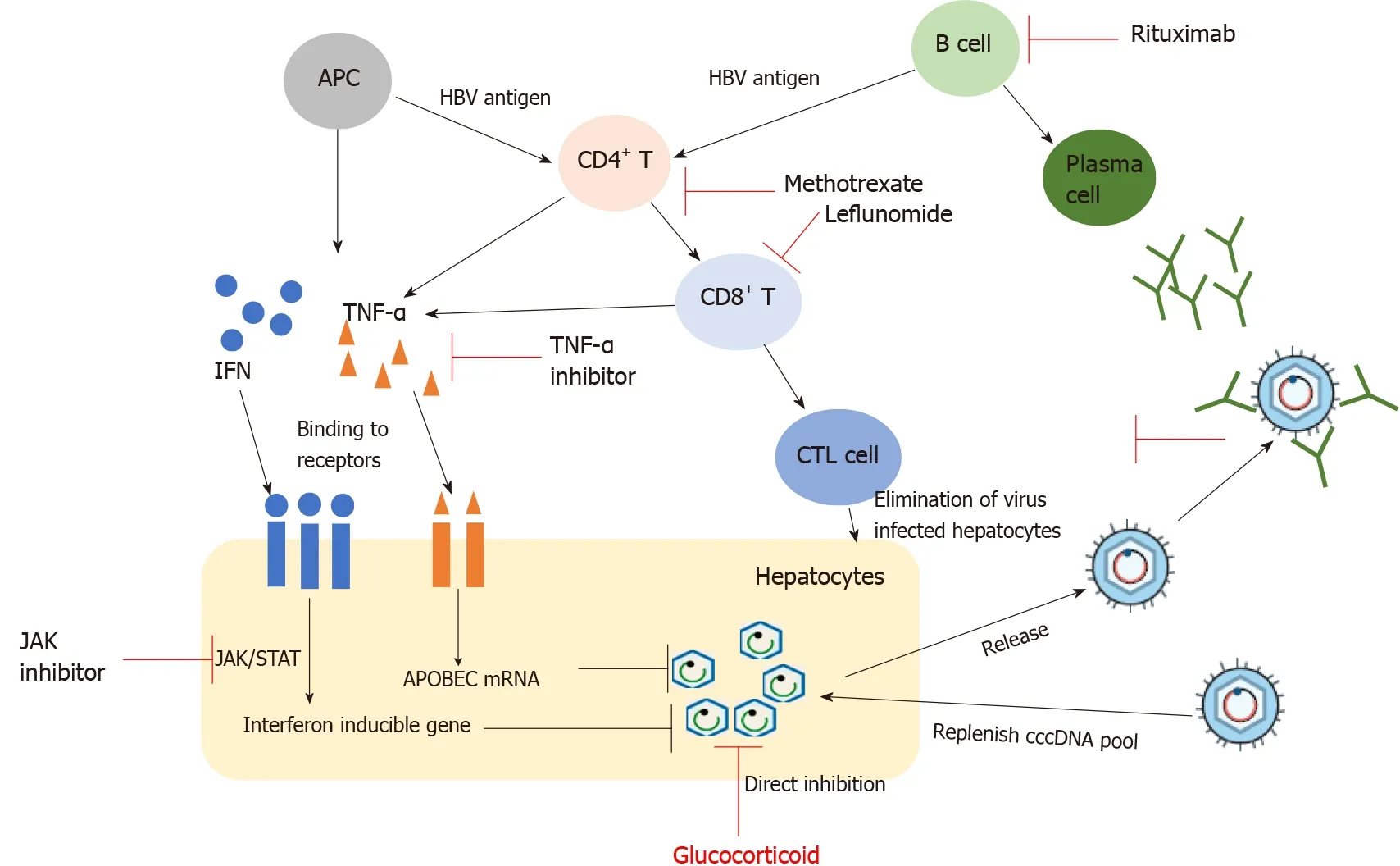
Studies have shown that the host immune response plays an important role in anti-HBV infection (Figure 1). After HBV infection, the release of antigenic substances induces B cells to produce corresponding antibodies, such as HBV surface antibody (anti-HBs), antibody to HBV core antigen (anti-HBc), and antibody to HBV envelope antigen (anti-HBe). They are important reference indicators for the clinical diagnosis of HBV infection stage. However, among these antibodies, only anti-HBs has a protective effect on the body. In addition to producing neutralizing antibodies, B cells also have the function of antigen presentation, which can present antigens to CD4T cells to exert anti-viral effects. CD4T cells can differentiate into helper T cells of different subtypes under antigen stimulation. These cells play an indirect anti-viral role by regulating the function of virus-specific CD8T cells and B cells. Cytotoxic T lymphocytes (CTL) play the most direct and critical anti-viral role in the anti-viral immune process. On the one hand, CTL directly kill infected hepatocytes by secreting substances such as granulomycin B and perforin, which promote cell lysis. On the other hand, CTL kill infected hepatocytes by secreting specific cytokines, including IFN-γ and tumour necrosis factor α (TNF-α), and then achieve the effect of clearing the virus, called the non-lysing pathway. Under normal circumstances, most adults with sound immune function can clear the virus through the coordination of the innate immune response and adaptive immune response and can obtain resistance to HBV re-infection. However, in HBV infection, CD8T cell function is exhausted under the combined action of multiple factors, which is one of the main causes of chronic infection[27].
In their hurry they had, however, forgotten two things: a bundle of keys which lay on the table, and the girl whom the pin had pricked, and who now stood pale and helpless beside the wood stack
RISK OF HBV REACTIVATION WITH DIFFERENT ANTI-RHEUMATIC DRUGS
ISDs, that is, conventional synthetic DMARDs (cDMARDs) and biologic DMARDs, target the synthesis of DMARDs and steroids used for RA and can cause HBVr (Table 1). Therefore, evaluating the risk of reactivation for each drug is very important.
Methotrexate (MTX) is a folic acid antagonist that was initially used to treat tumors and has now become the most widely used drug for RA. MTX can suppress the HBVspecific cytotoxic T cell response and inhibit the production of proinflammatory cytokines such as TNF-α, interleukin (IL)-1, and IL-6. At present, insufficient data are available to identify the risk of cDMARD-related HBVr. In one study, only 3 (2 were HBsAg-positive and 1 was HBsAg-negative/anti-HBc-positive) of 211 patients (23 were HBsAg-positive and 188 were HBsAg-negative/anti-HBc-positive) had HBVr with MTX treatment[29]. Similarly, only one case of HBVr was reported in a crosssectional study conducted in Thailand in HBsAg-positive patients[30]. For HBsAgnegative and anti-HBc-positive patients, although there have been a few case reports of HBVr using MTX alone or combined with other DMARDs, the risk of HBVr seems rather low in these patients and in a large cohort study conducted by Laohapand[30], HBVr was not detected in such patients[20,30]. In general, MTX is relatively safe in RA patients with chronic or past HBV infection.

Those who have gone have returned changed; even by a four-day weekend in Santa Fe, an Amtrak ride up the coast or an organized tour of Civil War battlefields
In addition, the application of different types of anti-rheumatic drugs also affects HBV reactivation. Lin[50] showed that TNF-α inhibitors significantly reduced the risk of HBV reactivation compared with other anti-rheumatic drugs (1.4%6.1%), which was consistent with Cantini[49]’s report. However, in other studies, TNF-α inhibitors such as infliximab and adalimumab have a relatively high risk (62.5%) of HBVr in HBsAg-positive patients[39].
CSs can produce powerful anti-inflammatory effects and immunomodulatory effects, so they are widely used in the treatment of RA. They can not only reduce the number of monocyte-macrophages in the circulatory system but can also reduce the synthesis of inflammatory factors. The link between CSs and HBVr has been strongly established in the literature. Fujita[15] and Chen[18] showed that CSs alone or in combination with other drugs would significantly increase the risk of HBVr in RA patients who received immunosuppressive therapy. This may be related to the mechanism of CSs[28]. In addition, the risk of reactivation varies with the dosage and course. The American Gastroenterological Association (AGA) guidelines have proposed that the risk of HBVr in RA patients be divided into different levels according to the amount of CSs and the duration of treatment: High risk - dose > 10 mg and treatment duration > 4 wk; medium risk - dose < 10 mg and treatment duration > 4 wk; and low risk - dose < 10 mg and treatment course < 1 wk[34]. Wong[35] reported that among CHB patients, a peak daily dose > 40 mg< 20 mg CS was an independent risk factor for hepatitis flare, and under the same treatment dose, the risk of hepatitis flare was significantly higher in patients with a treatment course of > 7 d< 7 d (< 0.001)[35]. At present, little data exist on HBVr in HBsAg-negative and anti-HBc-positive patients after using CSs. However, according to the 2015 AGA guidelines, HBsAg-negative and anti-HBc-positive patients are considered to be a lowrisk group (< 1%) in terms of the occurrence of HBVr during CS therapy[34,36].
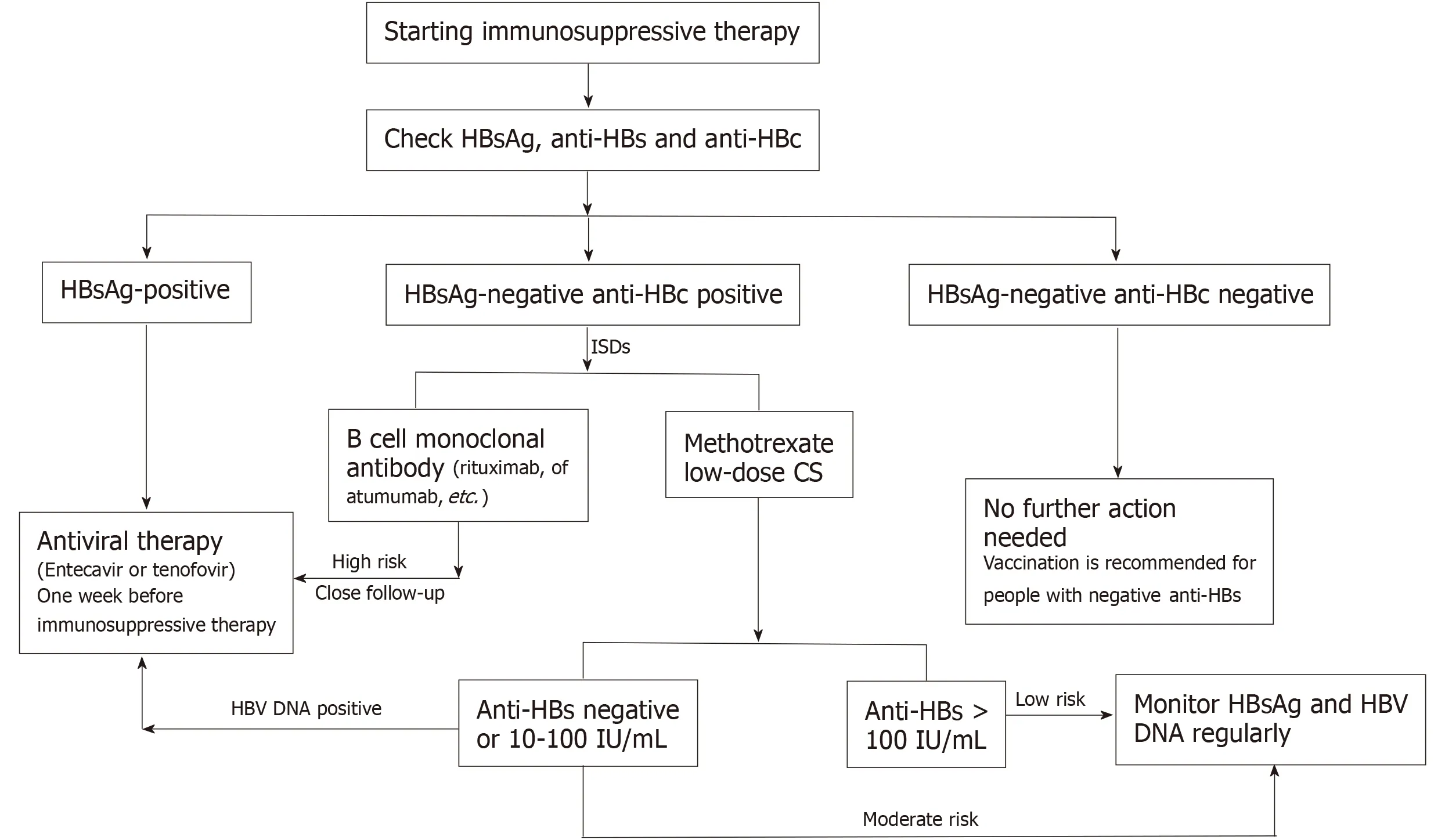
There is a risk of HBVr in RA patients when they are treated with ISDs. Therefore, it is necessary to identify patients at high risk before starting immunosuppressive therapy. We recommended that all RA patients be screened for HBsAg, anti-HBs, and anti-HBc when applying ISDs. According to the different detection results, the following three categories exist (Figure 2):
IL-6 is a key factor in the pathogenesis of RA. It promotes inflammatory cell aggregation, stimulates synovial pannus formation, and induces osteoclast activation, leading to joint inflammation and bone destruction. Tocilizumab (TCZ) is a humanized monoclonal antibody that blocks IL-6 signalling by inhibiting its receptor. However, there are currently insufficient data on the effect of TCZ on HBVr in RA patients with chronic HBV infection. Only one study reported that in RA patients with past HBV infection, the HBVr rate was 8%[40].
The JAK/STAT signalling pathway plays an important role in the pathophysiology of RA and has a regulatory effect on various cells and cytokines in the inflammatory process of RA. JAK inhibitors are a new kind of targeted synthetic DMARDs. The nonselective JAK inhibitor tofacitinib was the first approved treatment for RA. In a study, six HBsAg-positive RA patients were treated with JAK inhibitors, among which four were treated with antiviral prophylaxis and two were not, and the two who were not treated with antiviral prophylaxis showed HBVr[41]. However, in RA patients with previous HBV infection, no reactivation of HBV was observed.
In some ways the consequences have been quite dire22 and I no longer have contact with my mother. However, Dad s hug had a profound effect on me. It carried me along a path from childhood to adulthood23. At last I am my own woman and one who loves nothing better than a good old-fashioned hug.
Abatacept is a selective T cell costimulatory regulator. It is a fusion protein comprising the extracellular functional region of human cytotoxic T lymphocyte associated antigen-4 (CTLA-4, also known as CD152) and the FC segment of human immunoglobulin (Ig) G-1. It inhibits the activation of T cells by binding to CD80 and CD86 on the surface of antigen presenting cells. At present, abatacept has been approved for the treatment of active RA, including moderate and severe active patients with poor treatment effects of MTX and TNFi, which can be used alone or in combination with cDMARDs[42]. At present, there have been reports on HBVr in RA patients after the application of abatacept, but most of them come from case reports, and there are few relevant cohort studies. In one study, four inactive HBV carriers who received abatacept treatment developed HBVr within an average of 10 mo[43]. In contrast, in another study, none of the 38 inactive HBV carriers who received abatacept treatment developed HBVr[44]. At present, the only study collected 27 patients with HBsAg-negative and anti-HBC-positive RA treated with abatacept, of whom 19% received preventive antiviral drugs, and there were no cases of HBVr[45]. Although the current research data are limited, the AGA classifies abatacept as a medium-risk drug (1%-10%) for HBVr in HBsAg positive patients and a low-risk agent in HBsAg negative and anti-HBC positive RA patients (< 1%)[34].
RISK FACTORS FOR HBV REACTIVATION
HBVr is affected by many factors that can be roughly divided into three categories: Host factors, viral factors, and drug factors. Host factors, including male sex, advanced age, and the presence of cirrhosis, are risk factors for HBVr[9,15,46,47]. Different ISDs cause different risks of HBVr, as shown in Table 1.
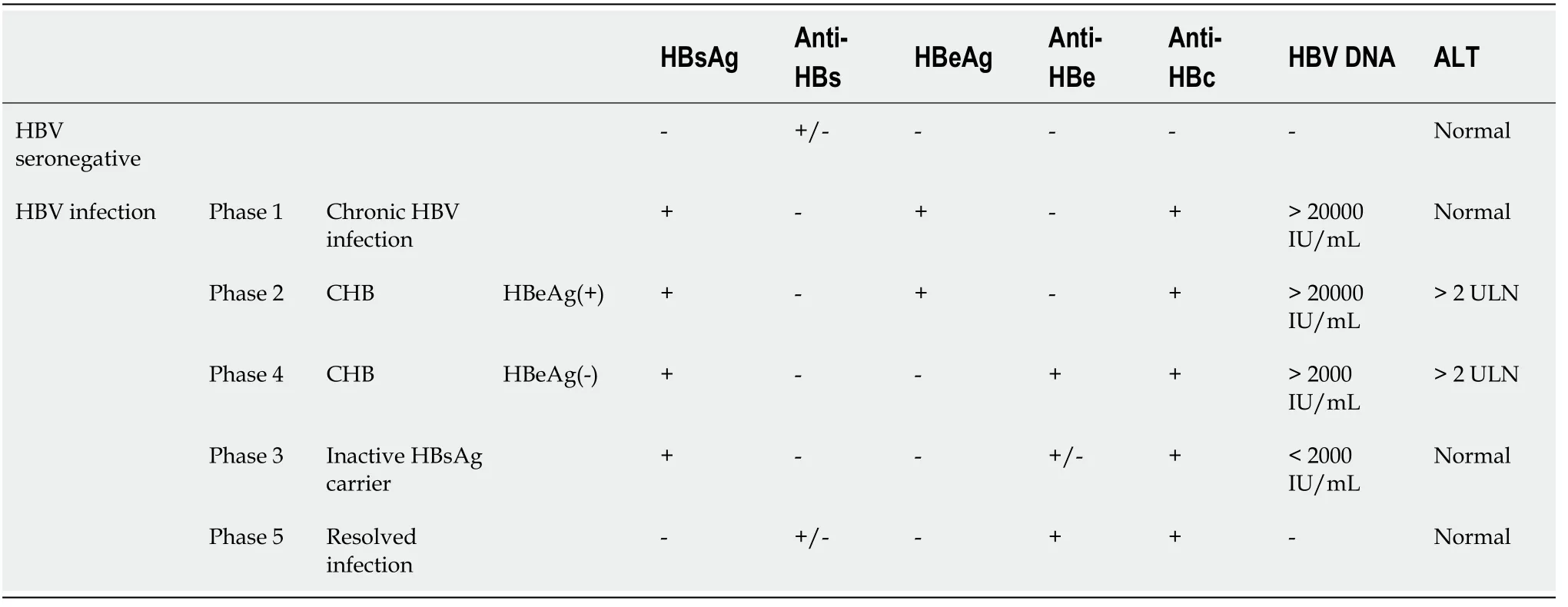
RA patients with different HBV infection statuses have different risks of reactivation[48]. According to natural history, HBV infection can be divided into the following five phases (Table 2). Phase 1 is hepatitis B e antigen (HBeAg)-positive chronic HBV infection, characterized by HBsAg and HBeAg positivity and high levels of HBV DNA but continuous normal alanine aminotransferase (ALT); in the liver, there is minimal or no liver inflammation. This phase is the stage of “harmonious co-existence” between the virus and the immune system. Phase 2 is characterized by HBeAgpositive CHB, serum HBsAg positivity, HBeAg positivity, HBV DNA positivity, and sustained or repeated increase in ALT, with accompanying moderate or severe liver necroinflammation. In this phase, the immune system of the body is activated, and the virus is quickly eliminated, but at the same time, it also causes damage to liver tissue. Phase 3 is HBeAg-negative chronic HBV infection and was previously termed the “inactive carrier” phase; it is characterized by HBsAg positivity, HBeAg negativity, undetectable or low (< 2000 IU/mL) HBV DNA, and normal ALT. In this phase, HBV DNA is in a low replication stage, and the immune system returns to normal again. However, when the body’s immunity is reduced for any reason, it will also cause a large amount of virus replication, thus entering Phase 4, or HBeAg-negative CHB, characterized by serum HBsAg positivity, HBeAg negativity, HBV DNA positivity, sustained or repeated elevations in ALT, and liver histology showing necroinflammation and fibrosis. In Phase 5, the HBsAg-negative phase is characterized by serumnegative HBsAg and anti-HBc positivity, with or without anti-HBs, and ALT is within the normal range. This stage is currently the “optimal endpoint” for CHB treatment, but cccDNA can still be detected in most liver tissues. It is generally believed that CHB (HBeAg positive and negative) has the highest risk of reactivation, followed by inactive carriers. Although HBsAg clearance is currently called a “functional cure”, there is still a risk of reactivation, but compared with other phases, the risk of reactivation is relatively low. In the study of Cantini[49], 21 studies were included in a systematic review, and 10 were eligible for meta-analysis. The results showed that the pooled prevalence of reactivation was 3.0% for patients with occult infection and 15.4% for chronic HBV infection[49]. The study by Lin[50] also showed that the HBV reactivation rate in inflammatory arthritis patients was low in resolved patients and moderate in chronic HBV infection patients. Furthermore, lower rates were observed in chronic HBV infection patients who used anti-viral prophylaxis.
Anti-HBs antibodies are protective antibodies produced by the body that can neutralize HBsAg. At present, many studies have found that anti-HBs levels affect HBVr in patients with RA treated with immunosuppressants[12,51,52]. In a retrospective study, 152 RA patients with resolved HBV were enrolled. During the observation period of 15 mo, 7 (4.6%) patients developed HBVr. Patients who were negative for anti-HBs showed a significantly higher incidence of HBVr (= 0.013)[12]. Similarly, in the study by Tien[51], 380 patients with RA were treated with biologics, and compared with the anti-HBs < 100 mIU/mL group, the anti-HBs > 100 mIU/mL group had no HBVr[51]. Therefore, we should pay more attention to the detection of anti-HBs when using ISDs for RA patients with HBV infection. In addition, we also need to pay attention to special situation-HBV S region mutants. HBV is a virus with a high mutation rate. In particular, regarding the “α” antigenic determinant in the HBV S region, protein structure changes caused by amino acid changes at this site may cause HBsAg antigenic changes or even decrease the antibody neutralizing ability[53], resulting in the failure of reagent detection[54]. Therefore, only when anti-HBs produced by the body can neutralize HBsAg does it have a protective effect.
Leflunomide (LEF) is the world’s first recognized DMARD specifically for the treatment of RA and can effectively control the course of disease and prevent bone destruction. Its role is mainly to inhibit the growth of activated lymphocytes by inhibiting dihydrolactic dehydrogenase. Although both MTX and LEF can cause HBVr, Mo[31] showed that MTX can be used as a therapeutic drug for RA patients in the HBV carrier state at low doses, while LEF increases the risk of HBVr (= 0.011), and it is best to prohibit its use. This may be related to the drug properties of LEF. Studies have shown that LEF may activate HBV replication through nucleoside reduction-related phosphorylation of mitogen-activated protein kinase P38[32]. In addition, liver damage may also occur during the application of LEF[33].
PREVENTION AND MANAGEMENT OF HBV REACTIVATION IN RA
TNF-α is a protein that promotes inflammation of the joints and plays an important role in coordinating innate immunity and adaptive immunity against HBV infection. In particular, TNF-α can activate apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC) proteins, which cause the degradation of cccDNA in HBVinfected cells[37]. Thus, blocking TNF-α signalling may lead to a higher HBV replication state and HBVr. Drugs or biological agents that block its pathway have been widely used in various inflammatory and autoimmune diseases. The association between TNF inhibitor (TNFi) use and HBVr has been well established. In general, the risk of HBVr is significantly increased in HBsAg-positive RA patients without antiviral treatment. Studies have shown that for HBsAg-positive RA patients treated with TNFi, the HBVr rate is 9.1%-75%[23,38]. When compared with HBsAg-positive patients, individuals who are HBsAg-negative and anti-HBc-positive appear to have a lower risk of HBVr when exposed to TNFi, and the HBVr rate is 0-8.3%[22,39]. The different reactivation rates of HBV may be related to the size of the study population, the definition of HBVr, and the different types of TNF-α inhibitors. For HBsAg-negative and anti-HBc-positive patients, the existing data show that the risk may be partly attributable to the concomitant use of other immune suppressive drugs that are in the low-risk category. In contrast, when high risk agents such as rituximab are used in HBsAg-negative, anti-HBc-positive patients, high rates of reactivation in excess of 10% occur and antiviral prophylaxis can be anticipated to result in similar absolute risk reduction as described for HBsAg-positive patients[34].
The reactivation of HBV mainly occurs during the application of various immunosuppressive agents in specific populations, among which HBVr is the most common phenomenon in patients with chemotherapy-treated diffuse lymphoma. When treated with immunosuppressive drugs (ISDs), the body’s immune system is suppressed, and HBV replicates and infects more liver cells; when the ISDs are reduced or stopped, the body’s immunity will gradually recover and quickly start immune killing of HBV-infected hepatocytes, resulting in varying degrees of liver damage. When the body’s immune response is too strong, it will lead to necrosis of a large number of liver cells and even to liver failure and death. For example, corticosteroids (CSs) are commonly used in the treatment of RA. When RA with HBsAg- and/or anti-HBc-positive patients are treated with high-dose, long-course CSs, they are more likely to experience HBVr. This is mainly because CSs can directly act on the glucocorticoid response element that controls viral replication and transcriptional activity in HBV, thus promoting HBV replication and increasing the risk of HBVr[28].
HBsAg-positive, anti-HBc-positive patients
HBsAg-positive patients have the highest risk of HBVr during immunosuppressive therapy. Timely antiviral therapy can effectively prevent HBVr[48]. Therefore, in HBsAg-positive RA patients, anti-viral prophylaxis is recommended before immunosuppressive therapy[55]. ETF and TDF can be used as the first choice[56].
HBsAg-negative, anti-HBc-positive patients
HBsAg-negative and anti-HBc-positive patients can be divided into different categories according to the level of antibodies and drugs. When RA patients are treated with rituximab, high-dose CS, or infliximab, HBVr is a high risk. Close followup, if necessary, is recommended before immunosuppressive treatment. For those who are treated with MTX or low-dose CS and have anti-HBs > 100 IU/L, the risk of HBVr is low. It is recommended that HBV DNA and ALT be regularly tested during therapy. If HBV DNA is elevated, it is also recommended that antiviral treatment be started. For those with negative or anti-HBs < 100 IU/L, there is a moderate risk of HBVr. Regular follow-up is also recommended.
We arrived at the stadium a few minutes before the players were due to take the field and I lined up with several other youngsters at the entrance to the Pirate locker7 room
I sang our favorites - Barbara Streisand, Linda Ronstadt and Bette Midler. My voice was quiet and hushed, commensurate with the dim light in the room. I made sure the sound didn t penetrate1 the walls. You listened with your eyes closed, then thanked me and told me how lovely and peaceful it was.
HBsAg-negative, anti-HBc-negative patients
For RA patients who have not been infected with HBV but are anti-HBs positive (preferably ≥ 100 IU/mL), no additional operation is needed. However, if they are anti-HBs negative, a hepatitis B vaccine is recommended.
Finally, the ideal time interval between antiviral therapy and immunosuppressive therapy was determined. For people at high risk of HBVr (such as HBsAg-positive and anti-HBC-positive), antiviral therapy should be applied 1 wk before the start of ISDs treatment. For HBsAg-negative and anti-HBC-positive patients, if B-cell monoclonal antibodies are used, antiviral drugs can be considered[34,57]. After the cessation of immunosuppressant treatment, NAs treatment should be continued for at least 6 mo. If B cell monoclonal antibodies are used, NAs treatment should be continued for at least 12 mo after stopping immunosuppressive treatment. After NAs are stopped, there may be recurrence and even worsening of the disease. Follow-up and monitoring should be considered[58].
CONCLUSION
The application of anti-viral drugs and anti-rheumatic drugs improves the prognosis of patients with HBV and RA. However, the reactivation of HBV in RA patients has not attracted enough attention. Although the infection rate of RA patients with HBV is not high, once HBVr occurs, it will cause serious consequences. Therefore, patients with RA should undergo comprehensive evaluation before anti-rheumatic drug treatment, including patient age, sex, anti-HBs level, HBV DNA load, and ISDs. Patients who are at high risk of HBVr need to undergo regular testing for ALT and HBV DNA. Once they meet the treatment indications, anti-viral treatment should be carried out in a timely manner. Drugs with strong anti-viral effects and high drug resistance barriers are the best choice.
World Journal of Clinical Cases2022年1期
- World Journal of Clinical Cases的其它文章
- Paradoxical role of interleukin-33/suppressor of tumorigenicity 2 in colorectal carcinogenesis: Progress and therapeutic potential
- Changes in rheumatoid arthritis under ultrasound before and after sinomenine injection
- Benefits of multidisciplinary collaborative care team-based nursing services in treating pressure injury wounds in cerebral infarction patients
- Outcomes and complications of open, laparoscopic, and hybrid giant ventral hernia repair
- Surgical resection of intradural extramedullary tumors in the atlantoaxial spine via a posterior approach
- Vancomycin lavage for the incidence of acute surgical site infection following primary total hip arthroplasty and total knee arthroplasty
