Novel m.4268T>C mutation in the mitochondrial tRNAIle gene is associated with hearing loss in two Chinese families
INTRODUCTION
Hearing loss is a very important health problem, influencing nearly 1 in 700 newborns[1,2]. Loss of hearing may be associated with gene modifications or environmental factors, which include ototoxic drugs such as aminoglycoside antibiotics[3,4]. Mutations of mitochondrial rRNA and tRNA genes have been accredited as one of the most important reasons for sensorineural hearing loss[5-7]. Therefore, the 12S rRNA 1555A>G and 1494C>T mutations account for both aminoglycoside-induced and nonsyndromic deafness in a large number of families worldwide. In addition, the 7472insC, 7510T>C, 7511T>C, 7445A>G and 7445A>C mutations in the tRNA(UCN) gene have been associated with non-syndromic deafness[5,8-11]. The mtDNA mutations such as 1555A>G and 7445A>G usually exhibit incomplete penetrance, as some individuals carrying the mutation (s) have normal hearing[10,11]. In fact, mutations in the GJB2 gene encoding connexin 26 (Cx26) are a common reason for non-syndromic deafness worldwide[12]. There are more than 150 different GJB2 variants that have been identified, which include missense, nonsense and frameshift mutations (The Connexin-deafness homepage, http://davinci.crg.es/deafness). The range of GJB2 mutations vary in different race groups[12,13]. Of those variants, c.35delG may be the most common GJB2-deafness-causing mutation in European people, while c.235delC and c.167delT are the most common variations in East Asian people and Ashkenazi Jewish families, respectively[14,15]. In addition, they exhibit different age-at-onset, severity, and penetrance of hearing impairment for matrilineal intrafamily or interfamily relatives, carrying the same deafness-associated mtDNA mutation (s). Thus, the phenotypic variability and penetrance of deafness associated with these mtDNA mutations may be modulated by other modifying factors, such as environmental factors, nuclear modifier genes and mitochondrial haplotypes.
Mutations such as 7471InsG, 7510T>C, 7445AN>G, 7505T>C and 7511T>C in the tRNA(UCN) gene, 4295A>G mutation in the tRNAt gene, and 12201T>C mutation in the tRNAgene[5,8-11], may change tRNA structure and functions, including modification of specific nucleotides, the processing of RNA precursors, aminoacylation and codon-anticodon interactions, maintaining the folded secondary and tertiary structures. Hence, the failure of protein synthesis and tRNA metabolism may lead to mitochondrial dysfunction associated with hearing damage. In order to further investigate the molecular mechanism of maternally transmitted deafness, we conducted an extended and systematic mutational screening of the tRNA gene in cohorts of hearing-impaired subjects. In the present study, we studied the clinical, molecular and genetic characterization of two Han Chinese pedigrees with maternally transmitted non-syndromic deafness. Three of the sixteen matrilineal relatives in these two families showed a variable severity and age-at-onset of hearing damage. The novel homoplasmic tRNA4268T>C mutation was identified by mutational analysis of the entire mtDNA, which disrupts a highly conservative base-pairing (6U-67A) on the ACC stem of tRNA. Also the 4268T>C mutational function value was evaluated by examining the steady-state levels of mitochondrial tRNA with cybrid cell lines carrying the 4268T>C mutation.
MATERIALS AND METHODS
Subjects and audiological examinations
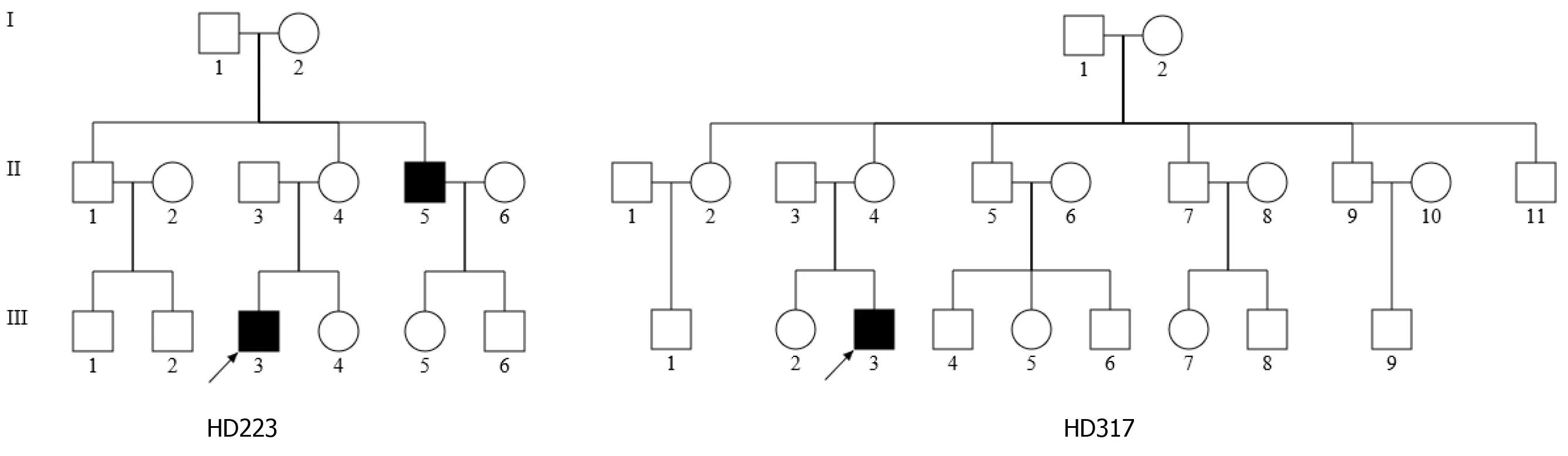
As part of the genetic screening program for hearing impairment, the two Han Chinese families, as shown in Figure 1, were examined at the Otology Department of the First Affiliated Hospital, Zhejiang University School of Medicine. A comprehensive history and physical examinations were performed to identify any syndromic findings, the history of the use of aminoglycosides, and genetic factors related to hearing impairment in members of this pedigree. An age-appropriate audiological examination was performed, which included pure-tone audiometry (PTA) and/or auditory brainstem response (ABR), immittance testing and distortion product otoacoustic emissions (DPOAE). The PTA was calculated from the sum of the audiometric thresholds at 500, 1000, 2000, 4000 and 8000 Hz. The severity of hearing impairment was classified into five grades: Normal < 26 decibel (dB); mild = 26-40 dB; moderate = 41-70 dB; severe = 71-90 dB; and profound > 90 dB. One hundred control DNAs were obtained from a panel of unaffected subjects of Han Chinese ancestry from the same region. Informed consent, blood samples, and clinical evaluations were obtained from all 36 subjects in these two families and 100 controls according to protocols approved by the Ethics Committee of Zhejiang University School of Medicine.
Mutational analysis of mitochondrial genome
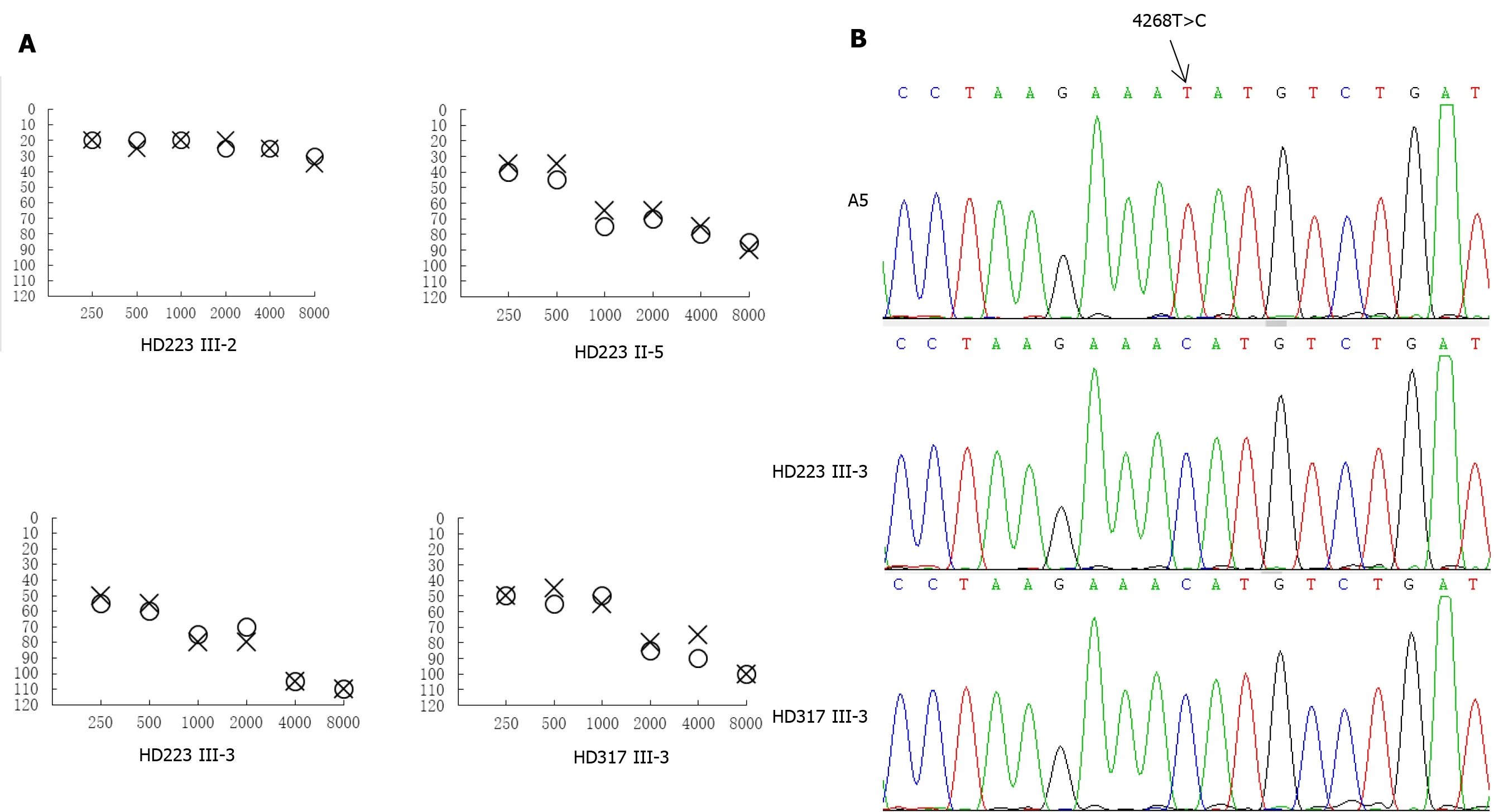
The Puregene DNA isolation kit (Biomega) was used to isolate total genomic DNA from the study participants, after which mitochondrial genomic DNA was assessedSouthern blotting as previously described[16]. A total of 24 overlapping PCR fragments were generated and amplified in order to provide full coverage of the mitochondrial genome, using appropriate pairs of light/heavy strand primers used in previous studies[17]. An ABI 3700 automated DNA sequencer was employed to sequence each of these fragments following purification with a Big Dye Terminator Cycle sequencing reaction kit. The revised Cambridge consensus sequence (GenBank accession number: NC_012920) was used for alignment of the sequenced fragments[16]. Detection of the 4268T>C mutation in family members and other controls was performed as previously described[18].
Mutational analysis of GJB2 gene
The DNA fragments spanning the entire coding region of GJB2 gene were amplified by PCR using the following oligodeoxynucleotides: forward: 5′-TATGACACT CCCCAGCACAG-3′ and reverse: 5′-GGGCAATGCTTAAACTGGC-3′. PCR amplification and subsequent sequencing analysis were performed as previously described[18]. The results were compared with the wild-type GJB2 sequence (GenBank Accession No. M86849) to identify the mutations.
Cell culture
For the family HD317, however, the proband III-3, was a 22-year-old man, who suffered from bilateral hearing damage since birth. As shown in Figure 2, auditory evaluation showed that he had severe deafness (79.2 dB in the right ear and 80 dB in the left ear), with a sloped-shaped pattern. Other members of this family showed normal hearing.
Well, said her godmother, be but a good girl,28 and I will contrive21 that thou shalt go. Then she took her into her chamber, and said to her, Run into the garden, and bring me a pumpkin22. 29
Northern blotting
This study had several limitations. First, this investigation was limited to a small number of patients from the same hospital. Second, the 4268T>C mutation from these Chinese families may be an inherited risk factor for the development of deafness, but may not lead to a clinical phenotype. The slight mitochondrial dysfunction, homoplasmic form, late onset, and incomplete penetrance of deafness may lead to eventual hearing loss. Finally, the involvement of nuclear modifier genes or tissue-specific RNA metabolism may be the cause of the tissue-specific effect of the 4268T>C mutation.
Assessment of ROS levels
Mutations in mitochondrial rRNA and tRNA genes have been identified as one of the most common reasons for sensorineural hearing loss. In this investigation, two Han Chinese pedigrees with maternally transmitted non-syndromic deafness were studied and the clinical, molecular and genetic characterization was investigated, which suggested maternally transmitted non-syndromic hearing impairment.
Statistical analysis
We identified the novel homoplasmic tRNA4268T>C mutation in two families,which was located at a highly conserved base-pairing (6U-67A) of tRNA. The abolishment of 6U-67A base-pairing likely changes tRNAmetabolism. Moreover, the proposed threshold of normal respiration in lymphoblastoid cells was more than the reduced tRNA level.
When the birds began to sing he could lie still no longer, and climbed out of his window into the branches of one of the great lime-trees that stood before the door
RESULTS
Clinical and genetic evaluation of two Han Chinese pedigrees with hearing loss
In the family HD223, the female proband (III-3) visited the otology clinic at the age of 28 years. As illustrated in Figure 2, auditory assessment showed that she had moderate-severe hearing loss (71.7 dB in the right ear and 67.5 dB in the left ear), with a sloped-shaped pattern. Moreover, her 48-year-old uncle (II-5) exhibited moderate hearing loss (66 dB and 61 dB in the right and left ears, respectively). Family history and clinical evaluations showed that the other members of this family had no hearing damage.
The Epstein-Barr virus was used to generate immortalized cell lines from the proband patient (HD223-III-3) bearing the 4268T>C mutation, as well as from a control individual (C2). These cells were cultured in RPMI 1640 containing 10% FBS. Cybrid cells were generated using previous protocols[19]. Briefly, bromodeoxyuridine (BrdU)-resistant 143B.TK− cells were cultured in DMEM containing 5% FBS, and the ρ206 cell line lacking mtDNA derived from these cells was also grown under these conditions in the presence of 50 μg uridine/mL. The patient and control cell lines were then enucleated and fused with the ρ206 cells. The resultant cybrid cell lines were selected in uridine-free DMEM supplemented with BrdU, allowing for donor-derived cybrid lines that could then be assessed for the 4268T>C mutation, amounts of mtDNA, and other cellular genetic features. The resultant cybrid lines were maintained in DMEM containing 10% FBS.
If any youth came within a hundred paces of the castle, he was obliged to stand still, and could not stir from the spot till she set him free; but if a pretty girl came within this boundary, the old enchantress changed her into a bird, and shut her up in a wicker cage, which she put in one of the rooms in the castle
Analysis of mitochondrial mutations
We sequenced the mitochondrial genome of these two probands to explore potential mitochondrial mutations linked with deafness. Altogether there were 34 evident mutations in the mitochondrial genome compared with the revised Cambridge consensus sequence (NC_012920). The mitochondrial haplotypes of these two patients belonged to the same D4j. These variants were evaluated for pathogenicity using the following criteria: (1) Potential structural and functional alterations; (2) Absence in the controls; (3) Conservation index (CI) from other 16 vertebrates > 75%; (4) Missense mutation; (5) Pedigree analysis. As shown in Table 1, 12 of 34 variants were known silent variants, seven of them were known D-loop variants, nine of them were known missense mutations affecting protein-coding genes, four of them were known 12S rRNA variants, one of them was a known 16S rRNA variant, and one was a novel homoplasmy 4268T>C mutation in the tRNAgene (Figure 2). Currently, the detected missense mutations were as follows: 4048G>A (Val260) in the ND1 gene, 8860A>G (Thr112Ala) in the ATP6 gene, 9612G>C (Val136Leu) in the mitochondrial CO3 gene, 11696G>A (Val313) in the mitochondrial ND4 gene, 13708G>A (Ala458Thr), 14766C>T (Thr7) and m.15326A>G (Thr>Ala) in the mitochondrial CYTB gene, 13928G>C (Ser531Thr) and 14002A>G (Thr556Ala) in the ND5 gene. The difference in these mutated RNA residues was compared across 16 different primate species, which showed that the tRNA4268T > C mutation had a 100% CI across species, increasing its likelihood of functional significance after mutation, as shown in the patient. Moreover, we did not detect this mutation when 20 unrelated other patients and 100 Chinese control subjects were assessed.
Mutation leads to decreased mitochondrial tRNAIle levels
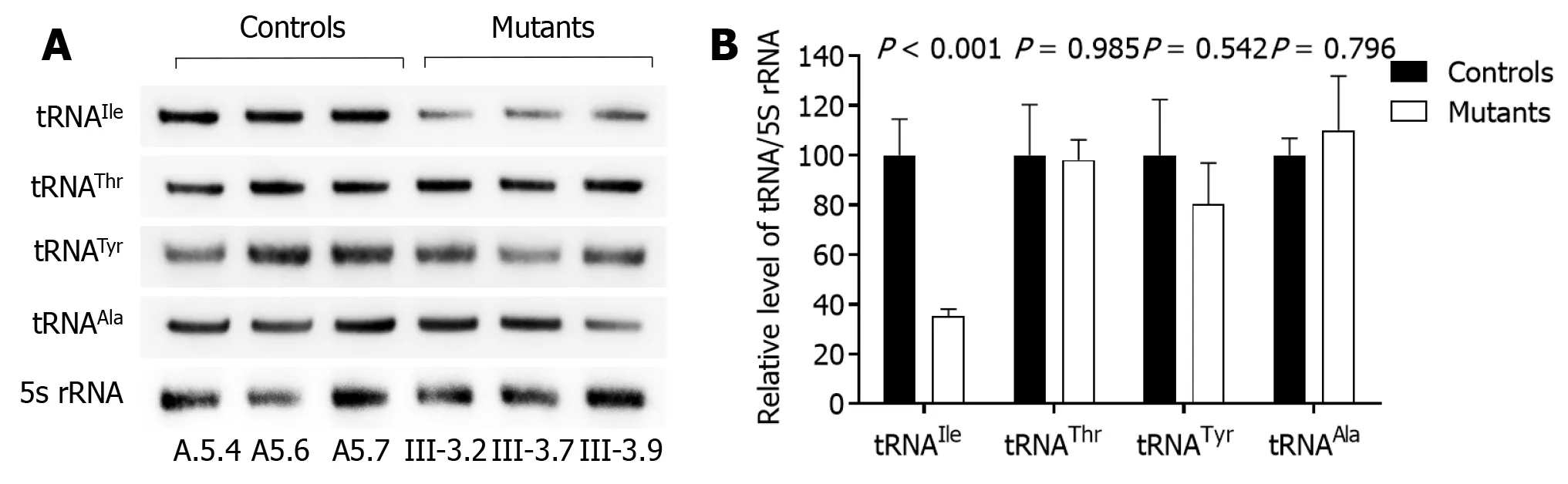
We examined how the 4268T>C mutation changed the metabolism of tRNA, which bears this mitochondrial mutation by subjecting cybrid cell lines to Northern blotting with probes specific to it and three other tRNAs. As shown in Figure 3, tRNAlevels in the mutant cybrid lines were obviously reduced compared with control wild-type cells, with baseline tRNAlevels in the mutant cells about 64.6% compared to those in control cells. The 5S RNA was applied for normalization. As a contrast, baseline tRNA, tRNA, tRNA, and tRNAlevels in the mutant cell lines were consistent with control cells (98.0, 80.4, and 110.1%, respectively).

Mutation contributing to increased ROS production
16.Both flew in brightness and in joy so high, so very high, and then above was neither cold, nor hunger, nor anxiety--they were with God: Andersen s religious beliefs included a firm belief that the innocent enter God s presence at death where there is no more suffering.Return to place in story.
Mutational analysis of GJB2 gene
We conducted mutational screening of the GJB2 gene in seven affected and two unaffected matrilineal relatives of the Chinese family to assess the role of GJB2 gene in phenotypic expression of 4268T>C mutation. We found none of the variants in GJB2 gene in these matrilineal relatives of the Chinese pedigree. We found that the GJB2 gene could not be a modifier of the phenotypic effects of the 4268T>C mutation in the subjects, as none of the GJB2 gene variants in the subjects with hearing loss was verified.
DISCUSSION
Hearing loss is one of the major sensory disabilities worldwide, which is often due to the damage of spiral ganglion neurons or sensory hair cells in the inner ear[23-25]. Hearing loss can be caused by gene alterations, aging and environmental factors, with chronic cochlear infections, noise exposure, ototoxic drugs, and genetic factors accounting for more than 60% of the cases with hearing loss[26-31]. Numerous genes are known to cause syndromic hearing loss[32-37]. In the present study, we examined the clinical, genetic, biochemical and molecular characterization of two Chinese families with non-syndromic deafness. Only two pedigrees from the members had hearing loss with a single clinical phenotype. There was also a possible maternal inheritance of deafness in the families, except for two possible etiological types. The tRNA4268T>C mutation was identified to belong to the Asian haplogroup D4j through the mutational analysis of mtDNA from the families. The 4268T>C mutation resided at position 6 of the ACC stem in the tRNAgene, and this position is very important for the structure and function of tRNA. A relationship was found between T>C transition at position 6 of tRNA(9996T>C), tRNA(5821G>A) and human diseases[5,6]. It is known that 7472insC, 7510T>C, 7511T>C, 7445A>G and 7445A>C mutations in the tRNA gene are associated with hearing loss[5,8-11]. Similarly, m.5587T>C mutation in the tRNAgene was associated with Leber’s hereditary optic neuropathy[38]. The altered secondary tRNAstructure caused by the 4268T>C mutation was thought to have led to a failure in tRNA metabolism. In the present study, an approximately 64.6% decrease in the tRNAlevel observed in cell lines with the mutation was consistent with our previous investigations, which showed the 4263A>G mutation at the ACC stem of tRNAin the reduced levels of tRNAin the cell lines. The mutant tRNAmay be less stable metabolically and easier to degrade, which can lower the level of tRNAs.
The involvement of nuclear genes in the phenotypic manifestation was reflected by the phenotypic variability of matrilineal relatives amongst families. To date, over 140 loci have been mapped for non-syndromic deafness, and 82 deafness-causing genes have been identified (The Hereditary Hearing loss homepage. http://hereditaryhearingloss.org). GJB2 is one of the most common disease-causing genes in hereditary deafness worldwide, and the coding region comprises the majority of known GJB2 sequence variations (The Connexin-deafness homepage. http://davinci.crg.es/deafness). Nevertheless, other nuclear modifier genes may have contributed to the phenotypic variability and tissue-specific effect in this Chinese family in view of the absence of GJB2 mutation.
ROS are mainly generated from leaking electrons in the mitochondrial electron transport chain. Under normal conditions, the balance between production and scavenging of ROS is subtly and dynamically maintained. Previous studies have reported that many stress stimulators such as excessive and persistent noise exposure, ototoxic drugs including cisplatin and aminoglycosides, and mitochondrial dysfunction can increase ROS production in hair cells[35,39-41], and eventually lead to hair cell apoptosis [42-47]. In this study, consistent with previous reports, we found that cybrids expressing the 4268T>C mutation elevated ROS production. Cellular macromolecules such as DNA and proteins can be disrupted by ROS production, which potentially lead to cellular dysfunction or asbestosis. As previously described for deafness-associated mitochondrial 7511A>G mutation, it could also potentially be attributed to the known deafness phenotype[48].
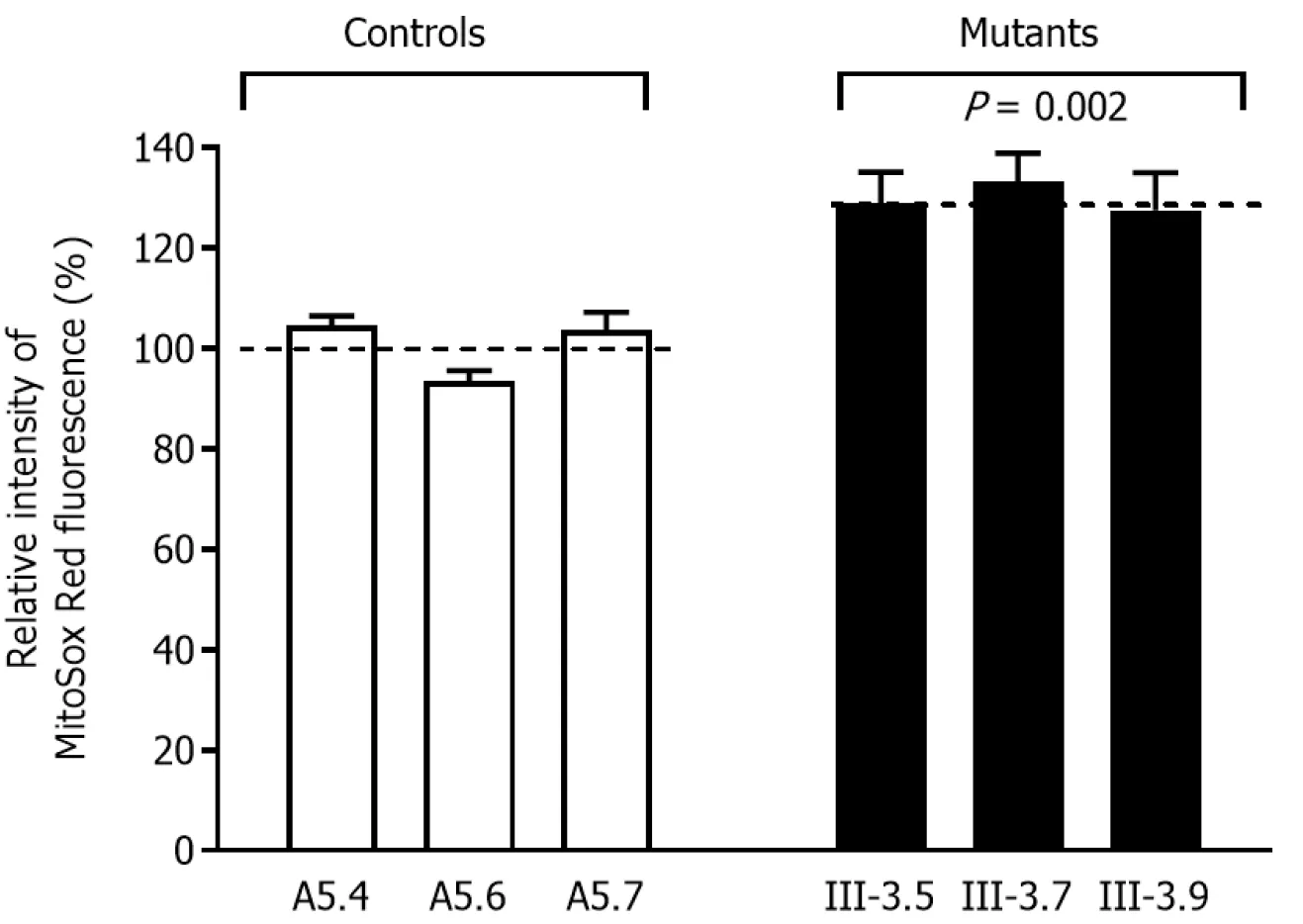
In order to obtain a ratio corresponding to ROS generation, we assessed ROS production at the mutant cybrid cell linesflow cytometry, in contrast to baseline staining intensity for every cell line after oxidative stress. As shown in Figure 4, slightly increased ROS generation was observed in the mutant cybrid cell lines with the 4268T>C mutation, and with ROS production 127.6-130.1% (average: 130.0%) for control cells.
These two Chinese families carrying the 4268T>C mutation with the maternal lineages had wide phenotypic variability. There was 33.3% and 10% penetrance of deafness in these Chinese families, respectively, which was similar to the Scottish family carrying the m.7445A>G mutation and some reported Chinese families with the m.1555A>G mutation with low penetrance of deafness[49,50]. The average age at deafness was 32.6 years (22-45 years) for matrilineal relatives with previous features. As a comparison, the average age was approximately 15 and 20 years old for 69 Chinese families and 19 Spanish families carrying the 1555A>G mutation[51,52], respectively, for those deaf individuals without aminoglycoside exposure, while some matrilineal relatives in a large Arab-Israeli family exhibited congenital profound deafness. Environmental factors, nuclear and mitochondrial genetic backgrounds and other modifiers may participate in slight mitochondrial dysfunction, which leads to late-onset hearing damage, similar to the homoplasmic nature of 4268T>C mutation in the phenotypic manifestation. Mitochondrial haplogroup did not play an important role in the phenotypic expression of functionally significant variants in the mtDNA. In addition, the phenotypic variability and tissue-specific effect may be caused by the nuclear modifier genes that were in synergy with the 4268T>C mutation. Alternatively, the deafness phenotype may develop due to tissue-specific disparity in tRNA metabolism.
CONCLUSION
The current study provides clinical, genetic, molecular and biochemical evidence that the tRNA4268T>C mutation may be related to late-onset deafness. Nevertheless, nuclear modifier genes or tissue-specific tRNA metabolism could cause the tissuespecificity of this variant. Also, the future molecular diagnosis of deafness could include the inherited factors of RNA4268T>C mutation, which may provide a new perspective for understanding the pathophysiology, management and treatment of maternally inherited deafness. Future research should include emerging linkages and their cause-effect relationship between deafness and mitochondrial dysfunction.
14.A pair of boots: Why boots? No reason is given for the cat s request for fancy footwear in Perrault s version of the tale. The two literary variants of the tale that predate Perrault, Straparola s Constantino Fortunato and Basile s Gagliuso, (also know as Caglioso) do not include boots in the story. Perrault s is the first literary version to include the boots. Some scholars believe the boots are not Perrault s invention but came from the French oral sources he had heard. Either way, Perrault s version gained such popularity that it later influenced other oral and literary versions with additions of the boots and the ogre we meet later in the story. In the end, the boots give Puss a sign of distinction and a level of respect. Jack Zipes reminds us that even during the time of this tale that clothes make the person (Zipes 2001, 390).
TRIzol was used to isolate total mitochondrial RNA from knockdown or control cell lines, as previously described[20]. Briefly, 2 μg of total mitochondrial RNA was electrophoresed through a 10% polyacrylamide/7 M urea gel in Tris-borate-Ethylenediaminetetraacetic acid (EDTA) buffer (after heating the sample at 65 °C for 10 min), and then electroblotted onto a positively charged nylon membrane for the hybridization analysis with oligodeoxynucleotide probes. This was followed by transfer onto a positively charged membrane, which was then combined with appropriate DIG oligodeoxynucleoside probes as previously described[20], using tRNA, tRNA, tRNA, and tRNAutilized in previous studies[21].
ARTICLE HIGHLIGHTS
Statistical analysis was performed using the Student’s unpaired, two-tailed-test in the SPSS version 17.0 program. Unless indicated otherwise, avalue < 0.05 was considered statistically significant.
Research background
The MitoSOX Red Mitochondrial Superoxide Indicator (Invitrogen, M36008) was used to assess reactive oxygen species (ROS) production in live cells based on a previously described protocol[22]. Briefly, approximately 2 × 10cells of each cell line were harvested, resuspended in PBS, supplemented with 100 μM of MitoSOX, followed by incubation at 37 °C for 20 min. The cells were then resuspended. Samples were analyzed by flow cytometer, with an excitation at 488 nm and emission at 529 nm, and 10000 events were analyzed in each sample.
Research motivation
In the present study, two Han Chinese pedigrees with maternally transmitted nonsyndromic deafness were studied and the clinical, molecular and genetic characterization was investigated. Mutational analysis of the entire mtDNA identified the novel homoplasmic tRNA4268T>C mutation, which disrupts a highly conservative basepairing(6U-67A) on the ACC stem of tRNA. The steady-state levels of mitochondrial tRNA were detected with cybrid cell lines for functional significance of the 4268T>C mutation.
Research objectives
To investigate the pathophysiology of hearing loss associated with mitochondrial tRNA mutations.
Research methods
Sixteen subjects from two Chinese families with deafness were assessed using clinical,genetic, molecular, and biochemical techniques. We measured the tRNA levels in lymphoblastoid cell lines derived from three control subjects and five affected matrilineal relatives of the families.
Research results
Variable severity and age-at-onset (8 years) of deafness were exhibited by three of the 16 matrilineal relatives from the families. The novel homoplasmic tRNA4268T>C mutation was identified by mutational analysis of mtDNA in two families that belonged to haplogroup D4j. The base-pairing (6U-67A) of tRNAwas a highly conserved gene where the 4268T>C mutation was located. The metabolism of tRNAcould be changed by the abolishment of 6U-67A base-pairing. Compared with the wild-type cell lines, the lymphoblastoid cell lines with the 4268T>C mutation were observed at the level of tRNAwith an approximately 64.6% reduction. Normal respiration in lymphoblastoid cells requires a higher threshold level than this reduced tRNA level. However, genotyping analysis did not detect any mutations in the prominent deafness-causing gene GJB2 in any members from the family.
I know. Let s go home. And home we went. All of my fear was gone. There would still be pain and trials that I could not even imagine. But I had a strong, loving family that I knew would always be there for me. Most of all, I was still Daddy s little girl, and armed with that knowledge, there wasn t a mountain I couldn t climb or a storm I couldn t weather.
Research conclusions
The novel tRNA4268T>C mutation may lead to maternally transmitted deafness.However, the phenotypic variability in the family may also be derived from epigenetic, other genetic, or environmental factors. These results may be of value in counseling, especially for families with maternally inherited deafness.
That year the Fort Wayne mayor officially proclaimed16 December 21 as Amy Jo Hagadorn Day throughout the city. The mayor explained that by daring to make such a simple wish, Amy taught a universal lesson.
When one of his comrades heard what had happened, he said, You blockhead, you can t have done it properly; just let me have a try, and with these words he seized his wife by the roots of her hair, cut her throat with a razor, and then took the pipe and blew into it with all his might but he couldn t bring her back to life
Research perspectives
Future molecular diagnosis of deafness can include the tRNA4268T>C mutation as one of the inherited risk factors. Thus, the results may have important value in deafness counseling, especially for families with maternally inherited deafness.
ACKNOWLEDGEMENTS
We thank all the people who participated in the study, including members of the two families.
World Journal of Clinical Cases2022年1期
- World Journal of Clinical Cases的其它文章
- Hepatitis B virus reactivation in rheumatoid arthritis
- Paradoxical role of interleukin-33/suppressor of tumorigenicity 2 in colorectal carcinogenesis: Progress and therapeutic potential
- Changes in rheumatoid arthritis under ultrasound before and after sinomenine injection
- Benefits of multidisciplinary collaborative care team-based nursing services in treating pressure injury wounds in cerebral infarction patients
- Outcomes and complications of open, laparoscopic, and hybrid giant ventral hernia repair
- Surgical resection of intradural extramedullary tumors in the atlantoaxial spine via a posterior approach
