Application of SOAT1 combined with multiple markers in the auxiliary diagnosis of hepatocellular carcinoma
ZHENG Jing, WEI Shang, DENG Hui-zi, NIU Hai-yan✉
1. Department of Pathology, the First Affiliated Hospital of Hainan Medical University, Haikou 570000, China
2. Department of Pathology, Hainan Medical University, Haikou 570000, China
Keywords:Hepatocellular carcinoma SOAT1 Immunohistochemistry Pathological diagnosis
ABSTRACT Objective: To explore the expression of sterol oxygen-acyltransferase 1 (SOAT1) in hepatocellular carcinoma tissues and to evaluate the application value of its combination with various markers (HSP70, GS, CD10, CD34) in the diagnosis of hepatocellular carcinoma.Methods: Totally 58 cases of liver cancer tissue and its corresponding adjacent tissue, and 12 cases of benign liver lesions tissue were collected, and tissue chips were made to detect the expressions of SOAT1, HSP70, GS, CD10 and CD34 (immunohistochemical staining method) which were analyzed by scientific method. Results: The expression of SOAT1 was up-regulated in hepatocellular carcinoma tissues, and the difference was statistically significant (P <0.01), and the expression level was closely related to the differentiation degree of hepatocellular carcinoma patients (P<0.05). SOAT1 was correlated with the expression of HSP70, GS, and CD34, with statistical significance (P<0.01). Positive percent agreement for the hepatocellular carcinoma was 100.00% for CD34, 89.66% for GS, 82.75% for HSP70,63.79% for CD10, and 63.79% for SOAT1; negative percent agreement (paracancerous tissues)was 100% for SOAT1, 98.27% for CD10, 96.55% for HSP70, 34.48% for CD34, and 5.17%for GS; Negative percent agreement (benign tissues) was 100% for SOAT1, 83.34% for CD10,100.00% for HSP70, 8.33% for CD34, and 0% for GS, respectively. Taking the positive expression of any three markers as the diagnostic criteria, 39 of the 58 cases of hepatocellular carcinoma could be diagnosed, and the diagnostic rate was 67.24% (SOAT1 was not included in the list of markers). If SOAT1 is included, 48 of the 58 hepatocellular carcinoma cases could be diagnosed, and the diagnosis rate could be increased to 82.76%. Conclusions: The expression level of SOAT1 was significantly up-regulated in hepatocellular carcinoma, and the expression level was higher in poorly differentiated HCC, suggesting that SOAT1 can be used as one of the indicators for the diagnosis and prognosis of hepatocellular carcinoma. Among the five markers above, CD34 and GS were more sensitive, while the specificity is better for SOAT1, CD10 and HSP70. The combined application of SOAT1 with HSP70, GS, CD10 and CD34 has certain application value in the diagnosis of hepatocellular carcinoma.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. The early detection and treatment is very important for the prognosis of patients with hepatocellular carcinoma[1]. Due to the lack of specific clinical symptoms,early small HCC is difficult to be detected and the clinical diagnosis often relies on the pathological diagnosis of needle biopsy[2]. However, the complexity and variability of liver cancer histomorphology can also make pathological diagnosis difficult.Well differentiated hepatocellular carcinoma often needs to be differentiated from normal liver tissue or benign liver nodular lesions. And poorly differentiated hepatocellular carcinoma needs to be differentiated from intrahepatic cholangiocarcinoma and metastatic adenocarcinoma. Therefore, immunohistochemical detection plays an important role in the auxiliary diagnosis of hepatocellular carcinoma. In the clinical application of various immunohistochemical staining antibodies, the use of only one antibody cannot meet the needs of diagnosis. Especially in the identification of benign and malignant hepatocellular proliferative lesions, which requires a combination of multiple antibodies to diagnose [3]. Relevant studies have shown that CD10, HSP70, GS and CD34 are of significance in the differential diagnosis of welldifferentiated HCC, focal nodular hyperplasia of the liver, liver cirrhosis and other benign liver diseases[4, 5]. However, in practical work, we often encounter situations in which the expression of markers does not match the morphology or the expression of markers is inaccurate and cannot play a role in differential diagnosis.And it is difficult to unify the specificity and sensitivity of the above markers in the diagnosis of hepatocellular carcinoma. Could a new marker be introduced as a key addition to the diagnostic markers for HCC? Sterol O-acyltransferase (SOAT), also known as acyl-CoA:cholesterol acyltransferase (Acyl-CoA: cholesterol acyltransferase,ACAT), is a key enzyme in cholesterol esterification[6]. Several studies have shown that SOAT1 is up-regulated in HCC and promotes tumor cell proliferation by altering the cholesterol homeostasis in tumor cells[7, 8]. In this study, the author attempted to explore the application value of SOAT1 combined with multiple markers in the diagnosis of hepatocellular carcinoma.
2. Materials and methods
2.1 Materials
Formaldehyde-fixed paraffin-embedded samples were collected from the First Affiliated Hospital of Hainan Medical College from August 1st, 2019 to October 30th, 2021, including HCC and paired adjacent liver tissue samples, a total of 58 cases. And 12 cases of benign liver lesions (including liver focal hyperplasia nodules and hepatic adenoma) were also collected.
2.2 Reagents
SOAT1 primary antibody was purchased from Thermo Fisher Scientific, USA, HSP70, GS, CD10, CD34 primary antibodies and immunohistochemical detection reagents were purchased from Fuzhou Maixin Reagent Company.
2.3 Methods
2.3.1 Making tissue microarray
A complete blank wax block without cracks was made first, and then the tissue location holes were drilled. The HCC tissues, adjacent liver tissues and benign liver lesions were placed in the blank wax block that had been drilled in sequence, and gastric mucosa tissues were selected as markers at appropriate positions. The surface of the wax block is drenched with hot wax to seal the edges, and the mold is placed in an oven (60 ℃) for 1h. Place at room temperature for about 10 min, then unmold after fully freezing in the refrigerator [9].
2.3.2 Immunohistochemical staining and result interpretation
Using the Envision System two-step method for immunohistochemical staining, the primary antibody SOAT1 dilution ratio was 1:50. The interpretation of immunohistochemical results is based on literature [10]. Details are shown in Table 1: staining score =staining expression intensity positive expression range. The scoring criteria are as follows: below 2 points are negative (-), 2 points and above are positive (+).

Tab 1 Interpretation criteria of SOAT-1 immunohistochemical results
According to the following four antibody expression patterns of HSP70, GS, CD10, and CD34[4, 5] , the negative or positive judgment is made.
HSP70 positive expression pattern: The cytoplasm and nucleus of cancer cells were diffusely stained with yellow or (and) brownyellow granules. HSP70 negative expression pattern: Cytoplasm was not stained, and the nucleus was light blue.
GS positive expression pattern: Cancer cells are mainly diffuse stained with varying intensity or no coloring at all; GS negative expression pattern: expressed in the cytoplasm of hepatocytes around the central vein, showing yellow or (and) brown-yellow granules staining. The cytoplasm of hepatocytes away from the central vein is not stained, and the nuclei are light blue.
CD10 positive expression pattern: decreased expression, no expression or strong expression of capillary bile ducts in HCC tissues; CD10 negative expression pattern: the cell membrane or cytoplasm of the capillary bile ducts between hepatocytes showed tubular, branched, comma-like yellow or (and) brown stained.
CD34 positive expression pattern: It is expressed in the vascular endothelial cells between the trabecular cords of cancer cells, and it is evenly distributed in the form of fissures or branches; CD34 negative expression pattern: Normal liver sinusoids do not show color.
All immunohistochemical results were independently interpreted by 3 senior pathologists, and the interpretation results of 3 pathologists were combined as the final result.
2.3.3 Data analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 software. Some theoretical frequencies in the chi-square test are less than 5, which are prone to deviations. Therefore, Fisher exact probability test is used for statistical analysis and P<0.05 is considered to be statistically significant. In this study, the sensitivity and specificity of various markers could not be assessed. The alternative method is to evaluate the positive percent agreement(PPA) of each marker immunohistochemical staining in HCC and the negative percent agreement (NPA) in non-HCC tissues according to the literature [11]. PPA is similar to sensitivity, while NPA is similar to specificity.
3. Results
3.1 SOAT1 protein expression
Immunohistochemical detection of SOAT1 was performed on the tissue chip, and it was found that SOAT1 was expressed in the cytoplasm and membrane of HCC tissues (Figure 1); 37 of 58 HCC tissues were positive for SOAT1. The positive expression rate was 63.79%. There was no SOAT1 expression in the 58 paired adjacent tissues, and the negative expression rate was 100%. The expression level of SOAT1 protein in cancer tissues was significantly upregulated, P<0.001(Table 2). There was no expression of SOAT1 in the 12 cases of benign liver lesions, and the negative expression rate was 100%(Table 3). And the expression of different degrees of differentiation of HCC is different. In well-differentiated HCC,SOAT1 generally showed low expression or no expression (Figure 1A), which meant the staining intensity was weak and only a few tumor cells were stained. SOAT1 generally showed high expression in poorly differentiated HCC (Figure 1B), which meant the staining intensity is strong and a large number of tumor cells are stained.
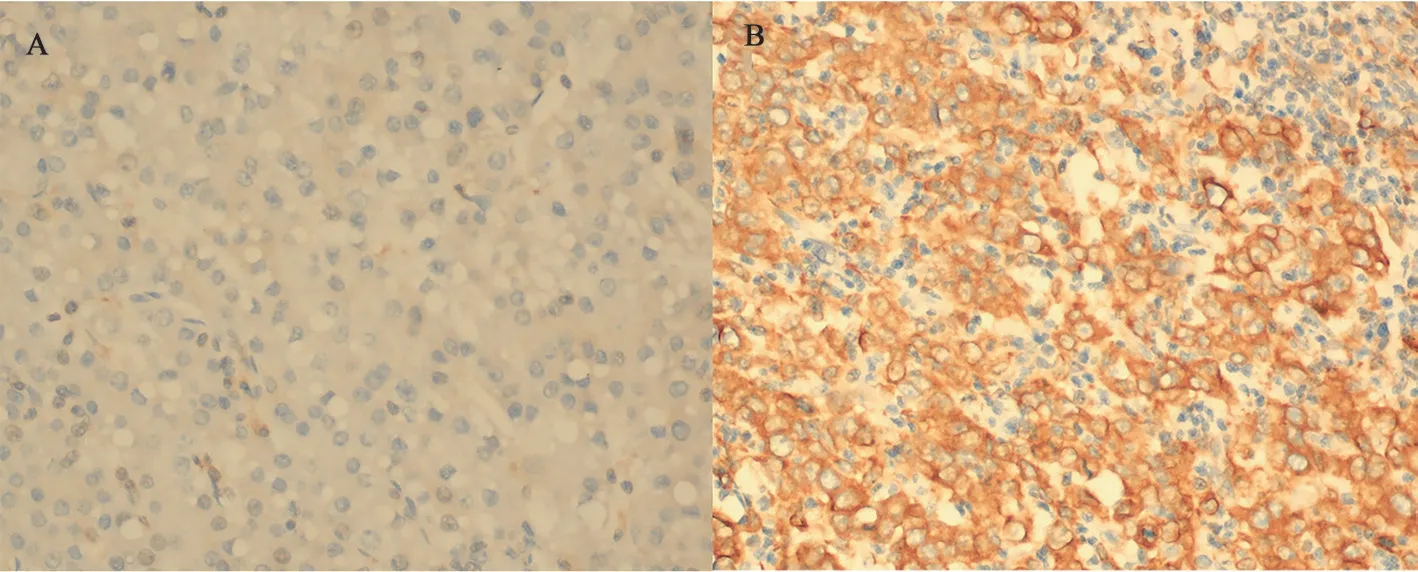
Fig 1 Expression of SOAT1 in hepatocellular carcinoma with different degrees of differentiation (×400)
3.2 Expression of HSP70, GS, CD10, CD34 and their relationship with expression
SOAT1 expressions of four markers, HSP70, GS, CD10, and CD34 in 58 hepatocellular carcinoma tissues are as follows (Figure 2 and Table 2): There were 48 cases of HSP70, 52 cases of GS, 37 cases of CD10 and 58 cases of positive expression among total 58 cases.And the PPA was 82.75%, 89.66%, 63.79% and 100% respectively.The four positive expression patterns were as previously described in materials and methods. (Fig 2 A-D). The expression of the above four markers in the paired paracancerous tissues of 58 cases was shown in Figure 2 and Table 2, and the negative expression pattern was also as described in the previous parts. The NPA of HSP70,GS, CD10 and CD34 was 96.55%, 5.17%, 98.27% and 34.48%respectively. The expressions of HSP70 and CD34 in HCC tissues were higher than those in the corresponding paracancerous liver tissues (P<0.001, Table 2). But the expression of CD10 in HCC tissues was lower than that in the corresponding paracancerous liver tissues (P<0.001, Table 2). While there was no significant difference in the expression of GS in HCC tissues and corresponding adjacent liver tissues (P=0.490, Table 2).

Tab 2 Expression of five markers in hepatocellular carcinoma and adjacent liver tissues(n=58)
In 12 cases of benign liver lesions, the NPA of was HSP70, CD10 and CD34 was 100.00%, 83.34% and 8.33%. All GS showed positive expression, and the NPA was 0%. The statistical analysis showed that the expression level of HSP70 in HCC tissue was higher than that in benign liver lesion tissue (P<0.001, Table 3) and the expression level of CD10 in HCC tissues was lower than that in benign liver lesions (P=0.004, Table 3), while the expression levels of GS and CD34 in HCC tissues were not significantly different from those in benign liver lesions (P=0.309, P=0.171, Table 3).
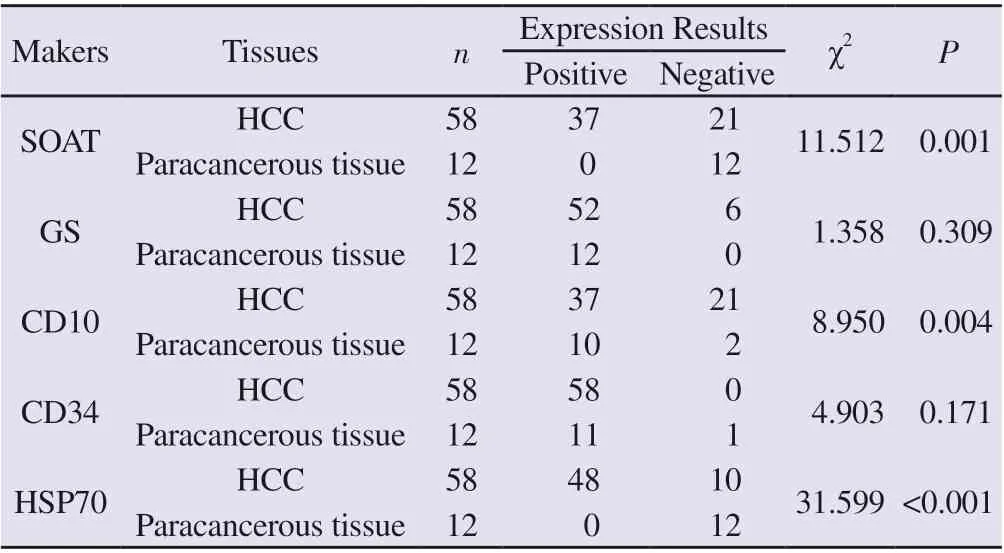
Tab 3 Expression of five markers in liver tissue of hepatocellular carcinoma and benign lesions
In hepatocellular carcinoma, SOAT1 expression had a certain correlation with the expression of HSP70, GS, and CD34 respectively (P=0.001, P<0.001, P<0.001, Table 4), but there was no clear correlation between SOAT1 and CD10 expression (P= 0.092,Table 4). Two of the 10 HSP70-negative hepatocellular carcinoma cases had high SOAT1 expression, 2 of the 6 GS-negative cases had high SOAT1 expression, and 10 of the 21 CD10-negative hepatocellular carcinoma cases had high SOAT1 expression.Among the above markers, if considering any of the three markers excluding SOAT1 positive expression as the diagnostic criteria, 39 of the 58 cases of hepatocellular carcinoma could be diagnosed,and the diagnostic rate was 67.24%. If SOAT1 was included in the list of markers, and also considering any of the three markers positive expression as the diagnostic criteria, 48 of the 58 cases of hepatocellular carcinoma can be diagnosed, and the diagnostic rate can be increased to 82.76%.
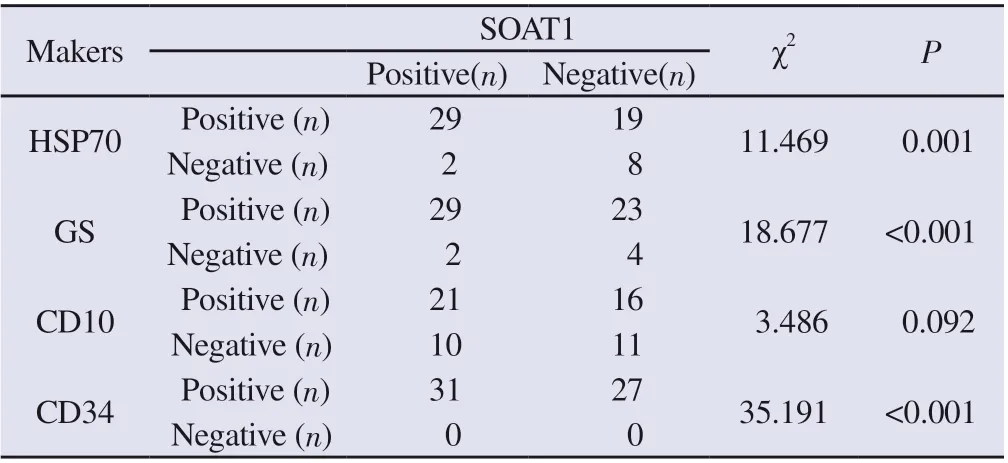
Tab 4 Relationship between SOAT1 and the expression levels of HSP70, GS, CD10 and CD34 in hepatocellular carcinoma
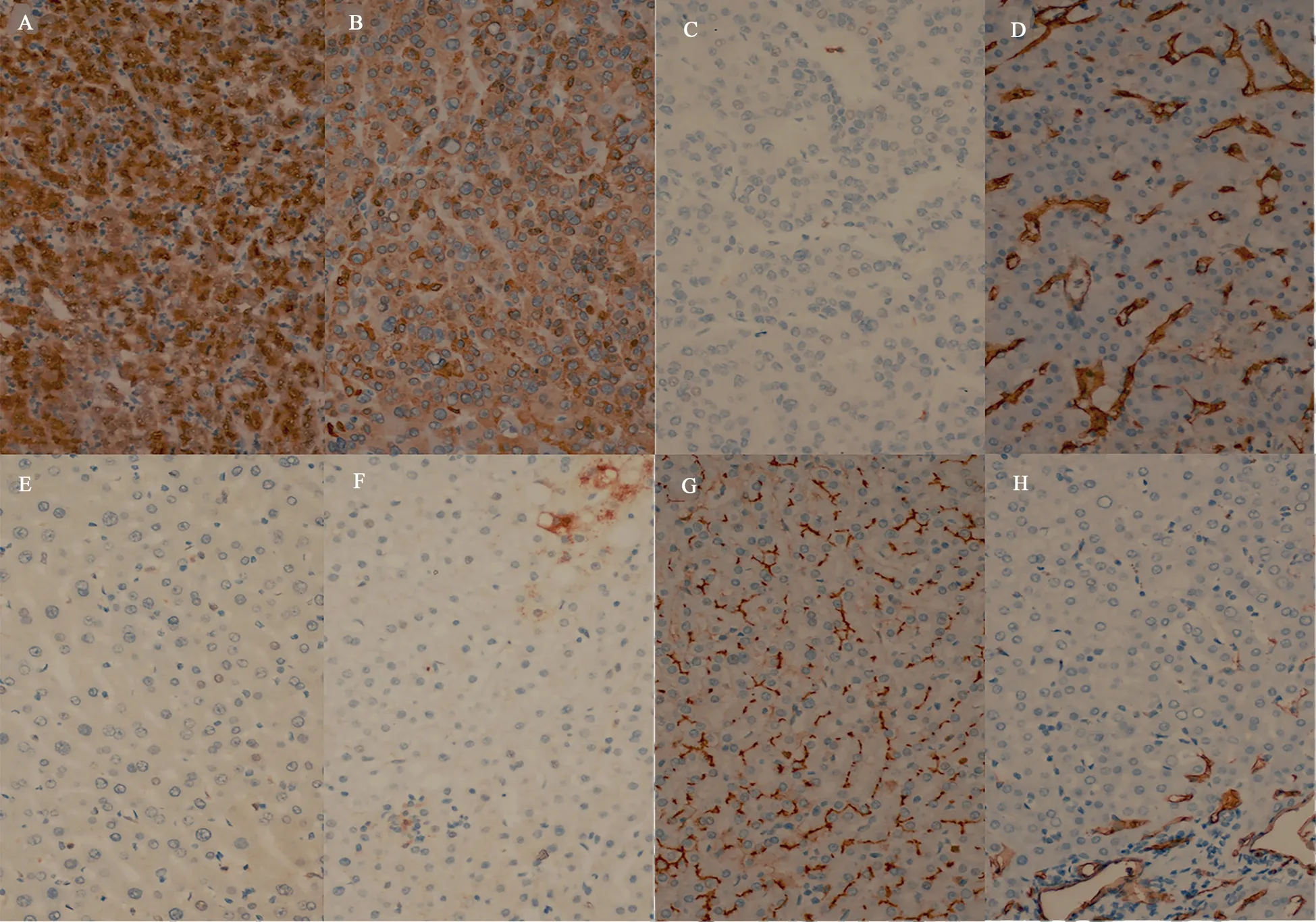
Fig 2 Expression of HSP70, GS, CD10, CD34 in paracancerous tissue and hepatocellular carcinoma (×400)
4. Discussion
Primary liver cancer is one of the most common malignant tumors worldwide. Globally, the incidence and mortality of primary liver cancer remain high, and the disease has a serious impact on all human beings [12, 13]. Asia, where China is located, is one of the regions with a high incidence of primary liver cancer in the world[14], and its incidence has increased in recent years. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for about 85% to 90% of all liver cancers [12, 15].Relevant studies have shown that the 5-year survival rate of patients with hepatocellular carcinoma in China is only 14%, and the 5-year recurrence rate after surgery is as high as 70% [16]. For the prognosis of patients with hepatocellular carcinoma, an important link is early detection and early treatment. Small hepatocellular carcinoma in the early stage is often difficult to be detected. When clinical symptoms are present, it is already in the middle and late stages. The clinical diagnosis often depends on the pathological diagnosis of needle biopsy, but the histological morphology of hepatocellular carcinoma is often complex and changeable. Well differentiated hepatocellular carcinoma often needs to be differentiated from normal liver tissue or benign liver nodular lesions. And poorly differentiated hepatocellular carcinoma needs to be differentiated from intrahepatic cholangiocarcinoma and metastatic adenocarcinoma. The most commonly used adjunctive technique for differential diagnosis is immunohistochemical staining. In the clinical application of various immunohistochemical staining antibodies, relying on the expression of one antibody alone to confirm the diagnosis is unreliable,especially in the differential diagnosis of hepatocellular carcinoma and benign liver lesions. It is necessary to combine the expression results of multiple antibodies to assist diagnosis [17].
Relevant study has shown that CD10, HSP70, GS and CD34 are of significance in the differential diagnosis of well-differentiated hepatocellular carcinoma and focal nodular hyperplasia of the liver and liver cirrhosis and other benign liver diseases [18]. The Guidelines for Standardized Pathological Diagnosis of Primary Liver Cancer (2015) [17] also recommends the use of the above markers for differential diagnosis. However, in practical work, we often encounter situations in which the expression of markers does not match the morphology or the markers cannot play a role in differential diagnosis. We were unable to assess the sensitivity and specificity of each of the above markers. An alternative approach in this study was to evaluate the positive percent agreement (PPA)of immunohistochemical staining for each marker in HCC versus the negative percent agreement (NPA) in non-HCC tissues based on literature. PPA is similar to sensitivity, while NPA is similar to specificity. In our study, PPA in hepatocellular carcinoma was:CD34 100.00%, GS 89.66%, HSP70 82.75%, CD10 63.79%, while in adjacent tissue, NPA was CD10 98.27%, HSP70 96.55%, CD34 34.48%, respectively, GS 5.17%. NPA in benign liver lesions was HSP70 100.00%, CD10 83.34%, CD34 8.33%, GS 0%, respectively.The sensitivity of CD34 and GS is good, but the specificity of the two is poor; while the specificity of CD10 and HSP70 is good, but the sensitivity of the two is lacking. Therefore, it is difficult to unify the sensitivity and specificity of the above four markers. In addition,the expression of the above four markers in three different tissues was compared in our study. The expression of HSP70 is basically negative in adjacent liver tissue and benign liver lesions, which is significantly different from that in hepatocellular carcinoma.The expression rate of CD10 in adjacent liver tissue and benign liver lesions was higher than that in hepatocellular carcinoma. The vascular expression of CD34 in hepatocellular carcinoma and benign liver lesions is not much different, but the expression in adjacent liver tissue is less. There was no significant difference in the expression of GS among the three tissues. The results showed that CD34 and GS could not distinguish hepatocellular carcinoma from benign liver lesions very well. The results well explained the aforementioned problems encountered in the routine pathological diagnosis of hepatocellular carcinoma. Based on the findings of this study and the problems encountered in the actual diagnosis work, we propose whether a new auxiliary diagnostic marker can be introduced as a key supplement in the diagnosis of hepatocellular carcinoma.
SOAT1 is a key enzyme in cholesterol esterification, which can esterify free cholesterol on the endoplasmic reticulum to form cholesterol lipids and store them in lipid droplets, thereby maintaining cholesterol homeostasis. Therefore, SOAT1 plays an important role in intracellular cholesterol balance [19]. Jiang’s team[7] used proteomics to study early hepatocellular carcinoma and hepatitis B virus infection-related hepatocellular carcinoma tissues and adjacent liver tissues, and found that a group of tumor tissues highly expressed SOAT1 and had cholesterol homeostasis in tumor cells destroyed. They also used SOAT1 inhibitor (avasimibe) to treat the mouse model of hepatocellular carcinoma cells transplanted,and the results showed that the tumor size of the SOAT1 high expression group was significantly reduced, indicating that targeted inhibition of SOAT1 can inhibit the proliferation of hepatocellular carcinoma[7]. Chen’s team[8]explored the relationship between SOAT1 gene mutation and hepatocellular carcinoma. They collected tumor tissue DNA from 221 hepatocellular carcinoma patients and genomic DNA from the blood of 229 healthy control individuals to evaluate the effect of three exonic SOAT1 variants (rs10753191,V323V; rs3753526, L475L; rs13306731, Q526R) on the risk of hepatocellular carcinoma. And they found that the rs10753191,V323V variant was associated with a reduced risk of hepatocellular carcinoma after adjustment for lipid levels. Therefore, SOAT1 may modify the risk of hepatocellular carcinoma through a lipid metabolism pathway. This study first revealed that SOAT1 gene variants altered susceptibility to hepatocellular carcinoma.
In our study, we performed SOAT1 immunohistochemical detection in 58 paired hepatocellular carcinoma, paracancerous liver tissue and 12 benign liver lesions. he results showed that SOAT1 was highly expressed in hepatocellular carcinoma tissues (37/58), while it was lowly expressed in corresponding adjacent liver tissues (0/58) and in 12 benign liver lesions (0/12). And correlation analysis showed that SOAT1 was correlated with HSP70, GS, CD34 expression levels, while SOAT1 had no clear correlation with CD10. Based on literature reports and the results of this study, we tried to introduce SOAT1 as one of the markers for the differential diagnosis of benign and malignant hepatocellular proliferative lesions, and to explore the application of its combination with CD10, HSP70, GS and CD34 in the diagnosis of hepatocellular carcinoma.
Some hepatocellular carcinoma cases in our study showed negative expression patterns of HSP70, GS or CD10, but these cases showed high expression of SOAT1. This indicated that SOAT1 could be used as a supplementary marker for auxiliary diagnosis of hepatocellular carcinoma. After we put SOAT1 into the markers list, the diagnosis rate of hepatocellular carcinoma increased from 67.24% to 82.76%.In conclusion, we believe that the combined application of SOAT1,HSP70, GS, CD10, and CD34 antibodies has certain feasibility in the diagnosis of hepatocellular carcinoma. The degree of SOAT1 expression has a certain correlation with the degree of tumor differentiation, that is, the lower the degree of differentiation,the higher the expression rate of SOAT1. The lower the tumor differentiation, the worse the prognosis of the patient. Therefore,SOAT1 can also be used as one of the prognostic indicators of hepatocellular carcinoma.
Author’s contribution:
Zheng Jing is responsible for experimental design, interpretation of section staining results and paper writing; Niu Hai-yan is responsible for interpretation of section staining results and article review and calibration; Wei Shang is responsible for case collection,immunohistochemical experiments and interpretation of section staining results; Deng Hui-zi is responsible for tissue collection, case data collection and analysis.
All authors declare no conflict of interest.
 Journal of Hainan Medical College2022年22期
Journal of Hainan Medical College2022年22期
- Journal of Hainan Medical College的其它文章
- Research advances in functional heartburn based on Rome Ⅳ criteria
- Research progress on modern pharmacological action of Radix bupleuri
- Tanreqing injection auxiliary in the treatment of heart failure with pulmonary infection: A systematic review
- Mechanism of total flavonoids in the treatment of rheumatoid arthritis based on network pharmacology
- Clinical characteristics of 72 cases with neuromyelitis optical associated optic neuritis
- Study on the relationship between psoriasis vulgaris and GC gene in Hainan Han nationality based on target gene capture sequencing
