Influence of aliovalent doping on the structure and property of Li2MnCl4chloride solid electrolyte
,
Department of Materials Science & Engineering,University of Science and Technology of China,Hefei 230026,China
Abstract:A series of Li2-xMn1-xGaxCl4 (x=0,0.1,0.3,and 0.5)materials were synthesized with the mechanochemical approach.As confirmed by X-ray powder diffraction and Rietveld refinements,Ga3+can be successfully incorporated into the octahedral sites that are partially occupied by Mn2+.The as-milled materials with relatively low crystallinity generally exhibit higher ionic conductivity than the well crystallized ones produced by annealing at 250 ℃.Among all the materials studied,the as-milled Li1.9Mn0.9Ga0.1Cl4shows the highest ionic conductivity (8.3×10-5 S·cm-1),which is two orders of magnitude higher than that of the as-milled Li2MnCl4(7.12×10-7 S·cm-1).While the unit cell volume does not vary significantly with the composition,the appropriate Li vacancy content should play an important role in the optimized ionic conductivity of Li1.9Mn0.9Ga0.1Cl4.
Keywords:aliovalent doping;chloride solid electrolyte;ionic conductivity
1 Introduction
Recently,chloride solid-state electrolytes (SSEs)have been proven a promising candidate for application in all-solid-state Li batteries (ASSBs),because they show a rare combination of high ionic conductivity,excellent deformability,and good compatibility with the 4 V-class cathodes[1-6].Nevertheless,recent studies are almost exclusively focused on the materials based on Li3YCl6and Li3InCl6[7],while another large family of chloride systems,Li2MCl4(M=Mn,Fe,Co,etc.),was barely investigated.
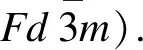
In this work,we explore the influence of aliovalent doping on the ionic transport behavior of Li2MnCl4.With divalent Mn2+partially substituted by trivalent Ga3+,aliovalently-doped Li2-xMn1-xGaxCl4(x=0.1,0.3,0.5)were synthesized and orders of magnitude conductivity improvement with respect to unmodified Li2MnCl4was observed.The mechanism behind such optimized conductivity is also discussed based on the crystal structures acquired from Rietveld refinement.
2 Experiments
2.1 Synthesis of Li2-xMn1-xGaxCl4 (x=0,0.1,0.3,0.5)
The samples were prepared by high energy ball-milling and annealing methods.The starting materials are LiCl (Alfa Aesar,99.99%),MnCl2(Aladdin,99.9%)and GaCl3(Aladdin,99.99%).For the ball-milling process,stoichiometric amount of LiCl,MnCl2and GaCl3were hand-mixed in the mortar by pestle for homogenization.Subsequently,the as-mixed stoichiometric mixture was loaded into tungsten carbide (WC)pots with 5 mm-diameter WC balls.The ball to the powder weight ratio was 25∶1 and the rotating speed was set to 500 r/min.All the aforementioned procedures were conducted in the glove box filled with Ar to avoid possible water-absorption of raw materials.After milling over 20 h,corresponding as-milled Li2-xMn1-xGaxCl4(x=0,0.1,0.3,0.5)powder samples were obtained.To synthesize the well-crystallized samples,the as-milled powder was conducted with annealing.Prior to annealing,the as-milled powder was cold-pressed into a pellet and then loaded into the borosilicate glass tube in the glove box.Subsequently,the glass tube loaded with the as-pressed pellet sample was evacuated and sealed by vacuum sealing apparatus.Finally,the sealed glass tube with the as-pressed pellet was transferred to furnace for annealing.Specifically,the Li2-xMn1-xGaxCl4(x=0,0.1,0.3,0.5)pellets were heated at 250 °C for 5 h.
2.2 Structure characterization
The structure information was obtained from X-ray powder diffraction (XRD)by means of X-ray powder diffractometer (Rigaku,Ultima IV)using Cu Kα1 radiation.Prior to XRD measurements,the powder was sealed in the sample holder by Kapton film in the glove box filled with Ar atmosphere considering its hygroscopic nature.The scanning speed was 5 degree per minute and the scanning range was from 20° to 80°.Rietveld refinements were conducted using the GSAS-II software[17].The structure was refined with theUisolimited to positive values and stoichiometry confined to the nominal ones;under these restraint conditions,the occupancy of each atomic site is allowed to vary freely during refinement.
2.3 Electrochemical characterization
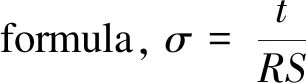
3 Results and discussions
XRD patterns of the as-milled Li2-xMn1-xGaxCl4powder samples are shown in Figure 1(a).For each XRD pattern of as-milled Li2-xMn1-xGaxCl4,it can be well indexed into PDF#51-0304 corresponding to diffraction pattern of Li2MnCl4.Based on the analysis of XRD measurements results,two points can be concluded.One is that Ga3+have been successfully incorporated into crystal lattice of Li2MnCl4since there are no appreciable impurity peaks in the XRD patterns of the as-milled samples and the diffraction peaks of the doped samples are in the same position as Li2MnCl4.The other one is the low crystallinity inferred from the broad diffraction peaks,making it hard to obtain detailed information on the crystal structure.Thus,the annealed samples with high crystallinity were conducted with XRD measurements and the results are shown in Figure 1(b).All the XRD patterns of annealed Li2-xMn1-xGaxCl4(x=0,0.1,0.3)can be in line with PDF#51-0304 whereas some unindexable tiny impurity peaks were observed for the annealed Li1.5Mn0.5Ga0.5Cl4.Therefore,we can conclude that the mutual solubility of Li2-xMn1-xGaxCl4is somewhat larger than 0.3 but less than 0.5.As for the reason why no such impurity peaks were observed in the XRD patterns of the as-milled Li1.5Mn0.5Ga0.5Cl4,a plausible explanation is that the diffraction intensity of impurities is so weak for the as-milled sample that the impurity peaks are covered by noises from the X-ray powder diffractometer and thus cannot be distinguished.
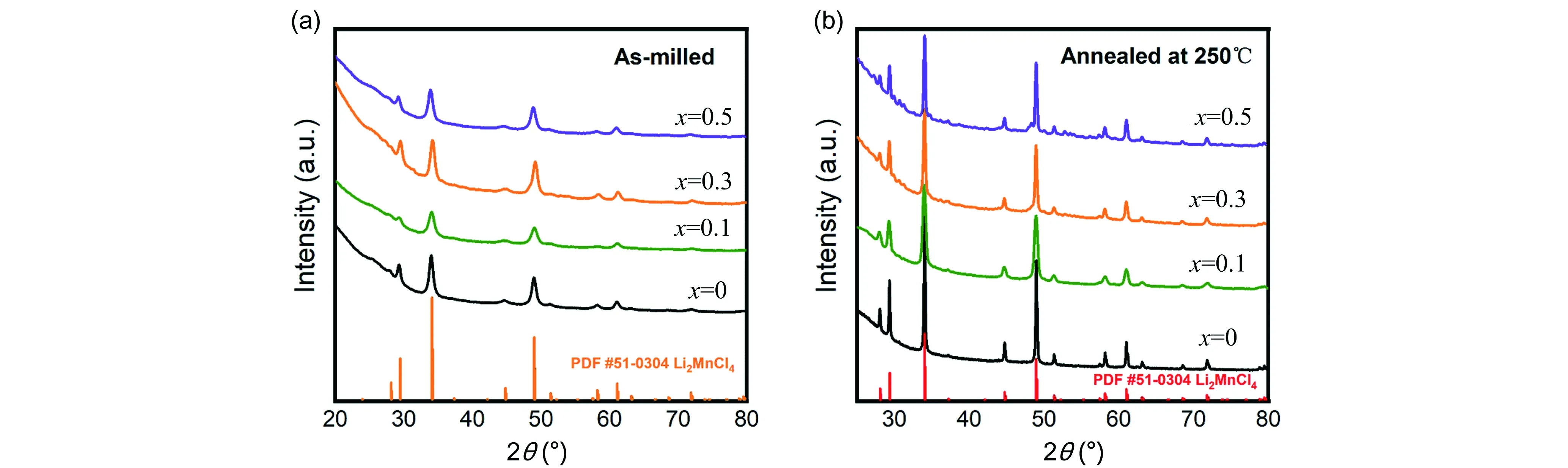
Figure 1.XRD patterns of ball-milled (a)and annealed (b)Li2-xMn1-xGaxCl4 powder samples. I both Figure1(a)and Figure 1(b),the black curve,gree curve,yellow curve and violet curve correspond to x=0,x=0.1,x=0.3 and x=0.5 for Li2-xMn1-xGaxCl4,respectively.

Figure 2.Rietveld refinements o XRD patterns of as-milled Li2-xMn1-xGaxCl4 powder samples. (a),(b),(c)and (d)correspond to x=0,x=0.1,x=0.3 and x= 0.5 for Li2-xMn1-xGaxCl4,respectively. Dark cross symbols correspond to experimental data. The red curve corresponds to the calculated pattern. Pink lines correspond to Bragg positions. The light gree curve corresponds to the difference betwee calculated patterns and experimental data.
From the aforementioned discussions,we preliminarily confirm that the aliovalently doped Li2-xMn1-xGaxCl4samples have been successfully synthesized as expected.To determine precise crystal structure parameters,Rietveld refinements were performed on the XRD patterns of both as-milled and annealed Li2-xMn1-xGaxCl4samples and the corresponding results are given in Figure 2 and Figure 3.All of the Rwpvalues of refinements for both as-milled and annealed Li2-xMn1-xGaxCl4are acceptably low,indicating high credibility of the Rietveld refinements results.In this case,we can obtain reasonably reliable information on the crystal structure of Li2-xMn1-xGaxCl4as shown in Tables 1-8.Note that the fitting uncertainty is given in brackets.For Li2MnCl4without substitution,there are two different sites of lithium.A part of Li+fully occupy the tetrahedral (8a)sites surrounded by four chloride ions while the rest are located at octahedral (16d)sites surrounded by six chloride ions.All the Mn2+are distributed over the same octahedral (16d)sites as the part of Li+.All the Cl-are located at 32esites.For doped Li2-xMn1-xGaxCl4,Ga3+are incorporated into octahedral (16d)sites.In the meantime,the tetrahedral (8a)sites are no longer fully occupied by Li+in order to keep the charge balance,leading to increased number of vacancies.Correspondingly,the octahedral sites are simultaneously accommodating Li+,Mn2+and Ga3+.Furthermore,with Ga3+incorporated into the crystal structure of Li2MnCl4,the number of vacancies increases while Li+concentration decreases at the same time.In addition to the change of cation site occupation,the unit cell parameter has also varied with the composition of Li2-xMn1-xGaxCl4.However,the difference of lattice parameters between the as-milled and annealed samples is insignificant for Li2-xMn1-xGaxCl4(x=0,0.1,0.3,0.5).
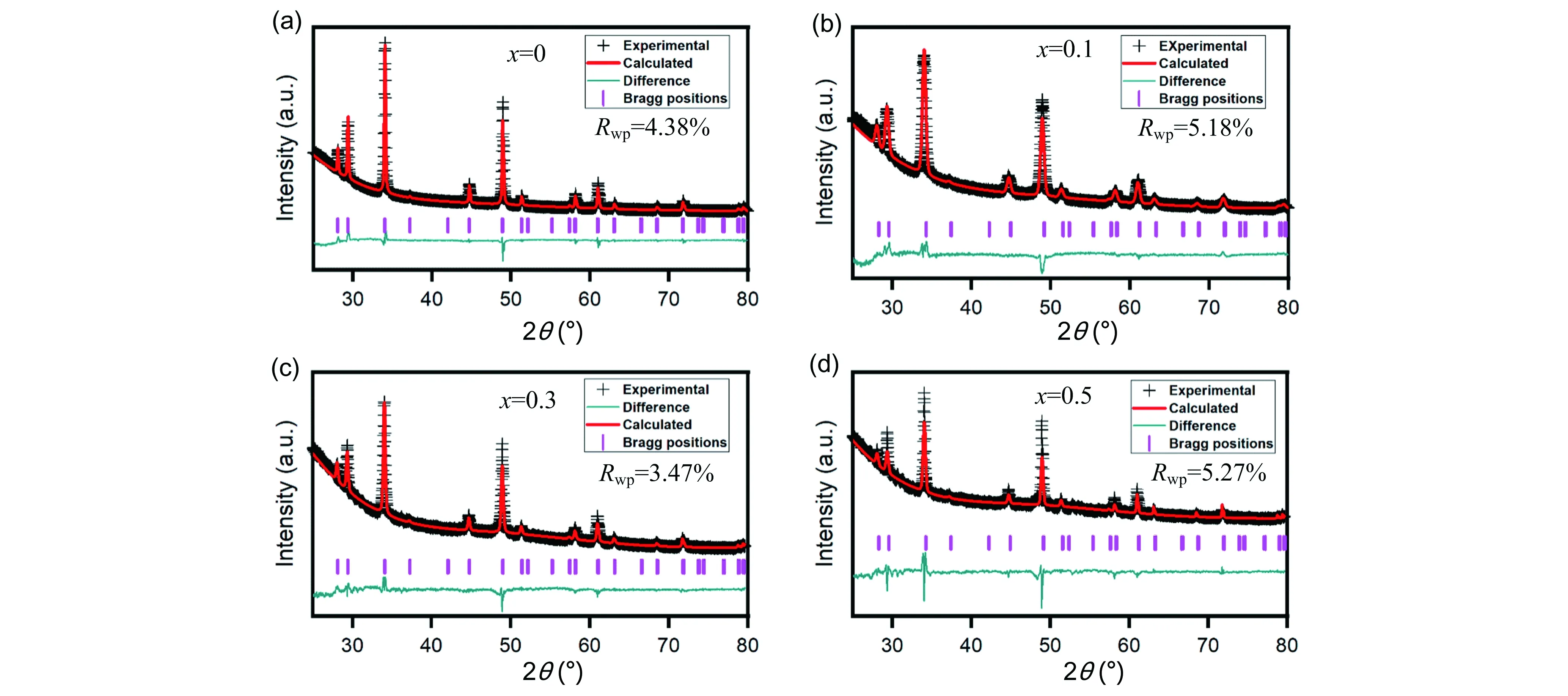
Figure 3.Rietveld refinements o XRD patterns of annealed Li2-xMn1-xGaxCl4 powder samples. (a),(b),(c)and (d)correspond to x=0,x=0.1,x=0.3 and x= 0.5 for Li2-xMn1-xGaxCl4,respectively. Dark cross symbols correspond to the experimental data. Red curve corresponds to the calculated pattern. Pink lines correspond to Bragg positions.The light gree curve corresponds to the difference betwee calculated patterns and experimental data.
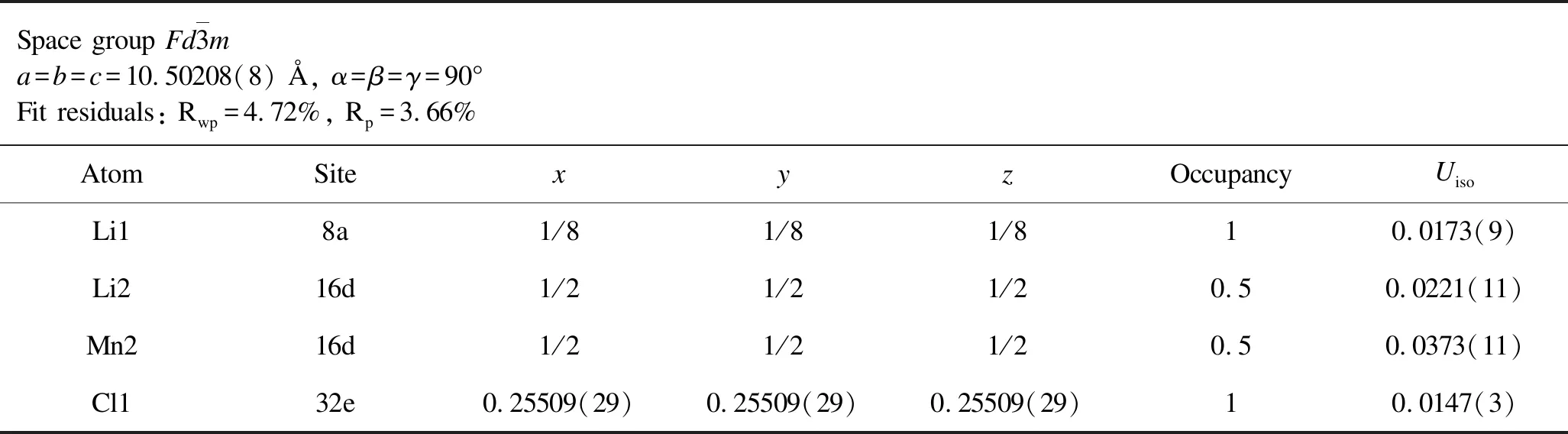
Table 1.Rietveld analysis results for the XRD patterns of as-milled Li2MnCl4.
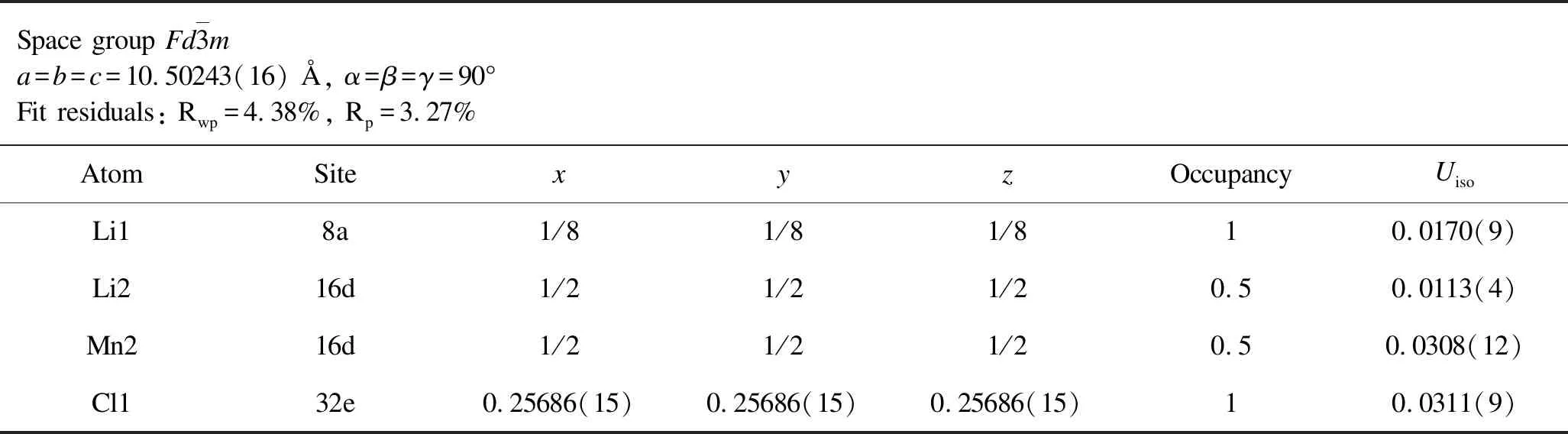
Table 2.Rietveld analysis results for the XRD patterns of annealed Li2MnCl4.

Table 3.Rietveld analysis results for the XRD patterns of as-milled Li1.9Mn0.9Ga0.1Cl4.

Table 4.Rietveld analysis results for the XRD patterns of annealed Li1.9Mn0.9Ga0.1Cl4.
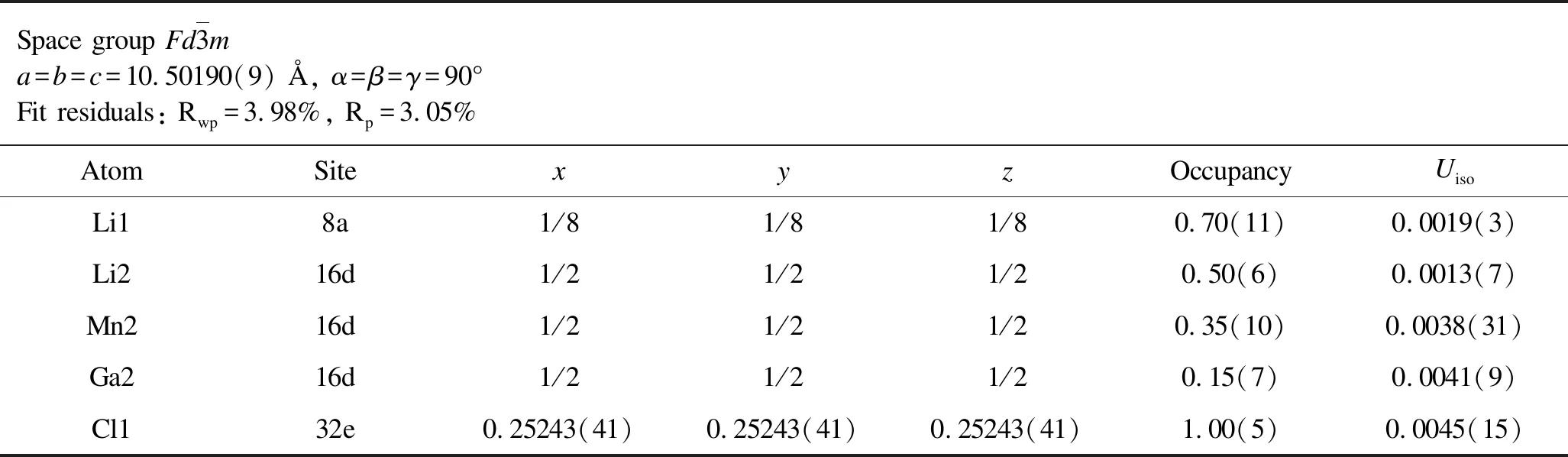
Table 5.Rietveld analysis results for the XRD patterns of as-milled Li1.7Mn0.7Ga0.1Cl4.

Table 6.Rietveld analysis results for the XRD patterns of annealed Li1.7Mn0.7Ga0.1Cl4.
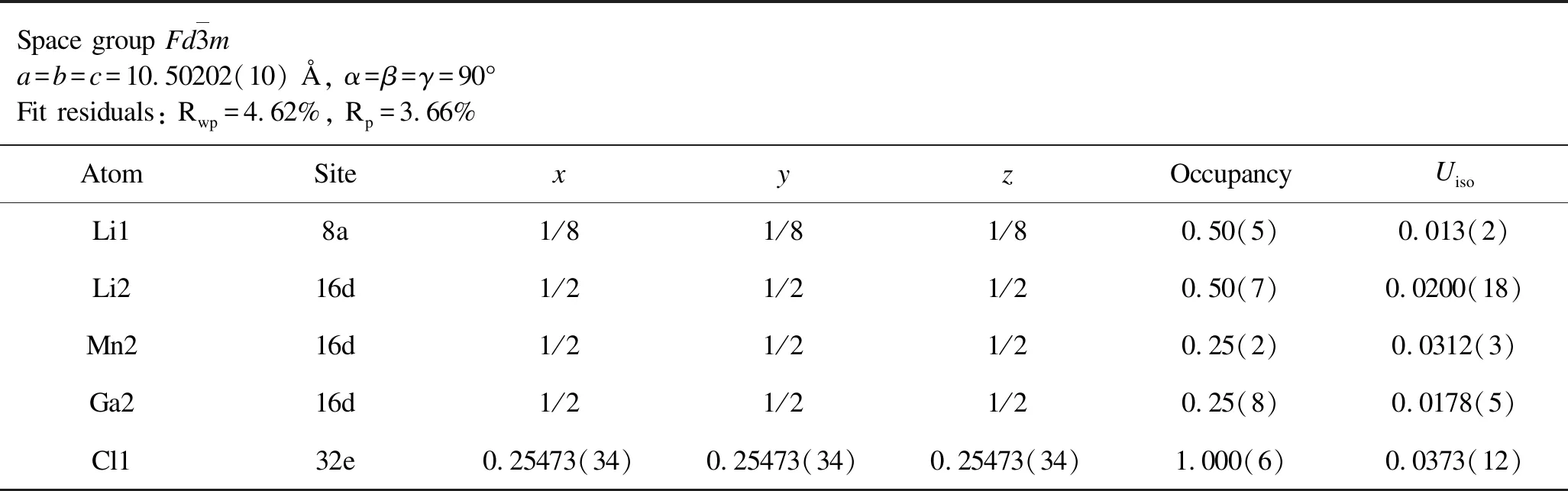
Table 7.Rietveld analysis results for the XRD patterns of as-milled Li1.5Mn0.5Ga0.5Cl4.

Table 8.Rietveld analysis results for the XRD patterns of annealed Li1.5Mn0.5Ga0.5Cl4.

Figure 4.Electrochemical measurements of ball-milled and annealed Li2-xMn1-xGaxCl4 samples. (a)Nyquist plots of ball-milled Li2-xMn1-xGaxCl4 samples;(b)Nyquist plots of annealed Li2-xMn1-xGaxCl4 samples;(c)ionic conductivities (red line)/cell volume (black line,the vertical line is error bar)versus compositions of ball-milled Li2-xMn1-xGaxCl4;(d)ionic conductivities (gree line,the vertical line is error bar)/cell volume (black line)versus compositions of annealed Li2-xMn1-xGaxCl4.

4 Conclusions
In summary,aliovalent doping has been demonstrated to be an effective way of improving ionic conductivity of Li2MnCl4.Using the X-ray diffraction,the partial substitution of Mn2+by Ga3+in the lattice has been confirmed.The as-milled materials generally show higher conductivities than the annealed ones with high crystallinity.The as-milled Li1.9Mn0.9Ga0.1Cl4was observed to show the highest ionic conductivity (8.3×10-5S·cm-1)among all the Li2-xMn1-xGaxCl4samples,two orders of magnitude higher than the 7.12×10-7S·cm-1conductivity for the as-milled Li2MnCl4.The highest conductivity achieved for Li1.9Mn0.9Ga0.1Cl4is due to the balance between the Li+concentration and vacancies available for Li+hopping,which should receive close attention in future studies of Li2MCl4-based solid electrolytes.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFA0209600,2017YFA0208300),the National Natural Science Foundation of China (51802302),and the Fundamental Research Funds for the Central Universities (WK3430000006).
Conflictofinterest
The authors declare no conflict of interest.
Authorinformation
DUANChaominreceived his Bachelor’s degree from Hefei University of Technology in 2018 and is currently a Master student at University of Science and Technology of China (USTC).His main research interests focus on all-solid-state batteries and halide solid state electrolytes.
MACheng(corresponding author)received his BS degree in Materials Science and Engineering in 2006 from Tsinghua University (Beijing,China)and PhD degree in Materials Science and Engineering in 2012 from Iowa State University.After completing his work as a postdoctoral researcher at the Oak Ridge National Laboratory in 2016,he joined USTC as a professor.His research interest lies in the critical materials and interfaces in all-solid-state Li batteries.
- 中国科学技术大学学报的其它文章
- 内皮功能失调与泛血管疾病
- LncRNA expression profiles of Schizosaccharomyces pombe in DNA damage inducing environments
- Development of an analysis method for determination of pectin in tobacco by solid state 13C CP/MAS NMR
- Highly perfluorocarbon loading efficiency of polymer biomimetic nanoparticle encapsulated by erythrocyte membrane to improve tumor phototherapy
- A new standard quadratic optimization approach to beam angle optimization for fixed-field intensity modulated radiation therapy
- Effects of specific amino acids on the metabolism of Drosophila melanogaster

