Methylprednisolone accelerate chest computed tomography absorption in COVID-19:A three-centered retrospective case control study from China
INTRODUCTION
As an emerging severe infectious respiratory disease,coronavirus disease 2019(COVID-19) has caused a pandemic outbreak with a high infection rate,high mortality and general population susceptibility,which makes this disease a major threat to international health and economy[1].In the absence of specific treatment methods and widespread mass vaccination,it is urgent to find clinically effective drugs to reduce mortality and shorter hospitalization stay.The therapeutic effect of corticosteroids in severe acute respiratory syndrome (SARS) has been confirmed before[2,3],but the use of corticosteroids in COVID-19 remains controversial due to the absence of evidence from randomized controlled trials(RCTs)[4].Clinically,we observed that some patients who received low-to-moderate dose short-term corticosteroids had better pulmonary imaging absorption.A retrospective analysis of clinical data was conducted to explore the optimal time,dosage,and course of corticosteroids in the treatment of COVID-19,expecting to evaluate the efficacy and safety profiles of corticosteroid therapy.
MATERIALS AND METHODS
Subjects
A total of 70 hospitalized patients who were admitted to Wuhan Union Hospital,Renmin Hospital of Wuhan University Hubei General Hospital and Wuhan Jinyintan Hospital from January 27,2020 to March 30,2020 were included in this study.The inclusion criteria were as follows:(1) The patients had confirmed COVID-19 and typical radiological characteristics;and (2) The patients were treated with Arbidol Hydrochloride Tablets (hereinafter,Arbidol) for 7-10 d before admission,and no obvious absorption was found on reexamination of chest computed tomography (CT)scan.The exclusion criteria were as follows:(1) Previous rheumatic immune system related diseases and long-term use of corticosteroids;(2) Use of corticosteroids within 2 mo before admission;(3) Serious cardiovascular and cerebrovascular diseases,refractory hypertension,epilepsy or delirium,glaucoma;(4) Active gastrointestinal bleeding in the recent 3 mo;(5) Combination with bacterial infection;(6) Mild and critical types;or (7) Patients received antiviral therapy other than Arbidol before admission.We collected the clinical data of patients,including sex,age,underlying diseases,clinical symptoms,epidemiological history,radiological characteristics,laboratory tests,.The diagnostic criteria and clinical classification referred to the Diagnosis and Treatment Protocol for COVID-19 (Trial version 7th)[5].The study was retrospectively analyzed and approved by the Medical Ethics Committee of Fujian Medical University Union Hospital (ethics approval No.2020KJTXGF001) and conformed to the principles of the Declaration of Helsinki.
Therapy and groups
All patients were received routine oxygen therapy and nutritional support.Some of them continued to be treated with Arbidol (200 mg tid) as the control group,and some were treated with methylprednisolone (orally or intravenously) as the corticosteroid group.Chest CT was reexamined to evaluate the absorption of pulmonary lesions after 7-10 d therapy.Two senior radiologists evaluated the chest radiological characteristics independently and contributed to confirming the degree of absorption,which was classified as four situations:no absorption,slightly absorption,obvious absorption and progression.
Efficacy evaluation
Routine blood tests,liver and kidney function tests,hypersensitive C-reactive protein(hs-CRP) levels,erythrocyte sedimentation rate (ESR),interleukin-6 (IL-6),serum ferritin (SF),lactate dehydrogenase (LDH),creatine kinase-MB (CK-MB),and D-dimer levels were evaluated before and after treatment.Throat swab samples were collected for detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA by real-time reverse transcription polymerase chain reaction (RT-PCR).During the treatment,the patient's temperature,respiration,pulse rate,blood pressure,blood glucose and oxygen saturation were closely monitored.The discharge criteria were as follows:(1) Body temperature that returned to normal for more than 3 d;(2)Respiratory symptoms that improved significantly;(3) Chest CT that showed significant improvement of acute exudative lesions;and (4) Two consecutive negative nucleic acid tests of respira-tory tract specimens (sampling interval of at least 24 h).
Statistical analysis
COVID-19 is an emerging respiratory infectious disease caused by the novel SARSCoV-2 that has declared a pandemic outbreaks.SARS-CoV-2 including new variants is characterized by strong infectivity,diverse transmission routes,and non-specific clinical manifestations,and people are generally susceptible.Currently,the treatment for COVID-19 is still mainly focused on antivirals,nutritional support,respiratory support,expectorants,antiasthmatics and immune enhancement.Unfortunately,there is no specific drug to treat COVID-19[1,4-5].Therefore,searching for effective treatment for COVID-19 has attracted considerable attention worldwide.
RESULTS
General Characteristics of the Patients
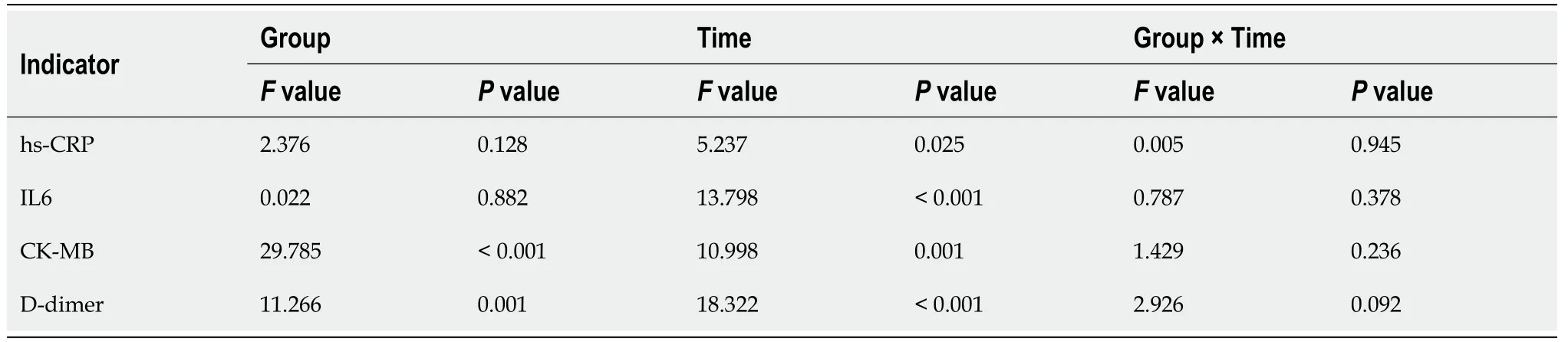
All 70 patients were local cases in Wuhan.The general characteristics of the patients are shown in Tables 1 and 2.There were 32 patients in the control group aged from 33 to 85 years old among whom 14 patients had underlying diseases (8 cases with of hypertension,4 cases of type 2 diabetes,2 cases of chronic obstructive pulmonary disease,1 case of hyperlipidemia,2 cases of gallstone,1 case of kidney stone,1 case of Parkinson's disease,1 case of rheumatoid arthritis and 1 case of systemic lupus erythematosus).There were 38 patients in the corticosteroid group aged from 27-91 years old among whom 15 patients had underlying diseases (8 cases of hypertension,7 cases of type 2 diabetes,1 case of rheumatoid arthritis,and 1 case of postoperative cervical cancer).There was no significant difference between the two groups in the gender distribution,classification,clinical symptoms or baseline data of underlying diseases (>0.05) (Tables 1 and 2).There was no significant difference between the two groups in the time of medication (>0.05) (Table 2).Since the time of admission did not conform to the normal distribution,the rank-sum test obtained Z=-0.132 and=0.898,suggesting that the days from the onset of illness to admission between the two groups was not statistically significant.
Use of methylprednisolone
The total days of methylprednisolone use in the corticosteroid group were 2-12 d,with the initial dose ranging from 24 to 80 mg.The patients in corticosteroid group were divided into the non-obvious absorption group (including no absorption,slightly absorption and progression) and obvious absorption group according to whether chest CT was obviously absorbed after medication.There was no significant difference in the course of corticosteroid therapy,total or daily corticosteroid dosage between the two groups (>0.05) (Table 3).
Therapeutic effect evaluation
In the whole treatment process of this study,we did not observe serious adverse reactions such as gastrointestinal bleeding,secondary severe infections,hypertension,diabetic ketoacidosis,mental disorders and electrolyte disorders.There were 11 cases(28.95%) of hyperglycemia in the corticosteroid group,which was statistically significant compared with the control group (0%) (=0.001).Spearman rank correlation analysis suggested that there was no correlation between elevated blood glucose and the existence of underlying diabetic diseases,indicating that the increase in blood glucose was caused by corticosteroids.However,only 18.18% (2/11) of patients with hyperglycemia needed short-acting insulin to control blood glucose.Thus,the above corticosteroid regimen was safe.During the treatment of COVID-19 with corticosteroids,we should monitor the patients' blood glucose more closely to avoid the occurrence of life-threatening situations such as hyperosmotic hyperglycemia coma and ketoacidosis.
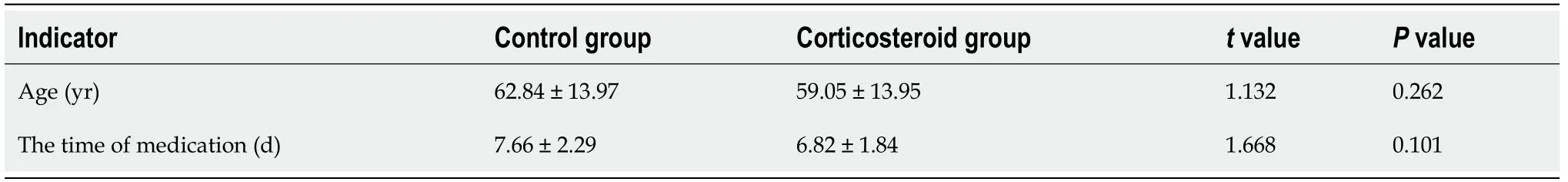
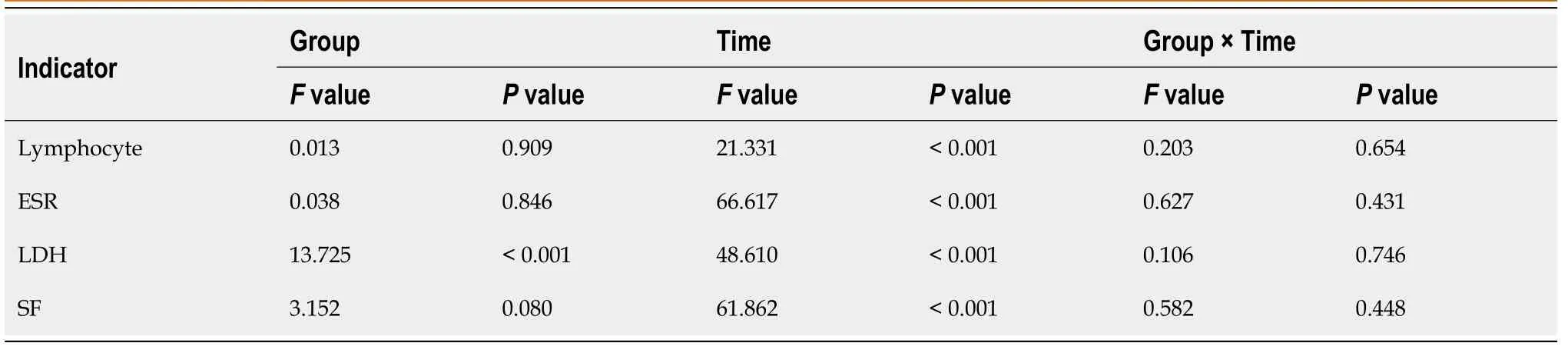
All the 70 patients completed the detection of peripheral blood indicators before and after medication.The results were as follows (Tables 6-9):(1) There was no significant difference in the lymphocyte count,ESR,SF,hs-CRP and IL-6 Level between the two groups.However,for the patients in the same group,the values of the above indexes varied at different time points with statistical significance (lymphocyte count:before the medication<after the medication;ESR,SF,hs-CRP and IL-6 Level:before the medication>after the medication).However,the interaction effects between the groups and time points did not exhibit significant differences.In other words,there was no significant difference in the gradient of each index.And (2) The LDH,CK-MB and D-dimer values of the two groups were significantly different (the corticosteroid group>the control group).Moreover,for the patients in the same group,the values of the above indexes varied at different time points with statistical significance (LDH,CK-MB and D-dimer levels:before the medication>after the medication).However,the interaction effects between the groups and time points also exhibited no significant differences.
But they soon found that they were left alone, and that their former friends even attributed their misfortunes to their own extravagance, and showed no intention of offering them any help
When at length he asked the father if he would give him one of his daughters to wife, the two eldest jumped up, ran into their bedrooms to put on splendid dresses, for each of them fancied she was the chosen one
The Prince was immediately seized with the most ardent79 desire to go on board the ship, and shouted loudly to attract the notice of her crew, but no one answered
Observation of adverse reactions to corticosteroid therapy
We observed no severe adverse reactions such as gastrointestinal bleeding,secondary severe infection,hypertension,diabetic ketoacidosis,mental disorders or electrolyte disorders during the whole corticosteroid treatment process (Table 10).There were 11 cases (28.95%) of hyperglycemia in the corticosteroid group,among which only 2 patients needed short-acting insulin hypodermic injections to control blood glucose,while no elevated blood glucose was observed among patients in the control group.The Chi-square test was used to compare the incidence of elevated blood glucose between the two groups (=10.990,28.95%0%,=0.001).Spearman rank correlation analysis showed no significant correlation between elevated blood glucose and the existence of underlying diabetic diseases (=0.052,=0.668).







DISCUSSION
Statistical analysis was performed using SPSS software (version 17.0).Enumeration data are represented by the number of cases and percentages.In addition,the differences in the variables between the corticosteroid group and control group were evaluated using the chi-square test or Fisher’s exact test for categorical variables.Ridit analysis was used for ranked data.Normally distributed measurement data are represented as the mean ± SD,and comparisons between groups were performed by thetest or two-factor repeated measurement analysis of variance.Nonnormally distributed data are represented by the median and mean rank,and comparisons between groups were performed by the rank-sum test.The index differences before initial medication,post initial medication and post-subsequent medication between the corticosteroid group and control group were compared by repeated-measures analysis.Spearman's correlation coefficient was used to determine the association between blood glucose variation and diabetes.<0.05 was considered to indicate a statistically significant difference.
Corticosteroids offer advantages over conventional therapy for alleviating clinical symptoms,reducing mortality and improving prognosis by inhibiting excessive inflammatory responses and cytokine release and reducing systemic toxic symptoms and pulmonary exudation[6].Clinical trials of dexamethasone have shown that it decrease 28-d mortality in patients with COVID-19 receiving respiratory support,but has no benefit in patients not require oxygen even may be harmful[7].A randomized clinical trial concluded that use of intravenous dexamethasone increased the number of ventilator free days over a 28-d in patients with COVID-19 and moderate or severe acute respiratory distress syndrome (ARDS)[8].Wu[9] reported that in patients with ARDS due to COVID-19,a standard dose of methylprednisolone significantly reduced the risk of death by 62%.
Examine the efficacy and safety of low-to-moderate dose short-term methylprednisolone on COVID-19 patients.
Oh, how enchanted19 the king was to see her again, and the whole town shared his joy!And the slave was called upon to tell how he had slain20 the monster, and when he had ended the king declared that he should have the princess to wife
The average daily methylprednisolone dose of the 34 patients with significant improvement in chest CT was (38.55 ± 13.17) mg,and the average course of methylprednisolone use was (6.44 ± 1.86) d;thus,this could be regarded as a low-to-moderate dose short-term regimen.There was no significant difference in the negative conversion rate and total clearance time of SARS-CoV-2 nucleic acid between the corticosteroid group and the control group (>0.05),indicating that the corticosteroid regimen did not affect the clearance time of the virus.
In this study,a total of 70 patients with no obvious absorption on chest CT after 7-10 d of Arbidol antiviral therapy were included in strict accordance with the recommended treatment regimen.Arbidol (as the control group) and methylprednisolone(as the corticosteroid group) were given respectively after admission.There was no difference between the two groups in the time of medication (>0.05).All patients in the corticosteroid group had varying degrees of CT absorption,and the CT absorption degree in the corticosteroid group was significantly better than that in the control group (CT obvious absorption rate:89.47%12.5%,<0.05).In the corticosteroid group,there was no significant difference in the course of treatment,total dosage and daily corticosteroid dosage between the patients with obvious CT absorption and those without obvious CT absorption (>0.05),indicating that there was no difference in the corticosteroid dosage and medication time between 34 patients with obvious CT absorption and 4 patients with slight absorption.
There were seven of them, but one of them was flawed4. It looked as good as others, almost the size of a tennis ball, nice red fading to light green, but where the stem5 had been there was now a break that went straight down into the heart of the seed.
Arbidol is a non-nucleoside broad-spectrum antiviral drug that has been proven to be effective against coronavirus in vitro[14,15].A retrospective study of 69 COVID-19 patients revealed that Arbidol treatment improved the discharge rate (33% in the-treated group19% in the-untreated group) and decreased the mortality rate[16].Arbidol treatment has been recommended as antiviral therapy according to the 7th trial version of Diagnosis and Treatment Protocol for COVID-19 released by the China’s National Health Commission:for adults,a dose of 200 mg tid for no more than 10 d is recommended[5].
The use of corticosteroids in COVID-19 has been included in World Health Organization guidelines,but still remains controversial.
Lymphopenia and elevated levels of LDH,hs-CRP,D-dimer,IL-6,CK-MB,ESR and SF can be regarded as risk factors for progression or predictors of disease severity of COVID-19[9,16-23].During the treatment,lymphocytes gradually increased and the ESR,SF,LDH,CK-MB,hs-CRP,IL-6 and D-dimer levels gradually decreased.It was suggested that both Arbidol and corticosteroids therapy can improve COVID-19 patients' condition.
During the treatment,the viral nucleic acid condition of the throat swab was dynamically monitored.If two consecutive nucleic acid tests of throat swab specimens(sampling interval of at least 24 h) were negative,the time of the first test turned negative was taken as the negative nucleic acid conversion time,and the time interval from the onset date to the negative nucleic acid conversion time was taken as the nucleic acid clearance time.The negative conversion rates of SARS-CoV-2 nucleic acid were 65.62% in the control group and 73.68% in the corticosteroid group before medication.The negative conversion rates of SARS-CoV-2 nucleic acid in the control group and the corticosteroid group were 93.75% and 97.37% after medication,respectively.There was no significant difference between the two groups in the negative conversion rate (>0.05) (Table 4) and total clearance time of SARS-CoV-2 nucleic acid (>0.05) (Table 5).After medication,all the patients in the corticosteroid group had varying degrees of CT absorption,with an obvious CT absorption rate of 89.47%.In contrast,40.63% patients in the control group showed no absorption in chest CT and the CT obvious absorption rate was only 12.5%.The CT absorption degree of the corticosteroid group was significantly better than that of the control group,with a statistically significant difference (<0.05) (Table 4).None of them developed into critical type.
CONCLUSION
Our study showed that the chest CT absorption in the corticosteroid group was significantly better than that in the control group.Low-to-moderate dose short-term methylprednisolone treatment can promote pulmonary radiological absorption and improve the indexes of lymphocyte count,ESR,SF,LDH,CK-MB,hs-CRP,IL-6 and Ddimer levels.The corticosteroid regimen was not associated with any serious adverse reactions and did not delay the clearance time of SARS-COV-2.COVID-19 has caused a lot of morbidity and mortality worldwide,occupying more medical resources.Lowto-moderate dose short-term methylprednisolone can rapidly improve symptoms,oxygenation and pulmonary function,alleviate the patients’ condition in a short term,reduce the hospital stay,avoid severe COVID-19 phases and save medical resources ultimately.Therefore,we suggest that confirmed COVID-19 patients with the common and severe types with no obvious improvement on chest CT after initial antiviral treatment with Arbidol can be treated with a low-to-moderate dose (30 to 40 mg/d)and short-term treatment (5-7 d) of methylprednisolone.A personalized regimen should be developed based on the underlying disease and infectious severity of the patient to fully demonstrate the advantages of corticosteroids in clinical use and to avoid adverse effects.Furthermore,RCTs need to be designed to further confirm the therapeutic effect of corticosteroids in the future.
ARTICLE HIGHLIGHTS
Research background
Coronavirus disease 2019 (COVID-19) has caused a pandemic outbreak with a high infection rate,high morbidity and mortality,occupying more public medical resources.Therefore,it is urgent to find effective treatment for COVID-19.
Research motivation
“I tried to be nice to her, Mom. Even when she called me a stupid dummy35 because the crib leg was short, I didn’t hit her. And every week, when we picked new names, I thought it would be over. But tonight, when I got her name again, I knew I couldn’t do one more nice thing for her, Mom. I just can’t! And tomorrow’s Christmas eve. I’ll spoil Christmas for everybody just when we’re ready to put Baby Jesus in the crib. Don’t you see why I had to leave?”
Research objectives
However,corticosteroids are a "double-edged sword".On the one hand,these drugs can help reduce excessive inflammatory responses;on the other hand,they may suppress immune function and delay the clearance of SARS-CoV-2 RNA[5,10].Whether patients with COVID-19 benefit from adjunctive corticosteroids still a debated issue.It has been reported that corticosteroids did not reduce mortality in patients with severe COVID-19 in intensive care units (ICUs)[11].Liu[12] carried out their study among 137 participants with 2019-nCOV infection found no significant benefits from systemic corticosteroid therapy.Evidence even showed that mortality benefit in severely ill COVID-19 patients treated with corticosteroids from a metaanalysis of 21,350 COVID-19 patients[13].In view of this,to make good use of the"double-edged sword" of corticosteroids to maximize the therapeutic effect while minimizing adverse effects,the timing,dosage and treatment course are of vital importance and should be carefully considered by clinicians.Few studies focused on the effects of corticosteroids on pulmonary imaging absorption in patients with COVID-19,so this study highlights this issue.
Research methods
Seventy COVID-19 patients received antiviral therapy with Arbidol for 7-10 d before admission but had no obvious absorption on chest computed tomography (CT)imaging were retrospectively analyzed.Arbidol (as the control group) and methylprednisolone(as the corticosteroid group) were given respectively after admission.After treatment,chest CT was reexamined to evaluate the absorption of pulmonary lesions.
Research results
The degree of CT absorption in the corticosteroid group was significantly better than that of control group (<0.05).The average daily dose and course of methylprednisolone in the patients with significant improvement on chest CT was (38.55 ± 13.17)mg and (6.44 ± 1.86) d respectively.
Bow-wow! That was the end of happiness! The Snow-man, however, was not listening to him any more; he was looking into the room where the housekeeper lived, where the stove stood on its four iron legs, and seemed to be just the same size as the Snow-man
Research conclusions
Low-to-moderate dose short-term methylprednisolone can accelerate the chest CT imaging absorption of COVID-19.
Research perspectives
The protocol has been proven to be effective and safe in clinical use,it can improve the condition,reduce the hospital stay,avoid severe phases and save medical resources.
When the message was delivered the princess sprang to her feet with delight, for she had been thinking that after all it was not much use to have a lovely mantle and elegant petticoats if she had no dress, and she knew the tailors would never be ready in time
We would like to express our heartfelt thanks to all the medical staff at Wuhan Union Hospital,Renmin Hospital of Wuhan University Hubei General Hospital and Wuhan Jinyintan Hospital.
 World Journal of Clinical Cases2022年2期
World Journal of Clinical Cases2022年2期
- World Journal of Clinical Cases的其它文章
- New trends in treatment of muscle fatigue throughout rehabilitation of elderlies with motor neuron diseases
- What emotion dimensions can affect working memory performance in healthy adults? A review
- Quadrilateral plate fractures of the acetabulum:Classification,approach,implant therapy and related research progress
- Analysis of photostimulable phosphor image plate artifacts and their prevalence
- N6-methyladenine-modified DNA was decreased in Alzheimer’s disease patients
- Inflammation-related indicators to distinguish between gastric stromal tumors and leiomyomas:A retrospective study
