THE EFFECT OF HYPOXIA ON HEART AND VASCULAR MORPHOLOGICAL DEVELOPMENT AND RELATED GENES EXPRESSION IN ZEBRAFISH EMBRYOS
JIANG Yu, YANG Ming-Yang, YE Qin, TAO Yi-Xi, XU Hao, and LI Yun,
(1. College of Fisheries, Institute of Three Gorges Ecological Fisheries of Chongqing, Southwest University, Chongqing 400715,China; 2. Key Laboratory of Freshwater Fish Reproduction and Development (Ministry of Education), Key Laboratory of Aquatic Science of Chongqing, Southwest University, Chongqing 400715, China; 3. School of Chemistry and Chemical Engineering,Southwest University, Chongqing 400715, China)
Abstract: In this study, we used transgenic zebrafish (Danio rerio) with green fluorescence protein labeling in the heart and vasculature as study models. Two dissolved oxygen conditions, hypoxia and normoxia, were set. The morphological structure of the embryo, heart and vascular shape, heart rate, and formation of the main vessel in the embryonic trunk were measured and analyzed by fluorescence microscopy. We found that the survival rate of embryos under hypoxia was reduced. Hypoxia not only delayed embryonic development but also caused abnormal morphology. Heart malformations in zebrafish embryos exposed to hypoxia mainly manifested as pericardial effusion and developmental arrest in the linear heart tube stage. Although the heart tube could differentiate into the atrium and ventricle, the right cyclization process could not be completed.Vascular development in zebrafish embryos was also affected by hypoxia. Hypoxia narrowed the diameter of the embryonic dorsal aorta and posterior cardinal vein, shortened the distance between the posterior cardinal vein and the dorsal longitudinal anastomotic vessel, caused abnormal blood vessel growth in the interstitial cells of the embryo, and led to the specific disappearance of the parachordal vessels and a lack of subintestinal venous vessels. The study aimed to elucidate the mechanism by which hypoxia impacts cardiovascular development. In situ hybridization and qPCR were performed to test the changes in cardiovascular development-related genes localization and quantity and explore the mechanism of cardiovascular development under different conditions of dissolved oxygen. Based on the functions of these genes in cardiovascular development, we speculated that hypoxia likely impacts cardiac looping via the Tbx5 gene. In addition, abnormal cardiac development under hypoxia might be attributed to impair cardiac looping. Hypoxia could impact vessel development through the VEGF/VEGFR and Notch signaling pathways. In conclusion, hypoxia caused embryo development arrest, heart and vascular abnormalities, and expression changes in cardiovascular development-related genes. Whether hypoxic stress in the early embryo development stage would affect the morphological structures and physiological functions of adult zebrafish remains to be further studied.
Key words: Hypoxia; Zebrafish embryos; Cardiovascular development; Gene expression
Dissolved oxygen (DO) in water is affected by many factors, including temperature, atmospheric pressure, circadian rhythm, seasonal change, water eutrophication, water pollution, dam overflow, and aquaculture density[1]. Excessive concentrations of DO in the water environment can also affect aquatic organisms, especially fish[2]. Hypoxic stress in fish results in a series of physiological responses. Although fish show compensation phenomenon when the stress is eliminated, the growth and development of fish are still affected. Due to abnormal amounts of DO, many problems of growth, development and disease arise in fish, including a large number of malformations and diseases. While previous studies on DO stress in fish have mainly focused on adult fish,few studies have focused on the effects of DO on the early growth and development of fish embryos. As a research model of cardiovascular development,zebrafish have the following advantage: the formation of the heart and blood circulation can be observed because of the transparency of the embryo body at early developmental stages. At the early stage of embryonic development, oxygen can be obtained from the environment by passive transportation without relying on the blood circulation system despite the absence of or damage to the cardiovascular system. Studies using zebrafish as a model have focused on the effects of different DO conditions on physiological and biochemical parameters, oxidative stress and related genes. Studies regarding that hypoxia affects the early growth and development of zebrafish embryos are rare. Ernens,et al. studied the formation of lymphocytes in zebrafish under hypoxia[3]. Zebrafish have emerged as an informative animal model to study the biological impact and molecular mechanisms of hypoxia. Kamei and Duan[4]have described a simple method to induce hypoxia in zebrafish embryos and larvae. Navina and Malathi[5]mimicked hypoxic condition and studied angiogenesis by inducing adenosine receptors using forskolin, a plant compound and NECA analogue of adenosine using zebrafish model. However, the effects of hypoxia on embryonic heart in zebrafish have not been reported. In this study, zebrafish embryos were observed under hypoxic and normoxic conditions to evaluate embryonic morphological development,heart and vascular development and the expression of related genes at 24—72 hpf. The goal of studying cardiovascular morphology and related gene expression in zebrafish embryos is to provide basic information on the effects of hypoxia on cardiovascular development in zebrafish embryos.
1 Material and method
1.1 Preparation of dissolved oxygen conditions
We used two DO conditions, hypoxia (2.0±0.5) mg/L and normoxia (6.0±0.5) mg/L. To maintain the preset DO concentrations, nitrogen and pure oxygen were injected directly into the water within the 1 L blue-mouth bottles in the hypoxia groups and normoxia groups, respectively. One hundred embryos were placed in each 1 L blue-mouth bottle, and the caps were tightened and sealed with parafilm to help stabilize the DO levels in each test group. Each experimental group was set up in triplicate. Zebrafish embryos were raised and kept under standard laboratory conditions (28℃; 14h/10h light/dark cycle). During the period of DO stress, the DO concentration in cultured water was detected every 2h, and the cultured water was replaced every 6h to ensure that the DO concentration was within the preset range. The measured DO concentrations were (2±0.14) mg/L for hypoxia and (6±0.26) mg/L for normoxia.
1.2 Determination of morphological and physiological parameters in embryos
The stages of embryonic morphological development were determined according to Kimmel,et al[6].
To determine the time window during which zebrafish embryos are susceptible to DO conditions,the embryos were treated under two DO conditions at 3 hpf for 15h and 27h at 24 hpf and 36 hpf for 12h and 24h, respectively. After treatment, the number of embryonic deaths was observed and recorded by stereomicroscopy.
To calculate the survival rate of zebrafish embryos, embryos at 6—48 hpf were evaluated by stereomicroscopy, and the number of embryonic deaths was recorded.
To determine the heart rate, 20 embryos were randomly selected from the embryos of 24 hpf, 36 hpf,48 hpf, 72 hpf and 24h-D (morphological development of embryo to 24 hours), 36h-D, 48h-D and 72h-D, respectively. The heart rate was observed and measured by counting heart beats under a stereomicroscope over a period of 1min.
To observe the morphological development of zebrafish embryos, 15—20 hpf, 36h-D and 48h-D embryos were randomly selected. The length of the embryo body was photographed and measured by a Leica DM 6000B fluorescence microscope.
To observe the development of the embryonic heart, transgenic zebrafish with green fluorescence protein-labeled hearts were selected. A total of 15—20 48h-D embryos were randomly selected. Embryonic heart development was observed and photographed by a Leica DM 6000B fluorescence microscope. To stabilize the embryo during imaging, the embryos were anesthetized with 0.03%—0.05% tricaine solution for 5—10min before photographing.After photographing, the embryos were washed 3 times with 1× ERS, and the embryos were returned to the incubator.
To observe the morphological development of vascular structures, transgenic zebrafish with vascular labeling by green fluorescence protein were selected. Embryos were randomly selected from among 48h-D and 72h-D embryos. The embryonic trunk vessels were observed and photographed by a Leica DM 6000B fluorescence microscope. The main parameters were the diameter of the dorsal aorta (DA), the diameter of the posterior cardinal vein (PCV), the distance between the adjacent intersegmental vessels(ISV), the distance from the dorsal longitudinal anastomotic vessels (DLAV) to the PCV and the growth angle of the ISV. Normally, the parachordal vessels(PAV) are formed at 36—48 hpf, but to avoid the possibility of delayed PAV growth, PAV development was observed in 72h-D embryos in this study.
1.3 In situ Hybridization (ISH)
PCR primers were designed using Prime Primer 5.0 software according to the known zebrafishCmlc2 gene CDS sequence in NCBI. The forward primer sequence is GGGAAGCTGAATGTGAGTGAT, and the reverse primer sequence is AGGGCCTTAAACC AAATGTCT. Cloning of the target gene cDNA,probe synthesis, and whole mountin situhybridization were performed as described by Li,et al[7].
1.4 qPCR
The cDNA reverse transcribed from 24h-D zebrafish embryo mRNA was used as a template, and the standard curve was generated with a 5-fold dilution gradient. Then, qPCR was performed. Total RNA was extracted as described by Li,et al[7]. After removing genomic DNA, cDNA was synthesized using the TaKaRa Reverse Transcriptase M-MLV (RNaseH-)kit.
The reaction system was configured according to the requirements of Promega GoTaq®qPCR Master Mix. Each group of 3 replicate wells was reacted on a Bio-Rad quantitative PCR instrument. The qPCR amplification curve and melting curve ofCmlc2,Tbx5,Aggf1,Flk1,igfbp-1genes andβ-actin(internal reference gene) were recorded and saved. The expression levels of the target gene were calculated by the 2-ΔΔCtmethod, and the changes in gene expression were analyzed by SPSS software. The primer sequences ofCmlc2,Tbx5,Aggf1,Flk1,igfbp-1andβactinare shown in Tab. 1.
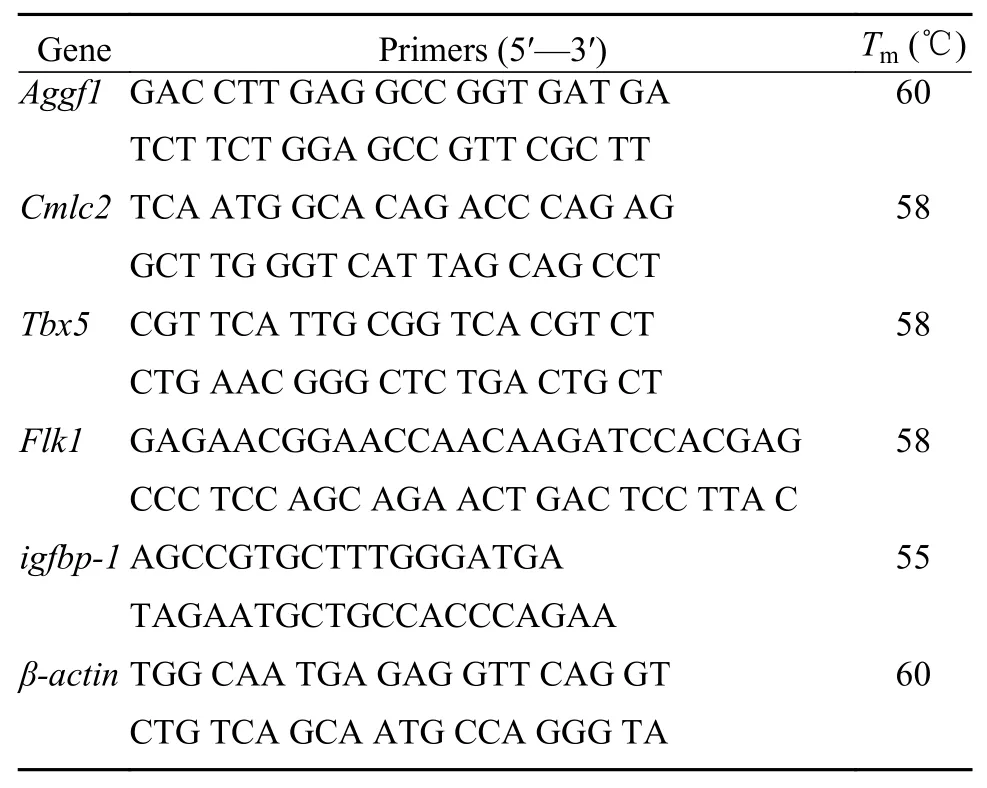
Tab. 1 Primers used for real-time fluorescent quantitative PCR
1.5 Statistical analysis
For qPCR data, the expression levels of the target gene were calculated by the 2-ΔΔCtmethod, and the changes in gene expression were analyzed by SPSS software. Other experimental data underwent varianceanalysis by SPSS. The results are expressed as the mean±standard deviation and were considered statistically significant atP<0.05 orP<0.01.
2 Results
2.1 Effect of hypoxia on the survival rate of embryos
The normal 3 hpf embryos were treated, the number of embryonic deaths within 48 hpf was recorded, and the survival rate (%) was calculated. The survival rate of embryos under normoxia was (74.18±4.23)%, and the survival rate of embryos under hypoxia was only (43.98±1.94)%. The survival rate of embryos under hypoxia was significantly lower than that under normoxia; hence, hypoxia reduced the survival rate of embryos.
2.2 Hypoxia-sensitive time windows for zebrafish embryos
Under the two DO conditions, the number of embryonic deaths was used to determine sensitivity to hypoxia. The results showed that the sensitive time window for embryos under hypoxia was 3—30 hpf.The mortality of the embryos decreased gradually with the delay in treatment initiation time (Fig. 1).The reason for this result may be that the additional development time allowed tissues and organs to be better developed, thus enhancing the adaptability and tolerance of the embryo to the external environment and increasing the survival rate.
2.3 Effects of hypoxia on the external morphology and body length of embryos
The 48h-D and 72h-D embryos were randomly selected for photography. At 48h-D, the embryos of the hypoxia group showed a small head (“→”), a large yolk ball, a short yolk extension (“*” mark),pericardial enlargement, and cardiac malformation(oval dotted line). At 72h-D, the embryos of the hypoxia group did not show improved pericardial edema; in addition, the head was short, the eye diameter was small, yolk absorption was lower than that in embryos of the normoxia group, the muscle compartment of the body was not clear, and the body was free of pigmentation (Fig. 2A). In summary, hypoxia caused a lag in zebrafish embryo development, and the heart and brain were greatly affected.
The body lengths of 36h-D and 48h-D embryos under normoxia and hypoxia were photographed and measured by a Leica DM 6000B fluorescence microscope. There was no significant difference between the two groups when the embryos developed to the 36h-D stage. At the 48h-D stage, the body length of zebrafish embryos in the hypoxia group was significantly shorter than that in the normoxia group (P<0.01;Fig. 2B).
2.4 Effects of hypoxia on embryonic heart morphology
To understand the effect of DO level on zebrafish embryonic heart morphology, this study allowed transgenic zebrafish with green fluorescence proteinlabeled hearts to develop to 48h-D under different DO concentrations and photographed them with a Leica DM 6000B fluorescence microscope. It was found that the embryonic heart developed an atrium and a ventricle under normoxia and hypoxia, the atrioventricular septum was connected to the atrioventricular septum, the atrium was connected to the inflow tract,and the ventricle was connected to the outflow tract.In the hypoxia group, the heart was an enlarged tube in the middle of the embryo body; the heart did not complete right heart looping, and the atrium and ventricle were small (Fig. 3).
Whether the heart develops normally is first indicated by whether the heart rate is normal. The zebrafish embryo showed heartbeats at 24 hpf. The heart rate of the hypoxia group was significantly lower than that of the normoxia group at 24—72 hpf(P<0.01). At 24 hpf, a heartbeat was not detected in the hypoxia because the embryo had not developed to the same stage as 24 hpf normal embryos, and the heart structure was not formed; 36—72 hpf, the heart rate was higher in the normoxia group than that in the hypoxia group (Fig. 4A). Hypoxia delayed heart development but did not affect heart development. At the same developmental stage of 24—72h-D, the heart rate of the hypoxia group was significantly lower than that of the normoxia group (P<0.01; Fig. 4B).Hypoxia had an effect on the heart and heart rate of zebrafish embryos, with a greater impact on embryonic heart rate. In summary, the differentiation of zebrafish embryos was not affected by hypoxia, but the development of cardiac organs was affected by hypoxia. Hypoxia had a marked effect on heart development, as it could delay the development of the heart and slow the heart rate.
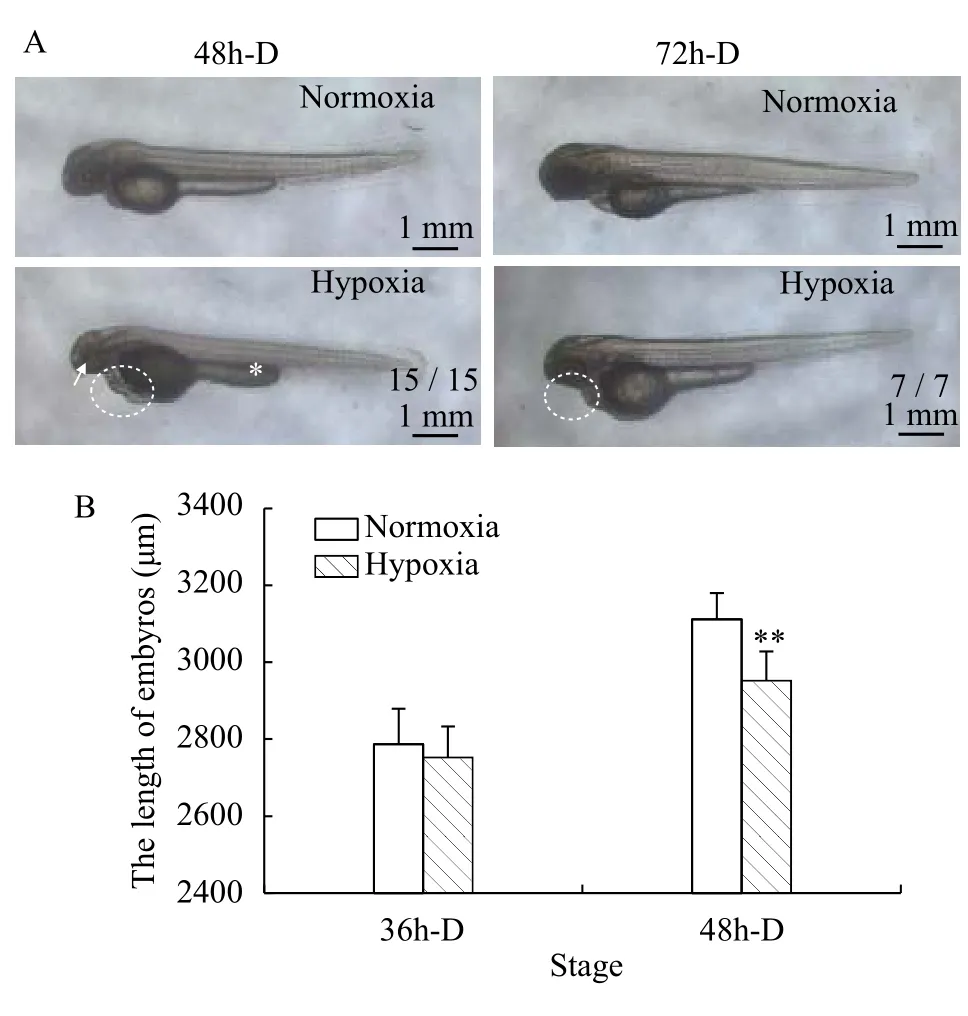
Fig. 2 Effects of hypoxia on the external morphology and body length of embryosA. External morphology differences of zebrafish embryos under normoxia and hypoxia, fraction indicate typical phenotype number/total samples number; B. Differences in embryonic body length under normoxia and hypoxia
2.5 Effects of hypoxia on embryonic vascular morphology
Under two DO conditions, the trunk vessels of the embryo were photographed with a fluorescence microscope at 72h-D. The results showed that under hypoxic stress, the main blood vessels in the trunk were extensively affected, with arteriovenous malformations, absence of subintestinal veins (SIVs;“★”), irregular ISV morphology, loosening of the DA, undeveloped caudal venous plexus (dotted box),and disappearance of PAV specificity as the main manifestations (Fig. 5). It is speculated that hypoxia inhibits SIV, PAV, and caudal venous plexus development.
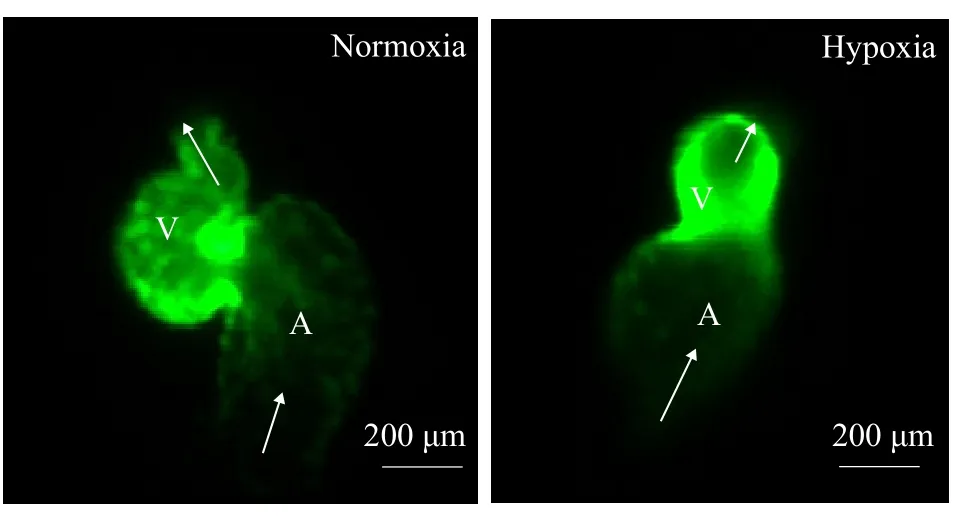
Fig. 3 Differences in the external heart morphology of zebrafish embryos under normoxia and hypoxiaThe developmental stage of the zebrafish embryonic heart was 48 h-D,and the ventricle and atrium are indicated as V and A, respectively
To more accurately understand the effect of DO concentration on the blood vessel system of zebrafish embryos, the related parameters of blood vessels were measured: the diameter of the DA and PCV, the distance from the DLAV to the PCV, the distance between two adjacent ISVs and the growth angle of the ISV. The diameter of the DA and PCV and the distance from the DLAV to the PCV were significantly longer in the normoxia group than in the hypoxia group (P<0.01; Fig. 6A and Fig. 6C). There was no significant difference in the distance between two adjacent ISVs (μm) under normoxia and hypoxia (Fig. 6D);however, the growth angle of the ISV (°) was extremely significantly larger in the hypoxia group than in the normoxia group (P<0.01; Fig. 6E). In summary, the vascular parameters of the zebrafish embryos under hypoxia showed notable changes, such as the decrease in the diameter of the main blood vessels (Fig. 6A and Fig. 6B), the shortening of the distance from the DLAV to the PCV, and the abnormal growth trajectory of the ISV. Therefore, the development of blood vessels in zebrafish embryos was greatly influenced by hypoxia.
2.6 Whole embryo in situ hybridization of cardiacrelated genes
The previous results showed that hypoxia hindered cardiac development in zebrafish embryos,but the initial mechanism by which DO levels affect morphological changes of zebrafish hearts remains unclear. Cardiac light chain 2 (Cmlc2), a cardiac marker gene, is mainly distributed in cardiomyocytes.To understand the earliest factor influenced by hypoxia in cardiac morphogenesis, a DIG-labeledCmlc2antisense probe was used for whole embryoin situhybridization. At 24h-D, the linearized heart tube of the normoxia group and the hypoxia group had formed and was gradually elongating (Fig. 7A, 7B).Cmlc2was expressed on the heart tube, but the expression level ofCmlc2in the hypoxia group was significantly lower than that in the normoxia group. At 36h-D, the linearized heart tube of the normoxia group had moved to the midline position of the embryonic body, and the heart tube had completed right heart looping (Fig. 7C); the ventricle (v) was located on the right side of the embryo body, and the atrium(a) was located on the left side of the embryo body.The embryonic heart tube of the hypoxic group did not undergo right heart looping and remained a linearized heart tube (Fig. 7D). In summary, hypoxia did not affect cardiac formation, but hypoxia affected the looping of the linearized heart tube, which is the first step in hypoxia-induced cardiac dysplasia.
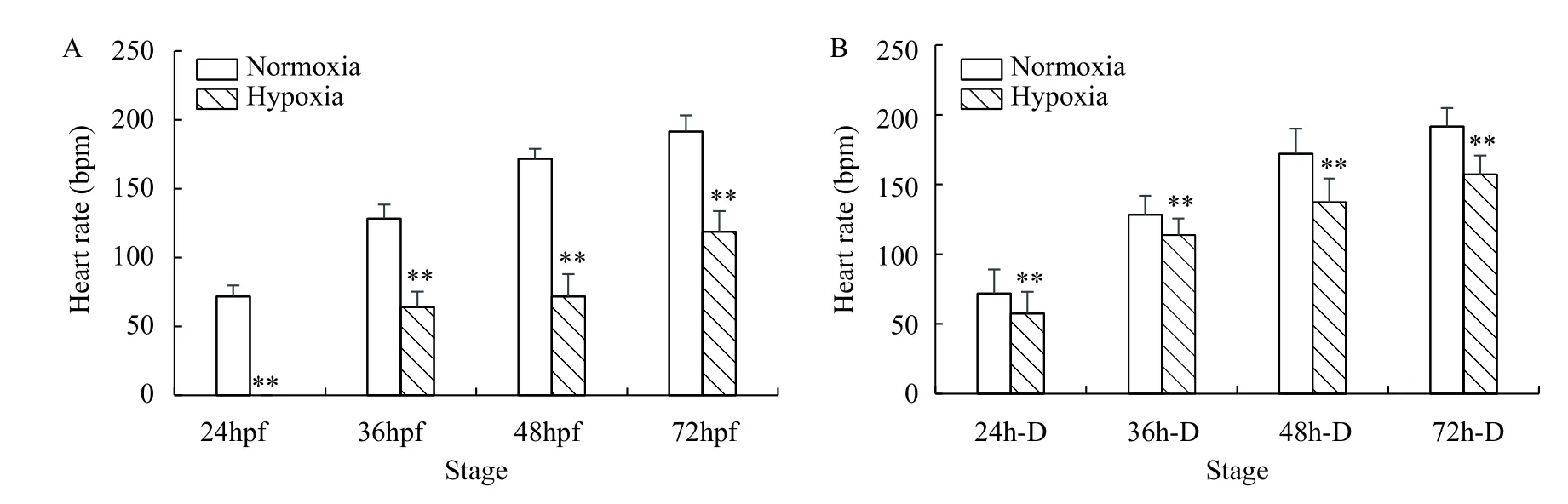
Fig. 4 Heart rate of zebrafish embryos under normoxia and hypoxia A. in the same treatment time; B. in the same development stage
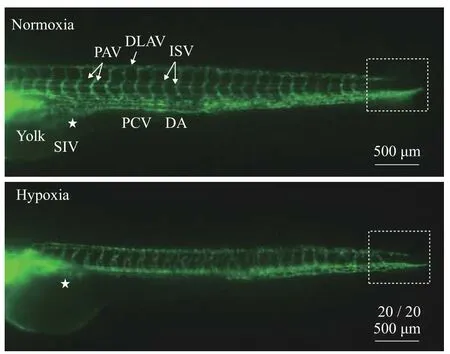
Fig. 5 Differences in the external morphology of trunk blood vessels in zebrafish under hypoxia and hyperoxiaThe developmental stage of the trunk blood vessels was 72h-D.DA. dorsal aorta; PCV. posterior cardinal vein; DLAV. dorsal longitudinal anastomotic vessel; PAV. parachordal vessel; ISV. intersegmental vessel; SIV. subintestinal vein. Fraction indicate typical phenotype number/total samples number
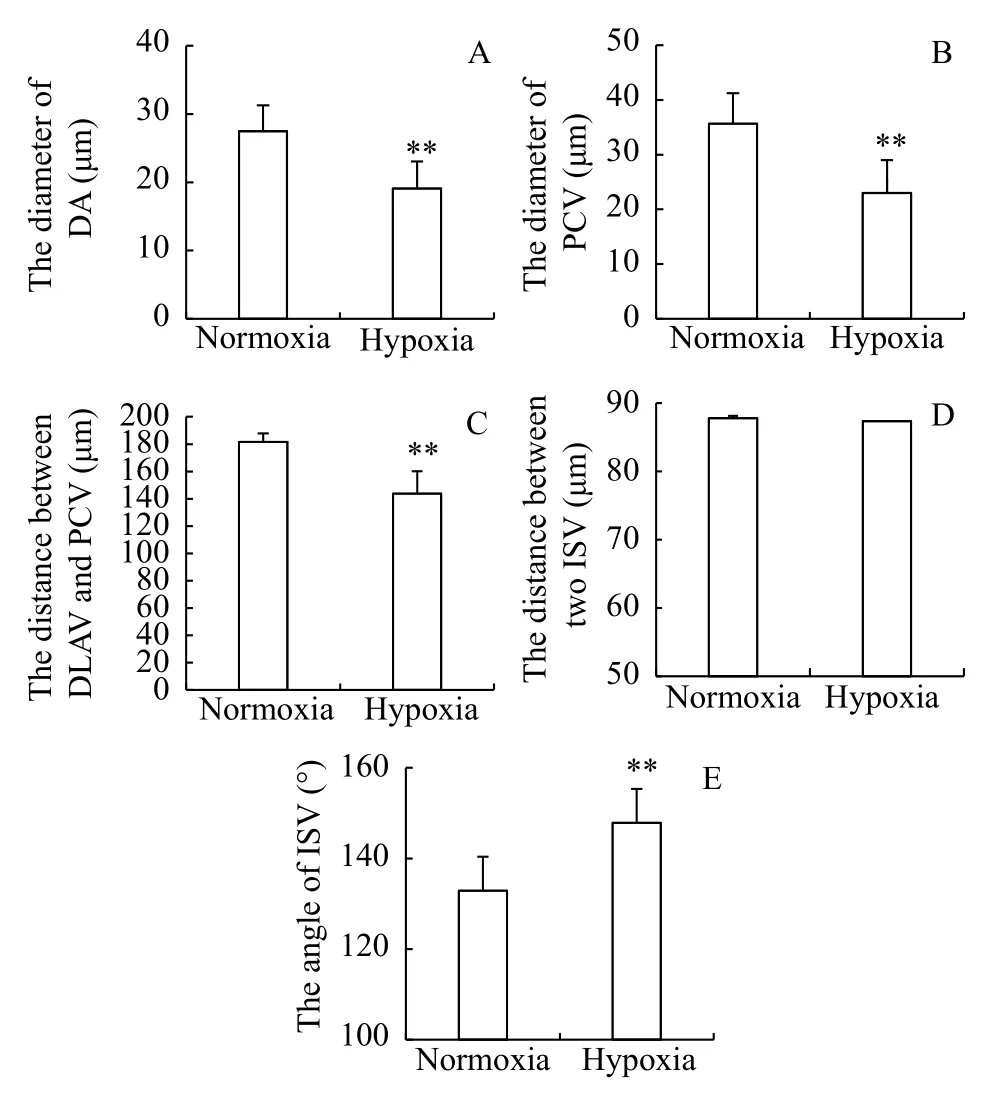
Fig. 6 Measurement of the blood vessel parameters in zebrafish embryosThe developmental stage of the trunk blood vessels was 72h-D.DA. dorsal aorta; PCV. posterior cardinal vein; DLAV. dorsal longitudinal anastomotic vessel; PAV. parachordal vessel; ISV. intersegmental vessel
2.7 Expression of genes involved in heart and vascular development as detected by qPCR
Subtle changes in genes expression cannot be detected byin situhybridization. To compensate for this limitation ofin situhybridization, the normal 3 hpf embryos were treated, 24h-D zebrafish embryo mRNA was used as a template, qPCR was performed to more accurately detect the expression of cardiovascular-related genes. The results showed that the expression level ofCmlc2was 1.00 in the normoxia group and 0.31±0.042 in the hypoxia group; the difference was highly significantly different (P<0.01;Fig. 8A); the expression of theCmlc2gene was downregulated by 69% in the hypoxia group, and hypoxia inhibited the expression ofClmc2. The expression level ofAggf1was 1.00 in the normoxia group, and its expression level in the hypoxia group was 0.64±0.043; the difference was significantly different (P<0.05; Fig. 8C). The expression ofAggf1was downregulated by 36% in the hypoxia group;hence, the expression ofAggf1was reduced by hypoxia. The expression level ofFlk1was 1.00 in the normoxia group and 0.77±0.075 in the hypoxia group;this difference was highly significantly different(P<0.01; Fig. 8D). The expression ofFlk1was downregulated by 23% in the hypoxia group; hence, the expression ofFlk1was reduced by hypoxia.
In recent years, it has been found thatTbx5in the T-box transcription factor family is also involved in the cardiac looping process[8]. To understand whether hypoxia-induced cardiovascular abnormalities were related toTbx5, the expression ofTbx5under hypoxia was detected by qPCR, and there was a highly significant difference between the normoxia and hypoxia groups (P<0.01; Fig. 8B).
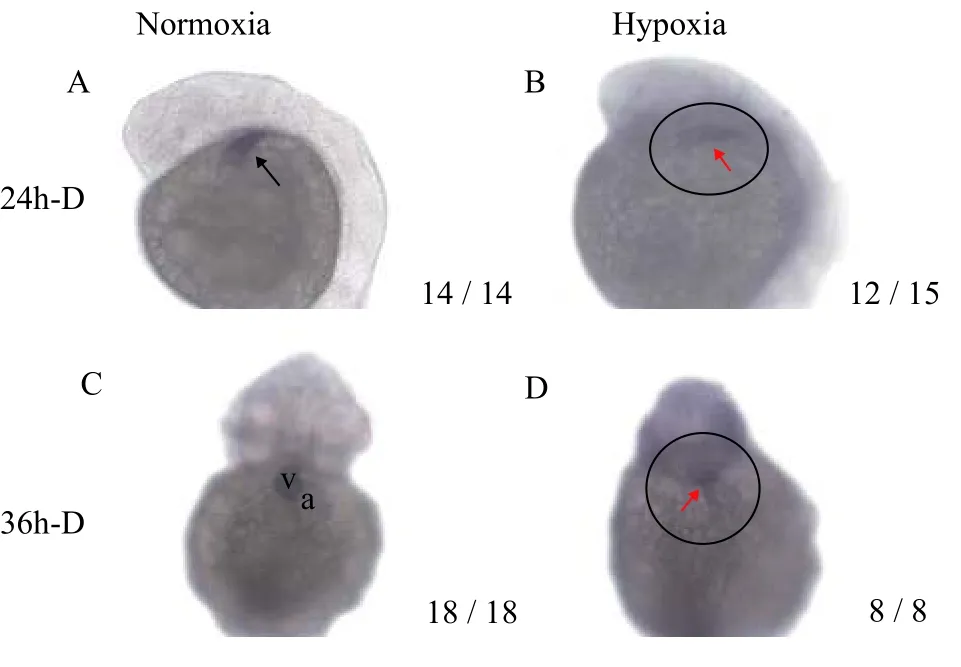
Fig. 7 Expression of Cmlc2 mRNA in zebrafish embryos under normoxia and hypoxia with ISHA and B are lateral views with the head pointed upward, and C and D are ventral views. v. ventricle and a: atrium. Fraction indicate typical phenotype number/total samples number
It has been found that exposure zebrafish embryos to hypoxia at 24 hpf activated the hypoxia-inducible factor-1(HIF-1) cellular pathway. Theigfbp-1gene is one of the HIF-1 target genes[9]. To understand whether hypoxic response activated by treatment in zebrafish, the expression level ofigfbp-1was detected by qPCR. The results showed that there was highly significantly different between the normoxia and hypoxia groups (P<0.01; Fig. 8E). The expression ofigfbp-1was upregulated in the hypoxia group;hence, the response of zebrafish embryos was activated by hypoxia.
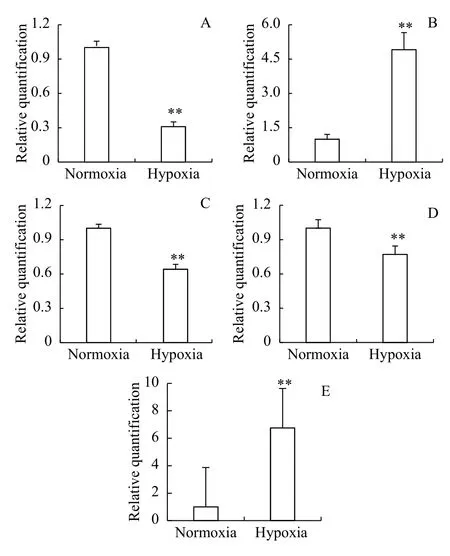
Fig. 8 Quantitative expression analysis of cardiovascular development genes and igfbp-1A, B, C, D and E show the expression of Cmlc2, Tbx5, Aggf1,Flk1, and igfbp-1 respectively
3 Discussion
3.1 Effects of hypoxia on the embryo survival rate and morphological development
In this study, zebrafish embryos were used as a model to investigate the effect of hypoxia on the early development of zebrafish embryos. The results showed that the survival rate of embryos under hypoxia decreased by 30.2%; hence, hypoxia significantly increased embryo mortality[10,11].
This study found that the sensitive time window for embryos under hypoxia was 3—30 hpf. Exposure to hypoxia at earlier time points in development resulted in greater affects in the embryos. Over time, the ability of the embryos to withstand adverse external environments was enhanced. Hypoxia not only delayed embryonic development but also caused irreversible damages. After hypoxia treatment, zebrafish embryos had vital signs, but there were many malformations in the fish body, such as stagnation of development,small size, small head, short eye diameter, black eyes,abdominal enlargement, yolk residue in the abdomen,pericardial edema, small heart tube, weak heartbeat,tail twist, no ingestive capacity, and no exercise capacity. These results were similar to reports on hypoxiainduced embryonic malformation[10,12,13].
3.2 Effects of hypoxia on embryonic heart development
Although certain vertebrate heart tissues are species-specific, early heart development processes in zebrafish are very similar to those in other vertebrates, including cardiac regionalization, cardiac primordia formation, tubular heart formation, cardiac cyclization, atrioventricular valve formation and the process of chamber septation[14]. In clinical medicine,abnormal looping of the human heart leads to two types of malformations: the right ventricle double exit malformation and the left ventricle double entrance malformation[15]. Zebrafish heart development is controlled by a variety of genes, andTbx5is involved in the developmental regulation of the heart[16]. Mutations in the humanTbx5gene result in Holt-Oram syndrome, which is manifested as defects in heart and upper limb development[17].Tbx5homozygous deletion results in severe malformations in the mouse,such as atrial and primary ventricular dysplasia and abnormal looping of the heart[18]. It should be noted thatTbx5expression begins very early, but it functions only during cardiac looping.
This study selected transgenic zebrafish with green fluorescence protein-labeled hearts as a model to study the effect of hypoxia on early embryonic heart development. It was found that hypoxia had a great effect on embryonic development. After hypoxic stress, embryonic heart development arrested at the heart tube stage. Although the heart differentiated into the atrium and ventricle, it could not complete the right heart looping process, and pericardial edema was also noted. The expression levels ofCmlc2andTbx5were detected byin situhybridization and qPCR. The results showed that hypoxia could markedly inhibit the expression ofCmlc2.Cmlc2is a cardiac marker gene that is mainly distributed in cardiomyocytes, and the expression ofCmlc2is blocked, resulting in abnormal heart development. The expression ofTbx5increased under hypoxia, and based on the morphological results, it was speculated that the failure to undergo embryonic heart looping after hypoxic stress was associated with increased expression ofTbx5.Mutations in zebrafishTbx5and overexpression of mouseTbx5can lead to circulatory disorders of the embryonic heart[8,19,20]. Therefore, we believe that the high level ofTbx5gene expression is one of the mechanisms of cardiovascular disorders after hypoxic stress. Long-term hypoxic stress during embryonic development leads to cardiovascular disorders,which in turn lead to abnormal embryonic heart formation and function. This result provides a potential mechanism underlying fetal heart disease caused by maternal uterine hypoxia in clinical medicine.
3.3 Effects of hypoxia on embryonic vascular development
The process of vascular network formation involves a series of cellular transformations that require the coordinated effort of adjacent endothelial cells.VEGFspecifically acts on vascular endothelial cells,and cardiomyocytes, vascular endothelial cells, the brain, the liver and kidneys can secreteVEGF; hypoxia, high glucose and inflammation can induceVEGFexpression[21,22].Flk1gene signal transduction can control endothelial cell proliferation, migration and survival[23,24]. In zebrafish embryos, at 24 hpf,Flk1is expressed in the main vessels of the trunk and in the internodes[25—27]. Endothelial cell migration and proliferation involve a variety ofVEGFRexpression patterns. The disappearance ofFlk1or the downregulation of thePLC-γ1effector affects only arterial development and differentiation; the head vein disappears whenFlk1is completely depleted. The embryonic veins in zebrafish develop normally after mutation of thePLC-γ1effector, and theFlk1 gene signal is significantly downregulated during arterial and venous development[28]. Recent studies have confirmed that the Notch controls the expression ofVEGFR[29].
Aggf1plays an important role in angiogenesis,especially venous development[30]. Overexpression ofAggf1induces excessive growth of ISVs, the formation of vascular filopodia, and excessive branching of ISV. Knockdown of theAggf1gene also affects the development of mesencephalic venous vessels and inhibits the accumulation of parachordal lymphatic cells in zebrafish embryos, suggesting thatAggf1may be involved in the development of lymphatic vessels[31,32].
Through the combination of morphological observations, hypoxia affected embryonic vascular development but did not affect angiogenesis. In the present study, we concluded that zebrafish embryonic angiogenesis was genetically determined, and environmental factors such as oxygen cannot affect the establishment of blood vessels. Hypoxia led to a decrease in the diameter of the DA and PCV, a shorter distance from the DLAV to the PCV, an abnormal trajectory of the ISV, and a loss of PAV specificity.These results confirmed that oxygen is involved in vascular development and that hypoxia has a significant inhibitory effect on vascular development. We hypothesized that hypoxia might regulate vascular development by altering the expression ofVEGF/VEGFR. Hypoxia reduced the expression ofFlk1andAggf1in present study, thereby reducing the proliferation of venous vascular cells and reducing the diameter of the PCV.Flk1was essential in the development of the DA. Hypoxia reduced the expression ofFlk1and affected the development of the DA. From a morphological perspective, the diameter of the DA appeared to be reduced. Related studies have shown that hypoxia plays an important role in the regulation of gene expression. HIF plays an important role in the response to hypoxic stress. The regulation ofVEGFby regulating the expression of the target geneVEGFis unclear.Flk1is aVEGFgene family receptor that affects the growth of vascular endothelial cells by binding to the correspondingVEGFligand. Therefore, we speculated that after hypoxic stress, the expression ofVEGFmight be regulated by the HIF gene to affect the binding ofFlk1andVEGF, thus affecting the development of embryonic blood vessels.
The expression of important ligands forFlk1,such asVEGF-A,VEGF-CandVEGF-Dgenes and proteins, and related pathway genes under hypoxia has not been reported. WhetherAggf1is directly regulated by hypoxia or by other genes requires further study. After embryos are stressed, whether other vascular development-related genes and aerobic-associated signaling pathways are involved in the regulation of vascular development needs further investigation.
4 Conclusion
Based on the results from this study, the survival rate of zebrafish decreased under hypoxia. Hypoxia caused embryonic development to be delayed by approximately 24—48h, yolk dysfunction, small eyes,abnormal heart looping, and slow heart rate. Hypoxia led to a decrease in the diameter of the DA and PCV,a shorter distance from the DLAV to the PCV, an abnormal trajectory of the ISV, a loss of PAV specificity, and a lack of SIVs. Hypoxia reduced the expression of the cardiac-specific geneCmlc2, that hypoxia downregulated the expression ofFlk1in the periocular region, and that the intervascular vascular expression site disappeared. Hypoxia also reduced the expression levels of theCmlc2,Aggf1andFlk1genes and increased the expression ofTbx5. According to the function of the above genes in heart and blood vessel development, it is suggested that hypoxia may affect heart looping throughTbx5, which is the origin of hypoxia-induced cardiac dysplasia. Hypoxia may affect the normal development of embryonic blood vessels through theVEGF/VEGFRpathway and the Notch signaling pathway. The effect of zebrafish early embryo development on adult morphological structure and physiological function after hypoxic stress needs further study.
Acknowledgements:
We thank Yu-Yu Chi for her help on qPCR.
References:Chemistry, 2017, 36(3): 682-690.
[1]Guan B, Ma H, Wang Y P,et al. Vitreoscilla hemoglobin(VHb) overexpression increases hypoxia tolerance in zebrafish (Danio rerio) [J].Marine Biotechnology, 2011,13(2): 336-344.
[2]Rees B B, Sudradjat F, Love J W. Acclimation to hypoxia increases survival time of zebrafish,Danio rerio, during lethal hypoxia [J].Journal of Experimental Zoology, 2001, 289(4):266-272.
[3]Ernens I, Lumley A I, Zhang L,et al. Hypoxia inhibits lymphatic thoracic duct formation in zebrafish [J].Biochemical and Biophysical Research Communications, 2017,482(4): 1129-1134.
[4]Kamei H, Duan C M. Hypoxic treatment of zebrafish embryos and larvae [J].Methods in Molecular Biology(Clifton,N. J.), 2018(1742): 195-203.
[5]Navina P, Malathi R. Targeting expression of adenosine receptors during hypoxia induced angiogenesis-A study using zebrafish model [J].Biomedicine & Pharmacotherapy,2018(99): 101-112.
[6]Kimmel C B, Ballard W W, Kimmel S R,et al. Stages of embryonic development of the zebrafish [J].Developmental Dynamics, 1995, 203(3): 253-310.
[7]Li Y, Xiang J H, Duan C M. Insulin-like growth factor-binding protein-3 plays an important role in regulating pharyngeal skeleton and inner ear formation and differentiation [J].Journal of Biological Chemistry, 2005, 280(5): 3613-3620.
[8]Garrity D M, Childs S, Fishman M C. The heartstrings mutation in zebrafish causes heart/fin tbx5 deficiency syndrome[J].Development, 2002, 129(19): 4635-4645.
[9]Cayleih E R, Patricia A W, Louise K,et al. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish,Danio rerio[J].Proceedings:Biological Sciences, 2014, 281(1786): 1-9.
[10]Andrade T S, Henriques J F, Almeida A R,et al. Zebrafish embryo tolerance to environmental stress factors-concentration-dose response analysis of oxygen limitation, pH, and UV-light irradiation [J].Environmental Toxicology and
[11]Bardon-Albaret A, Saillant E A. Effects of hypoxia and elevated ammonia concentration on the viability of red snapper embryos and early larvae [J].Aquaculture, 2016(459): 148-155.
[12]Mu J L, Jin F, Zhao H D,et al. Early life co-exposures to a real-world PAH mixture and hypoxia result in later life and next generation consequences in medaka (Oryzias latipes)[J].Aquatic Toxicology, 2017(190): 162-173.
[13]Ritchie H E, Oakes D J, Kennedy D,et al. Early gestational hypoxia and adverse developmental outcomes [J].Birth Defects Research, 2017, 109(17): 1358-1376.
[14]Schoenebeck J J, Keegan B R, Yelon D. Vessel and blood specification override cardiac potential in anterior mesoderm [J].Developmental Cell, 2007, 13(2): 254-267.
[15]Kathiriya I S, Srivastava D. Left-right asymmetry and cardiac looping: implications for cardiac development and congenital heart disease [J].American Journal of Medical Genetics, 2000, 97(4): 271-279.
[16]Ghosh T K, Song F F, Packham E A,et al. Physical interaction between tbx5 and mef2c is required for early heart development [J].Molecular and Cellular Biology, 2009, 29(8):2205-2218.
[17]Bruneau B G, Nemer G, Schmitt J P,et al. A murine model of holt-oram syndrome defines roles of the t-box transcription factor tbx5 in cardiogenesis and disease [J].Cell, 2001,106(6): 709-721.
[18]Chapman D L, Garvey N, Hancock S,et al. Expression of the t-box family genes, tbx1-tbx5, during early mouse development [J].Developmental Dynamics, 1996, 206(4): 379-390.
[19]Hatcher C J, Basson C T. Getting the t-box dose right [J].Nature Medicine, 2001, 7(11): 1185-1186.
[20]Liberatore C M, Searcy-Schrick R D, Yutzey K E. Ventricular expression of tbx5 inhibits normal heart chamber development [J].Developmental Biology, 2000, 223(1): 169-180.
[21]Nilsson M, Heymach J V. Vascular endothelial growth factor(VEGF) pathway [J].Journal of Thoracic Oncology, 2006,1(8): 768-770.
[22]Yang J G, Wang L L, Ma D C. Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases [J].British Journal of Haematology, 2018, 180(3): 321-334.
[23]Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial vegf receptor signaling [J].Nature Reviews Molecular Cell Biology, 2016, 17(10): 611-625.
[24]Hicklin D J, Ellis L M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis [J].Journal of Clinical Oncology, 2005, 23(5): 1011-1027.
[25]Fouquet B, Weinstein B M, Serluca F C,et al. Vessel patterning in the embryo of the zebrafish: guidance by notochord [J].Developmental Biology, 1997, 183(1): 37-48.
[26]Liao W, Bisgrove B W, Sawyer H,et al. The zebrafish gene cloche acts upstream of aflk-1homologue to regulate endothelial cell differentiation [J].Development, 1997, 124(2):381-389.
[27]Siekmann A F, Lawson N D. Notch signalling and the regulation of angiogenesis [J].Cell Adhesion & Migration, 2007,1(2): 104-105.
[28]Siekmann A F, Covassin L, Lawson N D. Modulation of VEGF signalling output by the Notch pathway [J].Bioessays,2008, 30(4): 303-313.
[29]Cao L, Arany P R, Wang Y S,et al. Promoting angiogenesis via manipulation of VEGF responsiveness with Notch signaling [J].Biomaterials, 2009, 30(25): 4085-4093.
[30]Chen D, Li L, Tu X,et al. Functional characterization of klippel-trenaunay syndrome geneAGGF1 identifies a novel angiogenic signaling pathway for specification of vein differentiation and angiogenesis during embryogenesis [J].Human Molecular Genetics, 2013, 22(5): 963-976.
[31]Ren B, Deng Y, Arpita M,et al. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish [J].Journal of Clinical Investigation, 2010, 120(4): 1217-1228.
[32]Hong C C, Peterson Q P, Hong J Y,et al. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and map kinase/ERK signaling [J].Current Biology,2006, 16(13): 1366-1372.

