An LC-MS/MS method for simultaneous analysis of the cystic fibrosis therapeutic drugs colistin,ivacaftor and ciprofloxacin
Huiya Yuan,Shihui Yu,Guihong Chai,Junting Liu,Qi(Tony)Zhou,*
aSchool of Forensic Medicine,China Medical University,Shenyang,110122,China
bDepartment of Industrial and Physical Pharmacy,College of Pharmacy,Purdue University,West Lafayette,IN,47907,USA
Keywords:
Cystic fibrosis
Ivacaftor
Colistin
Ciprofloxacin
HPLC-MS/MS
Lung epithelial cells
Plasma
Peer review under responsibility of Xi’an Jiaotong University.
A B S T R A C T
Inhaled antibiotics such as colistin and ciprofloxacin are increasingly used to treat bacterial lung infections in cystic fibrosis patients.In this study,we established and validated a new HPLC-MS/MS method that could simultaneously detect drug concentrations of ciprofloxacin,colistin and ivacaftor in rat plasma,human epithelial cell lysate,cell culture medium,and drug transport media.An aliquot of 200μL drug-containing rat plasma or cell culture medium was treated with 600μL of extraction solution(acetonitrile containing 0.1% formic acid and 0.2% trifluoroacetic acid(TFA)).The addition of 0.2% TFA helped to break the drug-protein bonds.Moreover,the addition of 0.1% formic acid to the transport medium and cell lysate samples could significantly improve the response and reproducibility.After vortexing and centrifuging,the sample components were analyzed by HPLC-MS/MS.The multiple reaction monitoring mode was used to detect the following transitions:585.5-101.1(colistin A),578.5-101.1(colistin B),393.2-337.2(ivacaftor),332.2-314.2(ciprofloxacin),602.3-101.1(polymyxin B1 as internal standard(IS))and 595.4-101.1(polymyxin B2 as IS).The running time of a single sample was only 6 min,making this a time-efficient method.Linear correlations were found for colistin A at 0.029-5.82 μg/mL,colistin B at 0.016-3.14 μg/mL,ivacaftor at 0.05-10.0 μg/mL,and ciprofloxacin at 0.043-8.58μg/mL.Accuracy,precision,and stability of the method were within the acceptable range.This method would be highly useful for research on cytotoxicity,animal pharmacokinetics,and in vitro drug delivery.
1.Introduction
Cystic fibrosis(CF)is an autosomal negative genetic disease characterized by a defect in the cystic fibrosis transmembrane conductance regulator(CFTR)protein[1].Cystic fibrosis can lead to recurrent respiratory infections[2].The production of sticky mucus in the lungs clogs the airways,causing chronic lung infections and excessive inflammation[3].Known CF pathogens include Staphylococcus aureus(SA)and Pseudomonas aeruginosa(PA).In the later stages of the disease,some patients are infected with more resistant and difficult-to-treat isolates[4,5].Ivacaftor is the first U.S.Food and Drug Administration(FDA)-approved CFTR protein used to treat CF patients who have a G551D mutation,which affects expression of the CFTR gene and changes the frequency of ion channel opening[6,7].
In recent years,colistin has been employed to treat infections caused by multiple drug-resistant Gram-negative bacteria(such as PA)in CF patients[8].Colistin,also known as polymyxin E,is a cationic lipopeptide antibiotic composed of cyclic decapeptide and fatty acyl chain[9].As a polycationic cyclic peptide,colistin can adsorb on the surface of electronegative bacterial membranes,killing the bacteria or promoting synergistic antibiotic uptake(when used together with a synergistic antibiotic)[10].In recent years,many other antibiotic drugs have been co-delivered with colistin to the lungs for synergistic treatment of CF or infections.Ciprofloxacin is a broad-spectrum quinolone antibiotic whose main antimicrobial mechanism inhibits bacterial DNA gyrase and topoisomerase IV[11].Literature shows that combining ciprofloxacin with colistin could maximize treatment efficacy and reduce drug resistance in chronic pulmonary infections caused by Gramnegative pathogens[12,13].Meanwhile,it was also reported that ivacaftor could be employed in combination with colistin.Ivacaftor demonstrates a certain degree of antibacterial activity against SA due to the existence of the quinoline ring structure[14].Moreover,it was demonstrated that the combination of ivacaftor and polymyxins exhibited an in vitro synergistic antibacterial activity against SA[15].Considering it is possible for CF patients to take colistin,ciprofloxacin and/or ivacaftor simultaneously,there is a demand for detecting these drugs in different media for research purposes.
Due to its amphipathic polypeptide structure,colistin tends to adhere with plasma proteins and form tight drug-protein bonds,which reduces the extraction recovery rate.In the previous studies,complicated sample pre-treatment procedures including solidphase extraction and the addition of derivatization reagents were needed for the analysis of colistin or polymyxin B[16,17].The previously reported HPLC-MS/MS method for the determination of colistin used different kinds of protein precipitation reagents[18-20].Therefore,it is necessary to establish a method with simple pre-processing steps and accurate analysis.In this study,a horizontal experiment was designed to compare the drug extraction recovery of three types of drug-protein bond cleavage agents in three different extraction solvents.
For further research in therapeutic options for CF patients,we established an HPLC-MS/MS method for the simultaneous determination of colistin,ivacaftor and ciprofloxacin.This modified method simplifies the pretreatment process while maintaining a high extraction recovery rate with short retention time.The method was validated in different media including rat plasma,cell culture medium,and drug transport medium.A two-stage mass spectrometry method was developed for the detection of ivacaftor based on the research by Schneider et al.[21],to better eliminate the interference of endogenous substances and to improve detection sensitivity.Our new method is reliable for the detection of three drugs in a complex matrix such as plasma and cell culture medium.The method also simplifies the extraction process of colistin in plasma and improves the recovery of drugs by screening different drug-protein bond cleavage agents[18,22,23].
2.Experimental
2.1.Equipment
The analyses were performed with Agilent 1200 HPLC system and Agilent 6460 QQQ mass spectrometer(Agilent Technologies Inc.,Palo Alto,CA,USA)with an electrospray ionization(ESI)source.Data processing was performed with a MassHunter B.07.00 workstation.A vortex mixer(Thermo Fisher Scientific,Waltham,MA,USA)and high-speed refrigerated centrifuge(Tomy MX-200,Thermo Fisher Scientific)were used for the pretreatment of samples.
2.2.Materials
Colistin sulfate salt,polymyxin B sulfate salt(as internal standard(IS)),and ciprofloxacin hydrochloride monohydrate were supplied by Betapharma Co.,Ltd.(Suzhou,China).Ivacaftor was purchased from Shanghai AOKChem Co.,Ltd.(Shanghai,China).The Sprague-Dawley rat plasma was purchased from Innovative Research,Inc.(Novi,MI,USA).
The Calu-3 human bronchial epithelial cell line was obtained from the American Type Culture Collection(ATCC,Manassas,VA,USA).The Hanks' balanced salt solution(HBSS),fetal bovine serum(FBS),and Dulbecco's modified eagle medium(DMEM)were purchased from Gibco Life Technologies Corporation(Grand Island,NE,USA).The RIPA lysis solution and extraction buffer were obtained from Thermo Scientific.Both trifluoroacetic acid(TFA)and trichloroacetic acid(TCA)were obtained from Sigma-Aldrich(St.Louis,MO,USA).All reagents were of analytical grade or better.
2.3.Cell culture
Calu-3 is the most commonly used human lung epithelial cell line for evaluating pulmonary drug transport in vitro[24,25].The Calu-3 cell lines(passage 25-35)were cultured in a 25 cm2flask in a culture medium consisting of DMEM supplemented with 10%(V/V)FBS,1%(V/V)non-essential amino acid solution,100 U/mL penicillin,and 100μg/mL streptomycin.The cells were incubated at 37°C in an environment of 5% carbon dioxide and 95% relative humidity,and subcultured according to the procedures recommended by ATCC[25].
2.4.Standard and quality control(QC)sample preparations
Colistin sulfate salt,polymyxin B sulfate salt(as IS),and ciprofloxacin hydrochloride monohydrate were dissolved in water separately to prepare stock solutions with a concentration of 1 mg/mL for each drug.Ivacaftor was weighed and dissolved in acetonitrile to an approximate concentration of 0.1 mg/mL.The IS stock solution of 1.0μg/mL polymyxin B sulfate salt was made in water.All stock solutions were stored at 4°C.
The composition of colistin sulfate salt varies with different production batches[26].We calculated that the apparent nominal concentration of colistin A and colistin B in the colistin sulfate used in this experiment was 58.2% and 31.4%,respectively[22,27].
Blank rat plasma was spiked with the stock solutions to make standard samples.For colistin A,the concentration ranges were 0.029,0.058,0.291,0.582,2.91,and 5.82μg/mL,and the three QC levels were 0.145,0.29 and 2.9μg/mL.For colistin B,the concentration ranges were 0.016,0.031,0.157,0.314,1.57 and 3.14μg/mL,and the three QC levels were 0.08,0.16 and 1.6μg/mL.Six calibration standard samples of ivacaftor at 0.05,0.1,0.5,1.0,5.0 and 10.0μg/mL in plasma were prepared,with the QC samples of 0.25,0.5 and 5.0μg/mL.Ciprofloxacin standard samples were set at concentration levels of 0.043,0.086,0.429,0.858,4.29 and 8.58μg/mL,and three levels of QC samples were prepared at concentrations of 0.215,0.43 and 4.3μg/mL.
For the standard samples and QC samples of cell culture medium,the concentrations of each analyte were prepared the same as those in the plasma samples by diluting the stock solutions with cell culture medium.
2.5.Sample preparation
2.5.1.Extraction from plasma and cell culture medium
An aliquot of 200μL drug-containing rat plasma or cell culture medium was transferred into a 1.5 mL polypropylene(PP)micro test tube with the addition of 600μL of extraction solution(acetonitrile containing 0.1% formic acid and 0.2% TFA).Then the sample was vortex mixed for 30 s and centrifuged at 10,000 r/min(7,200 g)for 5 min at 4°C.An aliquot of 200 μL of supernatant was withdrawn and transferred into an HPLC polypropylene vial for analysis.
2.5.2.Extraction from transport medium(HBSS)
After adding 0.1% formic acid,the HBSS sample was transferred into an HPLC PP vial.
2.5.3.Extraction from cell lysis sample
The cultured cells were digested with trypsin and centrifuged to remove the cell culture medium.RIPA lysate was added to form a density of 1×105cells/mL.An aliquot of 200μL of cell lysis solution was transferred into a 1.5 mL PP micro test tube,with the addition of 400μL of acetonitrile(containing 0.1% formic acid)to precipitate proteins.After vortex mixing for 30 s,the sample was centrifuged at 10,000 r/min(7,200 g)for 5 min at 4°C.The supernatant was collected for LC-MS/MS analysis.
2.6.Chromatographic and mass spectrometric conditions
A Kinetex C18(2.6 μm,100 Å,50 mm × 3 mm)column(Phenomenex,Los Angeles,CA,USA)was employed to separate sample components.The sample was stored in an automatic sampler at 4°C.The flow rate was 0.4 mL/min with an injection volume of 10μL.The mobile phase consisted of 0.1%(V/V)aqueous formic acid(solvent A)and 0.1%(V/V)formic acid in acetonitrile(solvent B).The gradient elution procedure was set as follows.The proportion of phase A from 0 to 0.5 min was 90%.The proportion of phase B rose to 60% from 0.5 to 1.0 min,then to 90% from 1.5 to 2.5 min,and was maintained at 90% for 0.5 min.Afterwards,the proportion of phase B decreased to 10% from 3.0 to 3.5 min,and was maintained until 6 min.
Each component was analyzed using multi-reaction monitoring.The positive ion mode of ESI source was adopted.The gas temperature was 350°C with a flow rate of 9 L/min.The nebulizer pressure was 35 psi.The sheath gas temperature was 300°C with a flow rate of 9 L/min.The capillary voltage was 4000 V and nozzle voltage was 1000 V.The MS/MS transitions of each compound analyzed are shown in Table 1.The typical chromatograms of drugs are shown in Fig.1.

Fig.1.Typical chromatograms of the drugs:(A)colistin A,(B)colistin B,(C)ivacaftor,(D)ciprofloxacin,(E)polymyxin B1,and(F)polymyxin B2.ESI:electron spray ionization;MRM:multiple reaction monitoring.

Table 1Mass spectrometric conditions for each compound.
2.7.Method validation
2.7.1.Specificity
Specificity was evaluated by comparing the chromatograms of six different batches of blank plasma and three different lots of HBSS.When the signal is less than 20% of the lower limit of quantification(LLOQ)peak area of the compound to be tested,the matrix can be considered to have no interference.
2.7.2.Carryover
When evaluating carryover,blank samples were injected in triplicate after the highest calibration standard.The signal in blank samples should be less than 20% of the LLOQ samples.
2.7.3.Matrix effect and extraction recovery
Matrix effects were assessed by comparing the peak area of the post-extracted samples to the peak areas of pure solutions at the same concentration.Extraction recovery of the analytes and IS were determined by comparing the peak area of the extracted samples to that of the non-extracted samples.The matrix effect and extraction recovery of colistins A and B,ivacaftor,and ciprofloxacin were verified at two QC levels(low QC(LQC)and high QC(HQC))and analyzed in triplicate,whereas the IS was determined at a concentration of 1.0μg/mL.
2.7.4.Linearity and LLOQ
The linearity standard was prepared according to the steps in Section 2.4.A weighting factor of 1/x2produced the best fit for analytes.The linear regression model y=ax+b was used,where the ordinate Y stands for the ratio of the peak area of each component's quantitative ion transition to the peak area of the IS(sum of polymyxins B1 and B2),and the abscissa X corresponds to the mass concentration of each analyte.The LLOQ was determined as the lowest concentration of the linearity standard sample where the intra-day accuracy and precision were below 15%.
2.7.5.Accuracy and precision
1.4 统计学分析 采用SPSS 22.0统计软件对数据进行统计学分析,计量资料数据以表示,数据比较采用t检验,计数资料数据以[例(%)]表示,数据比较采用χ2检验,P<0.05为差异有统计学意义。
Intra-day precision and accuracy were calculated by continuous determination of three different concentration levels of QC samples,with each sample tested six times.The inter-day precision and accuracy were tested on three consecutive days by analyzing the above QC samples.The result of precision was expressed by the coefficient of variation(CV).The accuracy was expressed by average relative error.
2.7.6.Stability
LQC and HQC samples were selected for short-term and longterm stability tests,respectively.The LQC samples and HQC samples were stored at 4°C for one week and one month respectively,with each concentration measured in triplicate with each concentration measured in triplicate.The peak area was compared with the freshly prepared samples.If the accuracy(±15%)of the analytical values was within the acceptable limits,the samples were considered stable.
3.Results and discussion
3.1.Pre-treatment of plasma samples
Due to colistin-protein bonding,the use of protein precipitation reagent alone in the pre-treatment would lead to low extraction recovery of colistin below the detection limit(as shown in Table 2).In order to solve this recovery problem,we added protein bond cleavage reagent during the pre-treatment process.Several different protein bond cleavage reagents were considered based on the literature.Sin et al.[23]found that using 4% TCA in acetonitrile was effective in the removal of milk protein.Ma et al.[22]demonstrated that 10% TCA solution mixed with an equal volume of methanol as the protein precipitating reagent provided satisfactory recovery performance.Jansson et al.[18]found that adding 0.1% TFA to the protein precipitation reagent acetonitrile could effectively reduce colistin-protein bonds.In this study,three different protein bond cleavage agents were tested:acetonitrile-10% TCA aqueous(50/50,V/V),4% TCA(m/V)in acetonitrile and 0.1% TFA in acetonitrile.Furthermore, nine extractants were formed by replacing acetonitrile with methanol or methanol-acetonitrile(50/50,V/V).Plasma samples were prepared and extracted with these nine extractants.The 0.1% TFA in acetonitrile performed best,as shown in Table 2.

Table 2Effects of different protein bond cleavage reagents and extraction reagents on extraction recovery of colistin A and colistin B.
To optimize the concentration of TFA,we tried three concentrations of 0.1%,0.2%,and 0.3%.The relative response strength of the four drugs was calculated by setting the highest response value as 100%.When acetonitrile was used alone,the proteins in the serum condensed into lumps that were most effective at bonding the drugs.With the addition of 0.1% TFA,the drug-protein bond was broken down and the drug was released into the extraction solvent,as shown in Fig.2.The drug-protein bond broke more completely as TFA concentration increased.Under the condition of 0.2% TFA,colistins A and B showed the highest MS response.However,the response value decreased at 0.3% TFA,possibly because a TFA concentration higher than 0.2% leads to stronger ion suppression.For this reason,the 0.2% TFA was selected for the pretreatment.The results are shown in Table 3.

Table 3The relative response strength of the four drugs in different extraction solvents.

Table 4Method validation results for plasma.
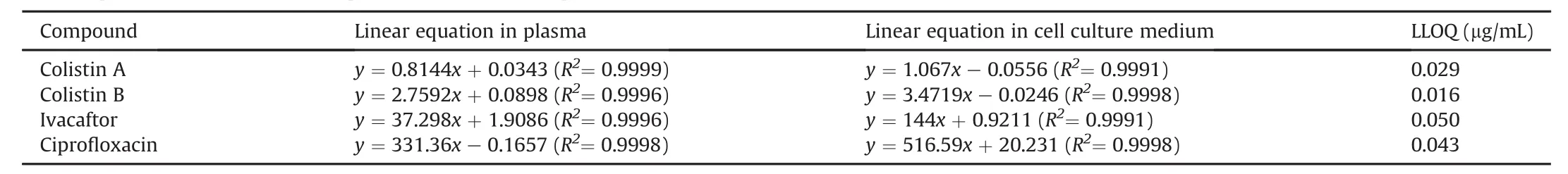
Table 5Linear equations and lower limit of quantification(LLOQ)in plasma and cell culture medium.

Fig.2.Cleavage of drug-protein bonds and mass spectrometry response percentage of each drug under different concentrations of trifluoroacetic acid(TFA):(A)0.1% TFA in comparison to acetonitrile(ACN)only and(B)concentrations of TFA were increased from 0.1% to 0.3%.
3.2.Optimization of drug extraction from the transport medium(HBSS)and cell lysis sample
Accurate quantification of drugs in epithelial cell samples and transport medium(HBSS in this case)is challenging because salts in the media can cause ion suppression during the LC/MS measurement.In our previous research,several additives were used to decrease the ion suppression caused by the salts in HBSS[25].During detection,the addition of 0.1% formic acid into the sample could significantly reduce the ion inhibition,promote the protonation,and improve the detection sensitivity.
The drug stock solution was added into the cell lysis sample,making concentrations of colistin A,colistin B,ivacaftor and ciprofloxacin at 0.582,0.314,0.500 and 0.429μg/mL,respectively.Two extraction solvents of acetonitrile alone and acetonitrile containing 0.1% formic acid were tested.After the addition of 0.1% formic acid,the extraction rates of colistin A and colistin B increased significantly to 207.9% and 221.3% of the original rates.The extraction rates of ivacaftor and ciprofloxacin decreased slightly to 71.35% and 85.83%,respectively.Therefore,acetonitrile with 0.1% formic acid was selected as the extraction condition for further studies.
3.3.Mass spectrometry
The precursor ion of colistin A was the doubly charged ion m/z 585.5[M+2H]2+.The dominant product ions for colistin A were m/z 241.0[6-methyloctanoicacid-L-Dab-γ-NH2+H]+and m/z 101.1[LDab-γ-NH2]+[28].The precursor ion of colistin B was the doubly charged ion m/z 578.5[M+2H]2+.The dominant product ions for colistin B were m/z 227.2 [6-methylheptanoicacid-L-Dab-γ-NH2+H]+and m/z 101.1[L-Dab-γ-NH2]+[28].These ions were consistent with those of the previous study that also used ESI as the ionization source[22].The main components and mass spectrometry MS structure analysis of polymyxin B sulfate salt are detailed in the literature[29].Polymyxin B sulfate salt is very similar in structure to colistin(as shown in Fig.3).Their recovery properties during the extraction process of the sample and the chromatographic behavior during the detection process were alike,which introduced a smaller detection error.Therefore,polymyxins B1 and B2 were chosen as the IS.The doubly charged polymyxin B1 ion m/z 602.3[M+2H]2+and polymyxin B2 ion m/z 595.4[M+2H]2+were measured.

Fig.3.Potential cleavage positions of each compound:(A)colistin,(B)ivacaftor,and(C)ciprofloxacin.m/z:mass-to-charge ratio.
For ivacaftor,the precursor ion was m/z 393.2[MH]+and the dominant daughter ion was m/z 337.2[MH-C4H9]+,which was the result after a tertbutyl cleavage.The ion m/z 288.2 originated from the breaking of the amide bond.The ions selected for ciprofloxacin were m/z 332.2,m/z 314.2 and m/z 288.2,which correspond to[MH]+,[MH-H2O]+and[MH-CO2]+,respectively[30].The potential cleavage positions of each compound are marked with red dashed lines in Fig.3.The typical product ions of the drugs are shown in Fig.4.

Fig.4.Typical product ions of the drugs:(A)colistin A,(B)colistin B,(C)ivacaftor,(D)ciprofloxacin,(E)polymyxin B1,and(F)polymyxin B2.
3.4.Methods validation
3.4.1.Specificity
In the blank plasma and HBSS,no obvious interference was observed at the retention time of the analyte and the IS,which indicates that the method has satisfactory selectivity.
3.4.2.Carryover
We optimized the ratio of needle washing solution as 50% water:25% acetonitrile:25% isopropanol.Both water-soluble colistin and ciprofloxacin or water-insoluble ivacaftor could be cleaned.After injection of the highest concentration of the analyte,no significant carryover was observed.
3.4.3.Matrix effects and extraction recoveries
As shown in Table 4,the results were within the acceptable limits and no significant matrix effect was observed.Using the protein precipitation method as previously described,a satisfactory and reproducible recovery was obtained for each analytical component.
3.4.4.Linearity and LLOQ
The linear equations are listed in Table 5.Deviations between the calculated and theoretical values were within 15%.
3.4.5.Accuracy and precision
For inter-day and intra-day experiments,the precision was within the acceptable range(CV≤15%)(Table 4).The accuracy was in the range of 85%-115% of the nominal concentrations,which also met the requirement.
3.4.6.Stability
For the stability experiment,the samples were stored at 4°C for one week and one month respectively,and compared with the freshly prepared samples.The accuracy of each drug was within±15%,which is considered to be relatively stable under such conditions.
4.Conclusions
The new HPLC-MS/MS method established here could be used for rapid and accurate analysis of colistin,ivacaftor and ciprofloxacin in various media simultaneously.The total retention time of less than 6.0 min is efficient for analyzing large quantities of samples.The sample pretreatment procedure could be applied to rat plasma,cells lysate,cell culture medium,and transport medium.This method would be useful in cystic fibrosis related pharmacokinetic,in vitro cytotoxicity and drug transport studies.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Research reported in this publication was supported by the National Institute of Health's National Institute of Allergy and Infectious Diseases under Award Nos. R01AI146160 and R01AI132681.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
 Journal of Pharmaceutical Analysis2021年6期
Journal of Pharmaceutical Analysis2021年6期
- Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
