Simultaneous determination of 35 constituents and elucidation of effective constituents in a multi-herb Chinese medicine formula Xiaoer-Feire-Kechuan
Zhnpeng Shng,Lulu Xu,Yi Kung,Yn Lin,Shung Liu,Long Sun,To Bo,Min Ye,**,Xue Qio,*
a State Key Laboratory of Natural and Biomimetic Drugs,School of Pharmaceutical Sciences,Peking University,Beijing 100191,China
bSchool of Pharmaceutical Sciences,Guizhou Medical University,Guizhou,550025,China
c Thermo Fisher Scientific,Beijing,100102,China
Keywords:
Multi-herb formulas
Effective constituents
Xiaoer-Feire-Kechuan formula(XFK)Parallel reaction monitoring(PRM)Cyclooxygenase-2(COX-2)
Peer review under responsibility of Xi’an Jiaotong University.
A B S T R A C T
Xiaoer-Feire-Kechuan(XFK)is an 11-herb Chinese medicine formula to treat cough and pulmonary inflammation.The complicated composition rendered its chemical analysis and effective-component elucidation.In this study,we combined quantitative analysis and bioactivity test to reveal the antiinflammatory constituents of XFK.First,UPLC-DAD and UHPLC/Q-Orbitrap-MS methods were established and validated to quantify 35 analytes(covering 9 out of 11 herbs)in different XFK formulations.Parallel reaction monitoring mode built in Q-Orbitrap-MS was used to improve the sensitivity and selectivity.Then,anti-inflammatory activities of the 35 analytes were analyzed using in vitro COX-2 inhibition assay.Finally,major analytes forsythosides H,I,A(8-10),and baicalin(15)(total contents varied from 21.79 to 91.20 mg/dose in different formulations)with significant activities(inhibitory rate≥80%)were proposed as the anti-inflammatory constituents of XFK.The present study provided an effective strategy to discover effective constituents of multi-herb formulas.
1.Introduction
Traditional Chinese medicines(TCMs)have attracted increasing attention due to their curative effects on complex and chronic diseases[1,2].In clinic,TCMs are mainly used in the form of formulas.The component herbs in a formula may target different symptoms of complex diseases,which might play synergistic roles in the drug efficacy[3,4].In total,1,933 TCM formulas are recorded in the Chinese Pharmacopoeia(2015 edition),and many of them are composed of more than 10 herbs[5],which are called “multi-herb formulas”or“Dafufang”[6].Up to now,only a few of reports have focused on the quality control of multi-herb formulas[6-9].For instance,12(6 out of 12 component herbs involved),16(5 out of 10 herbs involved),and 41(15 out of 19 herbs involved)analytes were determined in Kangjing formula,Yougui pill,and Niuhuangshangqing pill,respectively[6,7,9].However,due to their complex components,it is still challenging to comprehensively analyze the constituents and to discover the effective ones for multi-herb formulas.
Liquid chromatography with diode array detector(LC-DAD)and liquid chromatography tandem mass spectrometry(LC/MS)are popularly used methods to determine the contents of constituents in complex samples[9-14].Especially,parallel reaction monitoring(PRM)scan mode built in quadrupole(Q)-Orbitrap-MS combines the mass isolation capability of the quadrupole and the high resolution of Orbitrap detector,which could avoid false compliant and non-compliant results in complex samples[15-17].It emerges as a promising method to monitor characteristic analytes in multi-herb formulas.To further elucidate the effective constituents of multiherb formulas,feasible pharmacological models that are related to the therapeutic effect should be developed[18].For example,cyclooxygenase-2(COX-2)is one of the main isozymes responsible for inflammation[19].It has been proved to play roles in lung inflammation[20],such as pneumonia,bronchitis,and asthma[21,22].Therefore,COX-2 inhibitory assay can be used to reveal anti-inflammatory constituents from formulas.
Xiaoer-Feire-Kechuan(XFK)is a multi-herb formula composed of 10 herbal and 1 mineral herbs(Table 1).It is a patent TCM formula to treat bronchitis,pneumonia,and cough in children[5].Both oral solution and granule formulations have been developed.XFK oral solution is recorded in the Chinese Pharmacopoeia(2015 edition),while only ephedrine and pseudoephedrine from Mahuang(MH)are used as quality control markers[5].Several studies determined the contents of chemical constituents in XFK[23,24].For example,9 analytes from MH,Huangqin(HQ),Jinyinhua(JYH),Gancao(GC),and Lianqiao(LQ)were quantified using an 83-min HPLC-DAD method[24].To fully evaluate the quality of XFK formula,it is important to monitor characteristic analytes of each herb and to identify its major effective constituents.

Table 1The herbs of Xiaoer-Feire-Kechuan(XFK)formula.
In the present work,we reported an integrated method to reveal the anti-inflammatory constituents of XFK formula.The contents of 35 characteristic analytes in 18 batches of XFK formulations were determined using ultra performance liquid chromatography(UPLC)-DAD and ultra-high performance liquid chromatography(UHPLC)/Q-Orbitrap-MS.Furthermore,the COX-2 inhibitory activities of the 35 analytes,separate herbs,and different XFK formulations were investigated to discover the main anti-inflammatory constituents of XFK.
2.Experimental
2.1.Chemicals and reagents
Methanol,acetonitrile(Fisher Scientific,Fair Lawn,NJ,USA),and formic acid(Sigma-Aldrich,St.Louis,MO,USA)were of LC/MS grade.De-ionized water was prepared using a Milli-Q water purification system(Millipore,Burlington,MA,USA).COX-2 inhibitor screening kit was purchased from Beyotime Biotechnology(Shanghai,China).Reference standards 8-10,13,16,23,and 26 were isolated from LQ;15,17,18,and 34 were from HQ[11];20,21,and 22 were purchased from the National Institute for the Control of Biological and Pharmaceutical Products of China(Beijing,China);1,2,4,5,11,12,14,19,24,27,28,31-33,and 35 were purchased from Chengdu DeSiTe Biological Technology Co.,Ltd.(Chengdu,China);3,6,7,25,29,30,and internal standards(IS1 and IS2)were purchased from Chengdu Must Bio-technology Co.,Ltd.(Chengdu,China).Their structures are shown in Fig.1.Purity of all these standards was above 98% by HPLC analysis.

Fig.1.Chemical structures of analytes 1-35 and the internal standards(IS1 and IS2).
Herbs including Mahuang(MH),HQ,JYH,LQ,Kuxingren(KXR),GC,Zhimu(ZM),Banlangen(BLG),Maidong(MD),Yuxingcao(YXC),XFK oral solutions XFK1-XFK10(OS[a],10 mL/dose),and granules XFK13-XFK16(GR[a],3 g/dose)were supplied by company a.Granules XFK11 and XFK12(GR[b],3 g/dose)were from company b and granules XFK17 and XFK18(GR[c],4 g/dose)from company c.
2.2.UPLC-DAD method for major components
2.2.1.Preparation of calibration standard and sample solutions
Reference standards(1-14,16,17,and 19)were dissolved in 50% methanol to prepare a mixed standard solution 1.Reference standards 15 and 18 were dissolved in 50% methanol to prepare a mixed standard solution 2.Their concentrations ranged from 29.5-217.0μg/mL of each analyte.The mixed reference solutions were respectively diluted by 2,4,8,16,32,64,128,256,and 512-fold using 50% methanol to prepare a series of calibration samples.XFK oral solution(0.2 mL)was accurately diluted by 25-fold with 50% methanol.The fine powder of XFK granules(200 mg)were extracted using 10 mL of 50% methanol in an ultrasonic bath for 5 min.All samples were filtered through 0.22μm membranes before use.
2.2.2.Chromatographic conditions
A Waters UPLC H-Class system(Waters Technologies,Corp.,Milford,MA,USA)was employed.Samples were separated on an Acquity HSS T3 column(2.1 mm × 100 mm,1.8μm,Waters Corporation,Milford,MA,USA)and eluted using mobile phase A(water containing 0.1% formic acid)and B(acetonitrile).The gradient program was as follows:0 min,5% B;2 min,5% B;5 min,10% B;5.5 min,12% B;13 min,18% B;20 min,40% B;25 min,100% B.The flow rate was 400μL/min and the column temperature was set at 55°C.An aliquot of 2 μL was injected for analysis.The detector wavelengths were selected according to the UV absorption of each analyte(Table 2).
2.3.UHPLC/Q-Orbitrap-MS method for minor components
2.3.1.Preparation of calibration standard,IS,and sample solutions
An appropriate amount of each reference standard(20-35)was dissolved in 50% methanol to prepare a mixed standard solution containing 6-30μg/mL of each analyte.The mixed standard solution was then serially diluted by 2,4,8,16,32,64,128,256,512,and 1024-fold using 50% methanol.The series of calibration solutions were then diluted by 2-fold using the mixed internal standard solution(containing 400 ng/mL of IS1 and 400 ng/mL of IS2),respectively.The XFK oral solution(0.5 mL)was accurately diluted by 100-fold with 50% methanol.The fine powder of XFK granule(40 mg)was extracted using 10 mL of 50% methanol in an ultrasonic bath for 5 min.The sample solutions were then diluted with the mixed internal standard solution by 2-fold,respectively.All samples were filtered through 0.22μm membranes before use.
2.3.2.Chromatographic and mass spectrometry conditions
A Thermo Vanquish UHPLC system(Thermo Fisher Scientific,San Jose,CA,USA)was employed.Samples were separated on a Phenyl-Hexyl(2.1 mm × 100 mm,1.8μm,Agilent Technologies,Waldbronn,Germany)and eluted using mobile phase A(water containing 0.2% formic acid)and B(acetonitrile).The gradient program was as follows:0 min,2% B;2 min,2% B;7 min,13% B;13 min,28% B;16 min,80% B.An aliquot of 2μL was injected for analysis.The flow rate was 400μL/min and column temperature was set at 50°C.
Mass spectral data acquisition was performed on a Q-Exactive Focus hybrid Q-Orbitrap mass spectrometer equipped with a heated electrospray ionization source(HESI)(Thermo Scientific,San Jose,USA).The parameters were set as follows:spray voltage,-3.5 kV;sheath gas,45 arb;auxiliary gas,10 arb;capillary temperature,350°C;auxiliary temperature,300°C;S-lens RF level,55 V.Polarity switch negative(-)/positive(+)and PRM mode were employed.The scan windows of each analyte were set from 1.5 to 2.0 min based on their retention times to ensure sufficient data points. MS/MS resolution was set at 17,500 FWHM.Quantitative product ion and collision energy of each analyte were optimized using the MS Tune software(Thermo Scientific,Wilmington,DE,USA)and are provided in Table 2 and Fig.S1.Data were processed using XcaliburTM4.1 software(Thermo Scientific,Wilmington,DE,USA).

Table 2The information for UV and PRM parameters for quantitation of 35 analytes.
2.4.In vitro COX-2 inhibition assay
The inhibitory activities of different XFK formulations(10μg/mL),10 herbs(MH,HQ,GC,JYH,LQ,ZM,KXR,BLG,MD,and YXC,10 μg/mL),and 35 quantified analytes(10μM)were tested using a COX-2 inhibitor screening kit(Beyotime Biotechnology,Shanghai,China)according to the manufacturer's instructions.Celecoxib(50 nM)was used as the positive control.All experiments were carried out in triplicate.Preparation of XFK extracts is shown in Supplementary data.
3.Results and discussion
3.1.Selection of analytes
Only two analytes from MH were determined according to theChinese Pharmacopoeia (2015 edition) [5]. To comprehensively evaluate the quality of XFK, characteristic constituents from each herbwere selected for analysis. As a result, 35 analytes from 9 herbs in XFK formula were determined. (R,S)-goitrin, a characteristic component inBLG, was excluded due to its low concentration in XFK. In the presentmethod, 19 major analytes were quantified using UPLC-DAD(Figs. 2A-C), and 16 minor ones were determined by UHPLC/QOrbitrap-MS (Fig. 2D).

Fig.2.Typical UPLC-UV chromatograms and UHPLC-PRM-MS/MS ion chromatograms.(A)UPLC-UV chromatograms of mixed reference standards(1-19).(B)UPLC-UV chromatograms of oral solution XFK-8.(C)UPLC-UV chromatograms of granule XFK-13.(D)PRM ion chromatograms of XFK-13,showing minor analytes 20-35 and internal standards IS1 and IS2.
3.2.Optimization of separation method for UHPLC/Q-Orbitrap-MS analysis
Analytes 20-35 were determined using UHPLC/Q-Orbitrap-MS.They contain hydrophilic,hydrophobic,basic and acidic compounds,which are challenging for chromatographic separation.For example,the three ephedra alkaloids(20,21,and 22)are difficult to be fully separated using LC/MS-compatible mobile phases[5].Thus,the separation method was optimized.Different stationary phases were tested,including Acquity CSH C18(2.1 mm ×100 mm,1.7μm,Waters Corporation,Milford,MA,USA),Acquity HSS T3 C18(2.1 mm ×100 mm,1.8μm,Waters Corporation,Milford,MA,USA),Acquity Cortecs C18(2.1 mm×100 mm,1.6μm,Waters Corporation,Milford,MA,USA),Xterra MS C18(2.1 mm ×150 mm,3.5μm,Waters),and Phenyl-Hexyl(2.1 mm × 100 mm,1.8μm,Agilent).All columns exhibited good peak shape for analytes 24,26,27 and 34,but only the Phenyl-Hexyl column could effectively separate analytes 20,21,and 22(Fig.S2).By increasing the concentration of formic acid in the mobile phase(0.2%),the three analytes(20,21,and 22)showed satisfactory peak shape and resolution(Fig.S2F).The optimized stationary and mobile phases were used for the follow-up experiments.
3.3.Optimization of MS conditions for UHPLC/Q-Orbitrap-MS analysis
To improve the sensitivity,three different scan modes were compared,including full scan/data-dependent MS2(FS/ddMS2),target-selected ion monitoring(t-SIM)/ddMS2,and PRM(Fig.3).In FS/ddMS2,the precursor ions were detected by full scan mode and then delivered to the high energy collision-induced dissociation(HCD)cell via C-trap.The top N abundant ions in each scan were fragmented successively,and the fragment ions were detected by Orbitrap-MS to confirm the analyte.In t-SIM/ddMS2,precursor ions were selectively detected and delivered to the HCD cell,and their product ions were monitored by Orbitrap-MS for analyte confirmation.In PRM mode,predefined precursor ions were selected by the quadrupole and delivered directly to the HCD cell without detection.The product ions were then detected by Orbitrap-MS.
Data point(DP),sensitivity,and selectivity of the three scan modes were compared using representative analytes(Fig.3).The PRM mode allowed shorter scan time and duty cycle.For alkaloids 20 and 21,DP and signal-to-noise(S/N)values increased significantly when using PRM mode.The duty cycles(calculated as shown in Fig.S3)were 1.80,0.60,and 0.12 s for FS/ddMS2,t-SIM/ddMS2and PRM,respectively.Similarly,the S/N value for the glycoside 24 and the phenylethanoid 26 in PRM was much higher than that in t-SIM/ddMS2and FS/ddMS2.Our results also indicated the highest specificity of the PRM mode(Fig.3E).For analytes 28 and 31(precursor ion m/z 549.1602),false positive ions could be observed in FS/ddMS2and t-SIM/ddMS2modes,but not observed in PRM mode(Fig.S4).

Fig.3.Comparison of FS/ddMS2,t-SIM/ddMS2,and PRM scan modes by analyzing a mixed reference standard sample.(A-C)Working principles and sensitivities for the three scan modes.The flow of precursor ions(bigger dots)and product ions(smaller dots)are indicated using green and red arrows,respectively.(D)MS parameters and duty cycles for analytes 20,21,24,and 26.(E)Extracted ion chromatograms(FS/ddMS2and t-SIM/ddMS2)and PRM chromatogram for analytes 28 and 31.Concentrations of the four analytes were 100 ng/mL.Images for C-trap and Orbitrap detector were obtained from the producer’s website(http://www.thermo.com)and modified by the authors.DP:data point;S/N:signal to noise;CE:collision energy.
3.4.Method validation
3.4.1.Linearity,dynamic ranges,and limits of detection
Calibration curves of analytes 1-19 detected by UPLC-UV were constructed by plotting the peak areas(Y)against the concentrations(X).Calibration curves of analytes 20-35 detected by UPLC/QOrbitrap-MS were constructed by plotting the analyte/IS peak area ratio(Y)against the correspondent concentration(X).ISs were used to ensure precision of the analyses.Phenylpropanolamine(IS1)and daidzin(IS2)corresponded to the analytes 20-22 and 23-35,respectively.Standard calibration curves of the 19 analytes in UPLCDAD analysis showed good linearity within the range of 0.08-246.00μg/mL(r2> 0.9995).The analyte/IS peak area ratio of the 16 analytes in UHPLC/Q-Orbitrap-MS analysis showed good correlation with concentrations(r2>0.99)within the range of 2.00-27840.00 ng/mL(Table 2).The limits of detection(LOD,S/N=3)for UPLC-DAD and UHPLC/Q-Orbitrap-MS methods were 40.00 to 3760.00 ng/mL and 0.02 to 5.90 ng/mL,respectively.
3.4.2.Precision,repeatability,and stability
Intra-and inter-day precisions were assessed by testing a sample solution in the same day for six times and on three consecutive days.The relative standard deviation(RSD)values for intra-and inter-day precisions ranged from 0.2% to 5.6% and 0.8% to 7.2%,respectively,indicating acceptable precision of the method.The repeatability was described by analyzing six samples(XFK-6)prepared using the same method.The results indicated that the sample preparation method was repeatable with RSD values ranging from 0.1% to 8.6%.The stability was evaluated by analyzing the same sample solution at 10°C after 0,2,4,8,12,and 24 h.RSD values of the analytes within 24 h ranged from 0.1% to 5.8%,indicating the analytes were stable.The detailed data are listed in Table S1.
3.4.3.Accuracy
The accuracy was validated by spiking the reference solutions to a real XFK sample.For analytes 1-19,accuracy analysis was conducted at 100% concentration level.For analytes 20-35,recovery was conducted at 80%,100%,and 120% concentratio levels.Samples XFK-6 and XFK-5 were used for accuracy study of 1-14,16,17,19-35 and 15,18,respectively.Recoveries were calculated by the formula:recovery(%)= (detected amount-original amount)/spiked amount×100%.Recoveries of the 19 analytes detected by UPLC-DAD ranged from 84.5%-113.8% with RSD values ranging from 0.2% to 3.1%.Recoveries of the 16 analytes detected by UHPLC/Q-Orbitrap-MS ranged from 72.4%-118.5% with RSD values ranging from 0.5% to 10.7%.The data are shown in Table S2.
3.4.4.Sample analysis
The validated method was applied to analyze 18 batches of XFK formula,including 10 batches of XFK oral solution and 8 batches of granules from three pharmaceutical companies(Figs.4A and S5).Due to different formulation methods and packages,we converted the concentrations(mg/g or mg/mL)into the contents in a single dose(mg/3 g for GR[a]and GR[b],mg/4 g for GR[c],and mg/10 mL for OS[a])to facilitate the comparison among different formulations.All of the oral solution samples met the requirements of the Chinese Pharmacopoeia(20 and 21≥1.8 mg/dose)[5].The contents of analytes 20 and 21 varied from 1.09 mg/dose to 2.21 mg/dose in granules from different pharmaceutical companies.Baicalin(15)from HQ was the most abundant component in all samples(38.92±4.35 mg/dose for oral solution and 17.03±7.87 mg/dose for granules).The total contents of the 35 analytes in oral solutions showed slight variations ranging from 145.92-206.69 mg/dose,while significant variations (49.59-133.22 mg/dose) were observed in different granules.The results are shown in Table S3.
The quantitation results were then analyzed by principal component analysis(PCA)using SIMCA-P software(version 13.0).The first and second principal components accounted for 68.8% and 18.8% of the variation,respectively.Different formula samples were grouped in different clusters in Fig.4B.GR[a]and GR[c]were closer due to their similar chemical contents.Partial least squares discrimination analysis(PLS-DA)was then used to explore the variables that contributed to the grouping of the samples(Fig.S6).As shown in Fig.4C,contents of 5 and 33 from ZM,9 from LQ,15 and 34 from HQ,28 and 31 from GC,and 24 from KXR showed the highest intra-group variance,as suggested by the largest variable importance in projection(VIP)values(>1.20).For example,the contents of 5 and 33 from ZM were much higher in GR[b](16.90±1.66 mg/dose)than in other formulas(5.72±0.95 mg/dose for OS[a],2.87±0.17 mg/dose for GR[a],and 3.06±0.16 mg/dose for GR[c]).The data indicated the different qualities for the crude drugs used to prepare the XFK formula.

Fig.4.Contents of 35 analytes in XFK and their principal component analysis.(A)Contents of 35 analytes in four different XFK formulas;(B)PCA scatter plots for 18 batches of formulas;(C)variable importance in projection(VIP)values for 35 analytes in different XFK formulations.OS[a],oral solution from company a,GR[a],granules from company a,GR[b],granules from company b,GR[c],granules from company c.Red asterisk represented the analytes with the highest intra-group variance.
3.5.COX-2 inhibitory activities of chemical markers
In present study,in vitro COX-2 inhibitory activities of the 35 analytes,10 herbs,and 4 different formulations were investigated to discover the main anti-inflammatory constituents of XFK.XFK formula,along with JYH,LQ,HQ,YXC,and ZM,exhibited potent COX-2 inhibitory activities(inhibitory rate≥60%)(Fig.5A).These five herbs might be responsible for the anti-inflammatory activity of XFK.For single analytes,phenolic acids(1-4,11,12,14)from JYH,phenylethanoid glycosides(8,9,10,23,26)from LQ,xanthone(5)from ZM,and flavonoid glycosides(15 from HQ,25 and 29 from JYH,30 from YXC)exhibited significant inhibition activities at 10 μM level(inhibitory rate≥ 80%,Fig.5B).
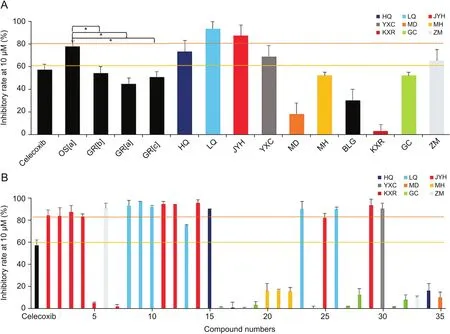
Fig.5.COX-2 inhibitory activities XFK formula,herbs,and 35 analytes.(A)COX-2 inhibitory activities of XFK formula and herbs at 10 μg/mL,*P<0.05;(B)COX-2 inhibitory activities of 35 analytes at 10μM.
Among the COX-2 inhibitors,8,9,10,and 15 were the major components.Their contents reached 81.70±7.47 mg/dose for OS[a],50.41±0.18 mg/dose for GR[b],23.28±2.00 mg/dose for GR[a],and 28.33±6.13 mg/dose for GR[c].Combining their contents and bioactivities,8,9,10,and 15 could be the major anti-inflammatory constituents for XFK.This was further supported by the activities of different formulations.For example,XFK oral solution exhibited higher inhibitory rate(78%±4%)than granules(average inhibitory rates of three granules at 50%±5%).Accordingly,the four effective constituents are higher in XFK oral solution.
4.Conclusions
In this study, quantitative analyses and bioactivity test werecombined to elucidate the anti-inflammatory constituents in XFK.Firstly, UPLC-DAD and UHPLC/Q-Orbitrap-MS methods were established and validated to quantify 35 analytes in different XFK formulations. The total contents of the 35 analytes in different XFKformulations showed significant variations ranging from75.69-269.46 mg/dose. Further COX-2 inhibitory assay revealed thatJYH, LQ, HQ, YXC, and ZM might be responsible for the antiinflammatory activity of XFK formula. Four major analytes 8, 9, 10,and 15 exhibited high abundance (total contents varied from 21.79 to91.20 mg/dose in different formulations) and potent COX-2 inhibitionactivities (inhibitory rate ≥ 80%) were proposed as the major effectivecomponents of XFK. The work also provided an effective strategy fordiscovery of effective constituents in multi-herb formulas.Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The work was supported by the National Key Research and Development Program of China(Grant No.:2018YFC1707304,2018YFC1707301),Beijing Natural Science Foundation(Grant No.:JQ18027),and National Natural Science Foundation of China(Grant No.:81725023).We thank Sunflower Pharmaceutical Co.,Ltd.for providing the samples.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.01.003.
 Journal of Pharmaceutical Analysis2021年6期
Journal of Pharmaceutical Analysis2021年6期
- Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
