Overoxidized poly(3,4-ethylenedioxythiophene)-gold nanoparticles-graphene-modified electrode for the simultaneous detection of dopamine and uric acid in the presence of ascorbic acid
Junqing Pn,Mei Liu,Dndn Li,Honn Zheng,Dongdong Zhng,*
aSchool of Pharmacy,Xi'an Jiaotong University Health Science Center,Xi'an,710061,China
bDepartment of Cardiovascular Medicine,Xi'an Central Hospital,Xi'an,710003,China
Keywords:
Graphene
Poly(3,4-ethylenedioxythiophene)Overoxidation
Dopamine
Uric acid
Ascorbic acid
Peer review under responsibility of Xi'an Jiaotong University.
A B S T R A C T
An innovative,ternary nanocomposite composed of overoxidized poly(3,4-ethylenedioxythiophene)(OPEDOT),gold nanoparticles(AuNPs),and electrochemically reduced graphene oxide(ERGO)was prepared on a glassy carbon electrode(GCE)(OPEDOT-AuNPs-ERGO/GCE)through homogeneous chemical reactions and heterogeneous electrochemical methods.The morphology,composition,and structure of this nanocomposite were characterized by transmission electron microscopy,scanning electron microscopy,X-ray diffraction,and X-ray photoelectron spectroscopy.The electrochemical properties of the OPEDOT-AuNPs-ERGO/GCE were investigated by cyclic voltammetry using potassium ferricyanide and hexaammineruthenium(III)chloride redox probe systems.This modified electrode shows excellent electro-catalytic activity for dopamine(DA)and uric acid(UA)under physiological pH conditions,but inhibits the oxidation of ascorbic acid(AA).Linear voltammetric responses were obtained when DA concentrations of approximately 4.0-100μM and UA concentrations of approximately 20-100μM were used.The detection limits(S/N=3)for DA and UA were 1.0 and 5.0μM,respectively,under physiological conditions and in the presence of 1.0 mM of AA.This developed method was applied to the simultaneous detection of DA and UA in human urine,where satisfactory recoveries from 96.7% to 105.0% were observed.This work demonstrates that the developed OPEDOT-AuNPs-ERGO ternary nanocomposite,with its excellent ion-selectivity and electro-catalytic activity,is a promising candidate for the simultaneous detection of DA and UA in the presence of AA in physiological and pathological studies.©2021 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Dopamine(DA)is an essential catecholamine neurotransmitter in the mammalian central nervous system,and its abnormal concentrations induce diverse neurological diseases,like Parkinson's disease and Schizophrenia[1].Uric acid(UA)is the end product of purine metabolism,and high levels of it have been linked to diseases such as hyperuricemia,gout,and angiocardiopathy[2].Therefore,the identification and detection of DA and UA have great clinical significance.Since DA and UA possess good electrochemical activities,electrochemical analysis is the preferred approach to identifying these compounds;it also has the added benefits of being simple,rapid,highly sensitive,and inexpensive[3,4].However,the development of this method is severely restricted by ascorbic acid(AA),which coexists with DA and UA in bodily fluids and interferes with their detection.This is due to AA being present in much higher concentrations than those of DA and UA(approximately 100 to 1,000 times greater)[5].Hence,specific nanomaterials are desperately needed to modify electrodes for the selective detection of DA and UA and their simultaneous detection in the presence of high concentrations of AA.Materials such as electrodeposited reduced graphene oxide(GO)combined with overoxidized polypyrrole[6],Pt/reduced GO composites[7],carboxyl-functionalized graphene, and silver-nanocubefunctionalized polydopamine nanospheres[8]have been successfully employed to prevent AA from reaching electrode surfaces,thus allowing for the individual or simultaneous determination of DA and UA.However,novel electrode-modification materials have yet to be exploited to further improve the analytical performance of DA and UA detection methods.
Poly(3,4-ethylenedioxythiophene)(PEDOT)is considered as one of the most promising conducting polymers due to its high conductivity,excellent processability,and exceptional stability.Over the past years,PEDOT has been largely explored for the construction of electrochemical biosensors for environmental monitoring,food and drug analysis,and health care[9].However,studies employing overoxidized PEDOT(OPEDOT)in electrochemical analysis are rare[10,11].When exposed to high positive potentials or excess amounts of powerful oxidants,PEDOT can be overoxidized,which causes it to undergo irreversible degradation to give OPEDOT[12-14].During the overoxidation process,electronegative oxygen groups such as sulfones,carbonyls,and terminal carboxylic groups can be introduced to decrease the hydrophobicity of the OPEDOT film[15],which may endow it with unique properties conducive to electroanalytical applications[9,16].However,the overoxidation process can also destroy the conjugation of the π-bonds of the PEDOT main chain and lead to unfavorable,low electronic conductivity [13,15].Therefore,the combination of OPEDOT with other nanomaterials possessing high electronic conductivities and electro-catalytic activities is necessary to compensate for this defect.
Metal nanoparticles,especially gold nanoparticles(AuNPs),have been extensively integrated into PEDOT materials because of their high affinity toward sulfur atoms and their commendable,high specific surface area,catalytic activity,and electrical conductivity[17-19].PEDOT-AuNPs nanocomposites can be simply and rapidly obtained by the direct chemical reaction between chloroauric acid and the 3,4-ethylenedioxythiophene(EDOT)monomer[20,21].This one-pot,green synthesis does not require any dispersing agents;however,it usually causes the agglomeration of the nanocomposites,which severely affect the electro-catalytic activity of the resultant nanocomposites[22,23].There have been reports of nanocomposites based on GO that show good stability and dispersity due to the abundance of hydrophilic groups and the great surface area of GO sheets[24,25].For example,Liu et al.[26]successfully prepared a stable,aqueous dispersion of PEDOT nanorods that were stabilized by the π-π stacking interactions and electrostatic adsorption between the positively charged PEDOT and negatively charged GO.
Herein, an innovative nanocomposite, OPEDOT-AuNPselectrochemically reduced GO (ERGO), was prepared by a combination of chemical and electrochemical methods. The PEDOT-AuNPswere first synthesized by a direct, homogeneous, chemical reactionbetween chloroauric acid (as an oxidant), a source of metal atoms, andthe EDOT monomer (as a reductant) at room temperature. Subsequently, GO was added to the PEDOT-AuNPs to form a homogeneousPEDOT-AuNPs-GO suspension, which was then dropped onto thesurface of a glassy carbon electrode (GCE) and dried. Finally, thismodified electrode was subjected to two heterogeneous electrochemical treatments via cyclic voltammetry that employed differentpotential windows to electrochemically reduce GO and overoxidizePEDOT. The fabrication procedure of the OPEDOT-AuNPs-ERGO/GCEis illustrated in Scheme 1. In short, this complicated, ternary nanocomposite can be constructed by a green, mild, and highly efficientsynthetic strategy. The morphology, composition, and structure of theOPEDOT-AuNPs-ERGO nanocomposite were characterized bytransmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The electrochemical properties of the modifiedelectrodes were investigated by cyclic voltammetry using potassiumferricyanide(K3Fe(CN)6)and hexaammineruthenium(III)chloride(Ru(NH3)6Cl3)redox probe systems.The electro-catalytic activities of the OPEDOT-AuNPs-ERGO/GCE towards AA,DA,and UA were investigated by cyclic voltammetry under physiological conditions.The analytical properties of the modified electrode towards DA and UA in the presence of high concentrations of AA were investigated by square wave voltammetry.Additionally,the developed method was applied to the simultaneous detection of DA and UA in human urine samples.
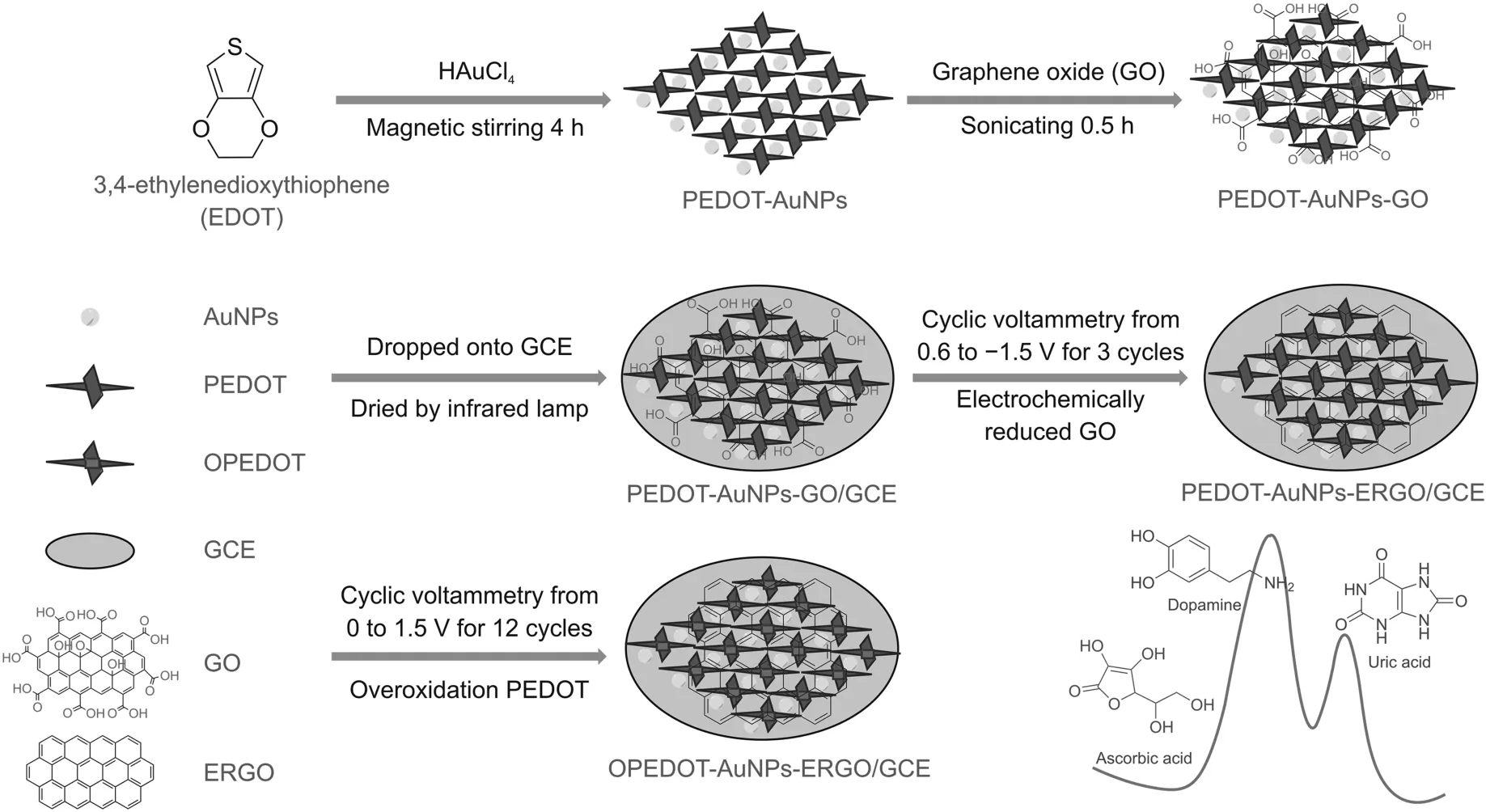
Scheme 1.Schematic diagram of the fabrication procedure of the OPEDOT-AuNPs-ERGO/GCE(OPEDOT:overoxidized poly(3,4-ethylenedioxythiophene);AuNPs:gold nanoparticles;ERGO:electrochemically reduced graphene oxide;GCE:glassy carbon electrode),which was then used to simultaneously detect dopamine(DA)and uric acid(UA)in the presence of ascorbic acid(AA).
2.Experimental
2.1.Reagents and apparatus
GO was purchased from Nanjing XFNANO Materials Tech Co.,Ltd.(Nanjing,China),while EDOT,DA,UA,AA,K3Fe(CN)6,and Ru(NH3)6Cl3were purchased from Sigma-Aldrich(St.Louis,MO,USA).HAuCl4was purchased from Strem Chemicals,Inc.(Newburyport,MA,USA).All reagents were of analytical grade,and 18.2 MΩ cm Milli-Q water was used throughout the experiments.
The surface morphologies of the nanocomposites were obtained by TEM(JEOL JEM-2100Plus microscope)and SEM(Gemini SEM 500 microscope).The crystal structures were characterized by XRD using a LabX XRD-6100 system(Shimadzu,Kyoto,Japan).The element compositions of the nanocomposites were analyzed by XPS(AXIS ULtrabld instrument;Kratos,Manchester,UK).Electrochemical measurements were obtained using a CHI760 electrochemical workstation with a conventional three-electrode system,where platinum wire served as the auxiliary electrode,a saturated Ag/AgCl electrode was used as the reference electrode,and a bare or modified GCE(diameter=3.0 mm)served as the working electrode.Sodium phosphate buffer saline(PBS;10 mM,pH=7.4)was used as electrolyte solution.
2.2.Preparation of the modified electrodes
Firstly,2.7 mL of an ethanol solution of EDOT(10 mM)was added to 15.3 mL of an aqueous HAuCl4solution(7.0 mM)under rapid stirring for 4 h at room temperature,and dark-blue PEDOT-AuNPs were obtained.In this process,the EDOT monomer was oxidized and polymerized to PEDOT by chloroauric acid,and HAuCl4was reduced by the EDOT monomer to give the AuNPs.Next,2.0 mL of an aqueous GO solution(2.0 mg/mL)was mixed with the PEDOT-AuNPs,followed by sonication for 0.5 h to give the PEDOT-AuNPs and a homogeneous GO suspension(PEDOT-AuNPs-GO).Subsequently,10μL of PEDOT-AuNPs-GO was dropped onto the clean surface of a GCE and dried with an infrared lamp to give the modified electrode(PEDOT-AuNPs-GO/GCE).The modified electrode was then immersed in 10 mM PBS(pH=7.4)and subjected to cyclic voltammetric scanning from 0.6 to-1.5 V for 3 cycles at a rate of 50 mV/s.This was done to electrochemically reduce GO,which gave the PEDOT-AuNPs-ERGO/GCE.Finally,the PEDOT-AuNPs-ERGO/GCE was placed in 10 mM PBS(pH=7.4)and further treated by cyclic voltammetry between 0.0v and 1.5 V for 12 cycles at a rate of 50 mV/s to electrochemically overoxidize PEDOT,giving the final,desired electrode(OPEDOT-AuNPs-ERGO/GCE).
3.Results and discussion
3.1.The preparation of the OPEDOT-AuNPs-ERGO/GCE
The electrochemical reduction process of the PEDOT-AuNPs-GO/GCE was investigated by cyclic voltammetry from 0.6 to-1.5 V in N2-saturated,10 mM PBS(pH=7.4).From Fig.1A,it can be seen that a broad,irreversible reduction peak appears between approximately-0.9 and-1.5 V during the first cycle,which is ascribed to the irreversible reduction of the GO functional groups[27].Moreover,this reduction peak disappears during the second and the third cycles,indicating the complete reduction of GO.The electrochemical overoxidation process of the obtained PEDOT-AuNPs-ERGO/GCE was investigated by cyclic voltammetry from 0 to 1.5 V in N2-saturated,10 mM PBS(pH=7.4).From Fig.1B,an irreversible oxidation peak can be seen at approximately 1.1 V during the first cycle.The oxidation peak current was noted to gradually decrease until disappearing as the scan cycles progressed,while the peak potential negatively shifted with increasing scan cycles.This phenomenon might be attributed to the overoxidation of the PEDOT film,which can result in the irreversible degradation of the film and the loss of the electro-activity of PEDOT.Furthermore,an irreversible reduction peak can be seen at approximately 0.52 V,the peak current of which also continuously declined with increasing scan cycles until it disappeared.This irreversible reduction is likely the ion-assisted reduction of the polymer double bonds owing to the presence of alkali metal cations,Na+and K+,in PBS[28].
3.2.The characterization of the OPEDOT-AuNPs-ERGO/GCE
Fig.2.displays the TEM images of the PEDOT-AuNPs and PEDOT-AuNPs-GO.In Fig.2A,the AuNPs with a particle size of approximately 5 nm randomly attached to the surface of PEDOT,and a porous network structure can be seen.In Fig.2B,while GO is introduced,in addition to AuNPs of similar size and PEDOT with porous network structure,PEDOT and AuNPs can also be seen adhering to the surface of the wrinkled GO sheets,indicating that GO can provide plenty of deposition sites for both PEDOT and AuNPs.

Fig.2.Transmission electron microscopy(TEM)images of the(A)PEDOT-AuNPs and(B)PEDOT-AuNPs-GO.
XRD and XPS were employed to characterize the components of PEDOT-AuNPs-GO.Fig.3A shows the XRD patterns of GO,the PEDOT-AuNPs,and PEDOT-AuNPs-GO.ForGO(curve a),the intense diffraction peak at 2θ=11.4°corresponds to the(002)plane of graphite[29].For the PEDOT-AuNPs(curve b),the diffraction peaks at approximately38.2°,45.2°,65.2°,78.5°,and 82.8°belong to the(111),(200),(220),(311),and(222)planes of Au,respectively[30].For PEDOT-AuNPs-GO(curve c),the(002)graphite plane and the typical diffraction peaks of Au can be clearly seen,indicating the simultaneous existence of GO and AuNPs in the nanocomposite.Additionally,a broad peak can be observed in all three curves at 25°,which may correspond to the carbon sources of GO and PEDOT[21].Fig.3B shows the XPS spectra of GO and PEDOT-AuNPs-GO.The sharp peaks at 278 and 524 eV are the signals of C1s and O1s,respectively.The high resolution S2p and Au4f spectra of PEDOT-AuNPs-GO are presented in Figs.3C and D,respectively.From Fig.3C,the typical peaks of a spinsplit doublet can be seen for S2p at 161.1(S2p3/2)and 165.7 eV(S2p1/2),which are derived from the thiophene ring of PEDOT.In Fig.3D,the Au4f spectrum exhibits the Au4f7/2peak at 81.4 eV and the Au4f5/2peak at 85.2 eV.These XRD and XPS characterizations significantly indicate the coexistence of GO,AuNPs,and PEDOT in the fabricated ternary nanocomposite.

Fig.3.(A)X-ray diffraction(XRD)patterns of GO(a),the PEDOT-AuNPs(b),and PEDOT-AuNPs-GO(c);(B)X-ray photoelectron spectroscopy(XPS)spectra of GO(a)and PEDOT-AuNPs-GO(b);high resolution(C)S2p and(D)Au4f XPS spectra of PEDOT-AuNPs-GO.
Fig.4.displays the SEM images of the PEDOT-AuNPs/GCE,PEDOT-AuNPs-GO/GCE,PEDOT-AuNPs-ERGO/GCE,and OPEDOT-AuNPs-ERGO/GCE.In Fig.4A,the PEDOT-AuNPs can be seen to exhibit a porous,sponge-like structure owing to the involvement of the AuNPs that serve as dopants and templates for the formation and growth of PEDOT[19].Fig.4B shows a homogeneous and curly morphology with a thin,wrinkled,paper-like structure.Fig.4C displays a rough and compact surface with plenty of bulges,which are mainly caused by the electrochemical reduction of GO in the nanocomposites[31].In Fig.4D,the bulges almost disappear and the surface becomes relatively flat,which is mainly due to the overoxidation treatment disrupting the thiophene α-α polymer bonds and the PEDOT films being subjected to heavy degradation and delamination[10].

Fig.4.Scanning electron microscopy(SEM)images of the(A)PEDOT-AuNPs/GCE,(B)PEDOT-AuNPs-GO/GCE,(C)PEDOT-AuNPs-ERGO/GCE,and(D)OPEDOT-AuNPs-ERGO/GCE.
The electrochemical activities of the modified electrodes were investigated in negatively charged Fe(CN)63-and positively charged Ru(NH3)63+probe systems,respectively.In the case of the Fe(CN)63-system(Fig.5A),a pair of reversible redox peaks can be seen for the bare GCE(curve a)with the peak potential separation(ΔEp)being 73 mV and the oxidation peak current(Ipa)and reduction peak current(Ipc)being 29.0 and-29.7μA,respectively.For the PEDOT-AuNPs-GO/GCE(curve b),the ΔEpvalue broadened to 100 mV,and the Ipaand Ipcvalues decreased by 41.9% and 35.0%,respectively.This was probably attributed to the poor electrical conductivity of GO.For the PEDOT-AuNPs-ERGO/GCE(curve c),the value of ΔEpdecreased to 76 mV.Both of the background charge currents and the redox peak currents significantly increased,and the values of Ipaand Ipcwere 29.4 and 38.9μA,respectively.This suggests that ERGO has a high electrical conductivity and an enhanced ability to accelerate electron transfer[32].For the OPEDOT-AuNPs-ERGO/GCE(curve d),the background charge currents dramatically decreased,which was due to the cleavage of the conjugation pathway in the PEDOT main chain during the overoxidation process.The values of Ipaand Ipcdecreased to 11.0 and-13.5μA,respectively,indicating that the electron transfer of the negativity charged Fe(CN)63-probe on the electrode surface was impeded due to the electrostatic repulsion of the negatively charged functional groups on the OPEDOT surface.

Fig.5.Cyclic voltammograms obtained in N2-saturated 10 mM PBS(pH=7.4)containing(A)2.0 mM K3Fe(CN)6and(B)2.0 mM Ru(NH3)6Cl3on a bare GCE(a),the PEDOT-AuNPs-GO/GCE(b),the PEDOT-AuNPs-ERGO/GCE(c),and the OPEDOT-AuNPs-ERGO/GCE(d),collected at a scan rate of 50 mV/s.
In the case of the Ru(NH3)63+system(Fig.5B),a pair of redox peaks appeared for the bare GCE(curve a)with the oxidation peak potential(Epa)being-0.106 V,the reduction peak potential(Epc)being-0.175 V,and the Ipaand Ipcvalues being 20.2 and-22.2μA,respectively.For the PEDOT-AuNPs-GO/GCE(curve b),the Epaand Epcvalues negatively shifted to-0.132 and-0.206 V,respectively,and the Ipaand Ipcvalues increased to 24.3 and-35.0μA,respectively.This is probably ascribed to the electro-catalytic activity of the AuNPs and the electrostatic adsorption of GO towards Ru(NH3)63+.For the PEDOT-AuNPs-ERGO/GCE(curve c),the background charge currents remarkably increased,but the lpaand lpcvalues decreased to 15.9 and-23.7μA,respectively.For the OPEDOT-AuNPs-ERGO/GCE(curve d),the oxidation peak split into two sub-peaks at approximately-0.123 and-0.250 V,and the broadened reduction peak negatively shifted to-0.252 V with the lpcvalue being-26.5μA.Regarding these two split oxidation peaks,the former wave at Epa=-0.123 is ascribed to the oxidation process of liquid-phase Ru(NH3)62+at the electrode surface,while the latter wave at Epa=-0.250 V is attributed to the oxidation process of solid-phase Ru(NH3)62+adsorbed onto the electrode surface.Moreover,the broadened reduction peak at-0.252 V is attributed to the merged reduction process of both dissolved and adsorbed Ru(NH3)63+at the electrode surface.This is supported by the cyclic voltammograms(Fig.S1)in which the former wave at-0.123 V was barely observable but the latter wave at-0.250 V was still clearly present when the modified electrode was re-cycled in the pure phosphate buffer in the absence of Ru(NH3)63+.These results suggest that the electron transfer of the positively charged Ru(NH3)63+on the electrode surface was promoted due to the strong electrostatic adsorption of negative charged functional groups on the OPEDOT surface.
These electrochemical characterization results reveal that the OPEDOT-AuNPs-ERGO nanocomposite film possesses excellent ion-selectivity.The oxygen-containing sulfone,carbonyl,and carboxylic groups introduced during the overoxidation process were found to hinder the approach of the anion to the electrode surface(for electron transfer)but promote that of the cation.In addition,upon comparing the electrochemical behavior of the OPEDOT-AuNPs-ERGO/GCE with that of the PEDOT-AuNPs-ERGO/GCE in both the Fe(CN)63-and Ru(NH3)63+systems,it could be seen that the electrochemical activity of the electrode modification material can be significantly changed through an electrochemical overoxidation process.
3.3.Electrochemical behaviors of AA,DA,and UA on the OPEDOT-AuNPs-ERGO/GCE
The electrochemical catalysis of AA,DA,and UA on the GCE,PEDOT-AuNPs-GO/GCE,PEDOT-AuNPs-ERGO/GCE,and OPEDOT-AuNPs-ERGO/GCE was investigated by cyclic voltammetry.In Fig.6A,the oxidation peak of AA on a bare GCE(curve a)was observed at+0.144 V,with the peak current being 4.8μA.The electrochemical behavior of AA on the PEDOT-AuNPs-GO/GCE(curve b)was very similar to that of AA on the bare GCE.For the PEDOT-AuNPs-ERGO/GCE(curve c),the oxidation peak potential negatively shifted to+0.012 V and the oxidation peak current increased to 10.3μA.For the OPEDOT-AuNPs-ERGO/GCE(curve d),the oxidation peak appeared at+0.077 V and the oxidation peak current dramatically declined to a level comparable to that observed with the bare GCE.This indicates that the OPEDOT-AuNPs-ERGO nanocomposite can effectively block the electron transfer of AA.

Fig.6.Cyclic voltammograms of(A)1.0 mM AA,(B)0.1 mM DA,(C)0.1 mM UA,and(D)a mixture of 1.0 mM AA,0.1 mM DA,and 0.1 mM UA on a bare GCE(a),the PEDOT-AuNPs-GO/GCE(b),the PEDOT-AuNPs-ERGO/GCE(c),and the OPEDOT-AuNPs-ERGO/GCE(d)in 10 mM PBS(pH=7.4),collected at a scan rate of 50 mV/s.
In Fig.6B,DA exhibits a pair of redox peaks at+0.197 and+0.147 V with a ΔEpvalue of 50 mV and a formal potential(E1/2)of+0.172 V at the bare electrode.The ΔEpvalues barely changed when the bare electrode was replaced with the other modified electrodes,but the oxidation peak current at the OPEDOT-AuNPs-ERGO/GCE(12.7μA)was 6.4,3.7,and 1.6 times greater than those at the bare GCE (2.0 μA), PEDOT-AuNPs-GO/GCE (3.4 μA), and PEDOT-AuNPs-ERGO/GCE(7.9μA),respectively.Additionally,the reduction peak current at the OPEDOT-AuNPs-ERGO/GCE(8.0μA)was 7.3,4.7,and 1.5 times greater than those at the bare GCE(1.1 μA), PEDOT-AuNPs-GO/GCE (1.7 μA), and PEDOT-AuNPs-ERGO/GCE(5.4μA),respectively.This suggests that the OPEDOT-AuNPs-ERGO/GCE shows electro-catalytic activity towards DA that is superior to those of the other electrodes.
As shown in Fig.6C,the oxidation peak current of UA at the OPEDOT-AuNPs-ERGO/GCE(9.7μA)is 5.3,3.7,and 1.4 times greater than those at the bare GCE(1.8μA),PEDOT-AuNPs-GO/GCE(2.6 μA),and PEDOT-AuNPs-ERGO/GCE(6.8 μA),respectively.Furthermore,a weak reduction peak of UA at+0.290 V can be observed when the PEDOT-AuNPs-ERGO/GCE was used.For the OPEDOT-AuNPs-ERGO/GCE,this reduction peak is more intense with a reduction peak current of 1.4μA,indicating the excellent electrochemical catalytic effect that the OPEDOT-AuNPs-ERGO/GCE has on the electrochemical redox reaction of UA.
In order to further investigate the electro-catalytic activity of OPETOT-AuNPs-ERGO,a system with AA,DA,and UA coexisting was tested by cyclic voltammetry,as shown in Fig.6D.When the bare GCE was used,it was difficult to distinguish AA,DA,and UA from one another,and only a broad peak was seen at+0.197 V.When the PEDOT-AuNPs-GO/GCE was employed,the oxidation peak of UA was slightly separated from the overlapping oxidation peak corresponding to the other two species.When the PEDOT-AuNPs-ERGO/GCE was used,three well-defined oxidation peaks were observed at approximately +0.034,+0.232,and+0.370 V,corresponding to AA,DA,and UA,respectively.More interestingly,with the OPEDOT-AuNPs-ERGO/GCE,AA did not exhibit an obvious electrochemical response,while the oxidation peaks of DA and UA could be clearly seen at+0.257 and+0.436 V,respectively,which is in accordance with the behavior of AA without UA and DA present,as can be seen in Fig.6A.These results demonstrate that the OPEDOT-AuNPs-ERGO/GCE has excellent electrocatalysis and selectivity towards DA and UA,which is mainly attributed to the increased hydrophilicity of the film,the electronegativity of the abundant oxygen groups of OPEDOT,the electrocatalytic activity of graphene and the AuNPs,as well as the synergetic effects among these nanomaterials.Hence,the electrochemical performance and application of the OPEDOT-AuNPs-ERGO/GCE in the selective and sensitive determination of DA and UA in the presence of AA are worthy of exploration.
The effects of pH on the anodic peak currents and peak potentials of DA and UA at the OPEDOT-AuNPs-ERGO/GCE were investigated by cyclic voltammetry in 10 mM PBS solutions with pH values ranging from 4.4 to 8.4.From Fig.7,it can be seen that the oxidation peak currents of DA decrease with an increase in pH,while the oxidation peak currents of UA increase with increasing pH.This can be attributed to the fact that the amino group of DA receives a positive charge under acidic conditions because the pKbof DA is 8.87,whereas UA possesses a neutral and an anionic charge under the same conditions because the pKaof UA is 5.76[33].However,a pH of 7.4 was still chosen for the supporting electrolyte in the following electrochemical measurements to reflect physiological pH.Both the redox peak potentials negatively shifted with increasing pH,and the oxidation peak potentials of DA and UA exhibited good linear relationships with pH;the linear regression equations are Epa(V)=0.57-0.047×pH(R=0.9953)and Epa(V)=0.71-0.049×pH(R=0.9950)for DA and UA,respectively.The corresponding slopes of 47 and 49 mV/pH,for DA and UA,respectively,are close to the theoretical value of 59 mV/pH.This indicates that an equal number of electron and proton transfer processes occur with both DA and UA[34].

Fig.7.Cyclic voltammograms and effects of pH on the oxidation peak currents(a)and oxidation peak potentials(b)of(A and B)0.1 mM DA and(C and D)0.1 mM UA for the OPEDOT-AuNPs-ERGO/GCE in 10 mM PBS with pH values of 4.4,5.4,6.4,7.4,and 8.4,collected at a scan rate of 50 mV/s.
The kinetics of the electrode reaction was investigated by exploring the dependence of the redox peak currents of DA and UA on the scan rate.Fig.8 shows the cyclic voltammograms of the OPEDOT-AuNPs-ERGO/GCE in 10 mM PBS with scan rates ranging from 20 to 500 mV/s.From Figs.8 A and B,it can be seen that both the absolute values of the oxidation and reduction peak currents linearly increase with increasing scan rate.The absolute values of the redox peak potentials increased with the log of the scan rate,remained nearly constant at low scan rates,and linearly increased at high scan rates.This indicates that the electrochemical process of DA at the OPEDOT-AuNPs-ERGO/GCE is a surface-confined process with relatively slow electron transfer[6,35].From Figs.8C and D,it can be seen that both the absolute values of the oxidation and reduction peak currents linearly increase with increasing scan rate,while the peak potentials remain nearly unchanged with increasing scan rate.This indicates that the electrochemical process of UA at the OPEDOT-AuNPs-ERGO/GCE is a surface-confined process with fast electron transfer[36].

Fig.8.(A and C)Cyclic voltammograms and(B and D)the plots of peak currents and anodic and cathodic potentials versus scan rate on the OPEDOT-AuNPs-ERGO/GCE in 10 mM PBS(pH=7.4)containing(A and B)0.1 mM DA and(C and D)0.1 mM UA at different scan rates(from inner to outer):20,40,60,80,100,120,140,160,180,200,250,300,350,400,450,and 500 mV/s.Lines a and b are the plots of the peak currents,and lines c and d are the plots of the peak potentials versus scan rate or the log of scan rate.
3.4.Simultaneous determination of DA and UA in the presence of AA
Fig.9.shows the square wave voltammograms at the OPEDOT-AuNPs-ERGO/GCE for the different concentrations of DA and UA in 10 mM PBS(pH=7.4)in the presence of AA.In Fig.9A,the oxidation peak currents can be seen to increase linearly as the DA concentration was increased from 4.0 to 100μM.This was in the presence of 1.0 mM AA and 100μM UA,and the corresponding linear equation is Ipa(μA)=0.41 × CDA(μM)-2.1(R=0.9985).From Fig.9B,the oxidation peak currents can be seen to increase linearly as the UA concentration was increased from 20 to 100μM.This was in the presence of 0.5 mM AA and 50μM DA,and the corresponding linear equation is Ipa(μA)=0.22 × CUA(μM)-2.5(R=0.9954).The corresponding detection limits(S/N=3)were 1.0μM for DA and 5.0μM for UA.From Fig.9C,it can be seen that the oxidation peak currents increase linearly with the concentrations of DA and UA in the presence of 0.5 mM AA.For DA,the corresponding linear equations are Ipa(μA)=0.94 × CDA(μM)-0.27(R=0.9826)in the range of 1.0-10 μM and Ipa(μA)=0.35 × CDA(μM)-2.1(R=0.9967)in the range of 20-80μM.For UA,the corresponding linear equations are Ipa(μA)=0.46 × CUA(μM)-0.65(R=0.9993)in the range of 2.0-12 μM and Ipa(μA)=0.21 × CUA(μM)+0.18(R=0.9950)in the range of 22-82μM.

Fig.9.Square wave voltammograms of DA and UA on the OPEDOT-AuNPs-ERGO/GCE in 10 mM PBS pH=7.4,collected at a scan rate of 50 mV/s.(A)DA concentrations of 0,4.0,6.0,8.0,10,12,14,16,18,20,30,40,50,60,80,and 100 μM in the presence of 1,000 μM AA and 100 μM UA;(B)UA concentrations of 0,20,30,40,50,60,70,80,90,and 100 μM in the presence of 0.5 mM AA and 50 μM DA.(C)DA concentrations of 0.0,1.0,2.0,4.0,6.0,8.0,10,20,30,40,50,60,70,and 80 μM and UA concentrations of 0,2.0,4.0,6.0,8.0,10,12,22,32,42,52,62,72,and 82μM in the presence of 0.5 mM AA.The insets show the linear relationships between the currents and concentrations of DA and UA.
Moreover,this modified electrode was successfully applied to the detection of DA and UA in human urine samples,which were diluted 250 times with 10 mM PBS(pH=7.4)before the measurements.When known amounts of DA and UA were added to the human urine samples,satisfactory,quantitative recoveries were obtained for both DA(96.7%-105.0%)and UA(98.7%-100.8%)(as shown in Table S1).This indicates the potential of the OPEDOT-AuNPs-ERGO/GCE to be used for the detection of DA and UA in the presence of AA under physiological and pathological conditions.
4.Conclusion
A novel, graphene-based, ternary composite, OPEDOT-AuNPs-ERGO,was prepared on a GCE to give a modified electrode(OPEDOT-AuNPs-ERGO/GCE)that was successfully applied to the simultaneous determination of DA and UA in the presence of AA under physiological pH conditions.This work demonstrates that the developed OPEDOT-AuNPs-ERGO/GCE displays electro-catalytic activities towards the redox reactions of UA and DA that are superior to those of other electrodes evaluated.This electrode also inhibited the oxidation of AA.It is worth noting that the electrochemical activity of the electrode modification material can be significantly changed through an electrochemical overoxidation process.Additionally,OPEDOT facilitated analytical application despite the overoxidation process resulting in mechanical degradation and decreased electronic conductivity.This work reveals that OPEDOT-AuNPs-ERGO,with its attractive features,is a promising candidate for electroanalytical and clinical applications.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Financial supports from the Natural Science Foundation of Shaanxi Province,China(Grant No.:2020JM-652),Fundamental Research Funds for the Central Universities of Xi’an Jiaotong University(Grant No.:xzy012020054),and Cultivation Project of Xi’an Health Committee(Grant No.:2020MS02)are gratefully acknowledged.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.09.005.
 Journal of Pharmaceutical Analysis2021年6期
Journal of Pharmaceutical Analysis2021年6期
- Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
