NiuHuangJiangYa capsule for hypertension:a Bayesian dose-response analysis of multiple N-of-1 trials
Jing-Bo Zhai
1Institute of Traditional Chinese Medicine,Tianjin University of Traditional Chinese Medicine,10 Poyanghu Road,West Area,Tuanbo New Town,Jinghai District,Tianjin 301617,China.
Abstract Background:There is the limited evidence available from randomized controlled trials on the dose-response relationship of NiuHuangJiangYa capsule for hypertension.The objective of this study is to investigate the dose-response relationship of NiuHuangJiangYa capsule for hypertension based on multiple N-of-1 trials.Methods:This study was a secondary analysis of the data from a series of N-of-1 trials examining the efficacy of high-dose versus low-dose NiuHuangJiangYa capsule for hypertension.Hierarchical Bayesian models were used to aggregate these N-of-1 trials for estimating the population and individual treatment effects synchronously.Results:It showed that overall population estimates of the posterior mean difference in Systolic Blood Pressure reduction,Diastolic Blood Pressure reduction,and traditional Chinese medicine symptom score reduction were 3.18 mmHg (95% CIs:-4.69 to 9.04,posterior probability (>0):83.33%),0.8636 mmHg (95% CIs:-5.19 to 6.79,posterior probability (>0):63.38%),and 0.8384 (95% CIs:-2.21 to 3.84,posterior probability (>0):77.05%) respectively.Individual posterior mean difference ranged from 1.237 to 5.628 with posterior probability (>0) ranging from 63.63% to 92.95% in Systolic Blood Pressure reduction,-0.714 to 3.423 with posterior probability (>0) ranging from 43.03%to 84.04% in Diastolic Blood Pressure reduction,and -0.5179 to 2.733 with posterior probability (>0) ranging from 27.02% to 97.73% in traditional Chinese medicine symptom score reduction.Conclusion:The efficacy of high-dose versus low-dose NiuHuangJiangYa capsule for hypertension may be various across patients.Further studies are warranted to investigate these findings.Moreover,Bayesian N-of-1 trial may be helpful to explore the optimal and personalized dosage of anti-hypertensive drugs.
Key words:Hypertension,N-of-1 trials,Dose-response,Bayesian models
Background
Hypertension is one of the most common public health issues worldwide [1].It has greatly contributed to the high prevalence of cardiovascular and renal disease[2].In China,the prevalence of hypertension has been increasing over the past decades [3,4].
Many new anti-hypertensive drugs and strategies have been developed in recent years [5].However,it is difficult to treat hypertension successfully.There are multiple reasons for the low rate of blood pressure control,such as adverse effects,treatment cost,complications,long-term treatment,and so forth [6,7].Therefore,the alternative anti-hypertensive interventions are still needed [6].
There has been an increasing interest in the use of complementary and alternative medicine to treat hypertension [8].Traditional Chinese medicine (TCM)belongs to complementary and alternative medicine and includes herbal medicine,acupuncture,Taichi,and so forth [9].NiuHuangJiangYa (NHJY) capsule is a specific TCM,consisting of cornu saigae tataricae,bovis calculus,etc [10].NHJY capsule has been used widely for treating hypertension in China.However,a systematic review indicates that the methodological quality of randomized controlled trials (RCTs) of NHJY capsule for hypertension is low [11].Moreover,there is the limited evidence available from RCTs on the dose-response relationship of NHJY capsule for hypertension.
RCTs are considered as the gold standard for assessing the efficacy of interventions [12].Nonetheless,the generalization of evidence from RCTs is challenging [13].Individual treatment effect is defined as the effect difference between two therapies in a patient [14].Heterogeneity of treatment effect is defined as the magnitude of variation of individual treatment effect in a population [14].When the heterogeneity of treatment effect is present,average treatment effects from RCTs may reflect a mixture of risks and benefits to individual patients [13,14].If so,caution is necessary when applying the evidence from RCTs to individualized clinical decision making.
It is potential to address above-mentioned questions via N-of-1 trials [13,15].They are often multiple crossover trials conducted in single individuals.A series of N-of-1 trials can be aggregated to estimate population and individual treatment effects synchronously using hierarchical Bayesian models[16].This design is named as Bayesian N-of-1 trial[16].It is useful for assessing the heterogeneity of treatment effect [15,17].
According to the clinical guideline,the dosage of initial drug can be increased if the expected blood pressure reduction is not reached after a month of the anti-hypertensive treatment [18].In this study,we hypothesized that high-dose NHJY capsule compared to low-dose NHJY capsule would significantly reduce blood pressure.Hierarchical Bayesian models were used to explore the dose-response relationship of NHJY capsule for hypertension.
Methods
Design
The present study was a secondary analysis of the data from multiple N-of-1 trials.They were a series of randomized,double blind,controlled,single center,single patient (N-of-1) trials to examine the efficacy of high-dose versus low-dose NHJY capsule for hypertension [19].The secondary analysis was approved by the medical ethics committee of Tianjin University of Traditional Chinese Medicine(registration number TJUTCM-EC20150002).Patient records were anonymised and de-identified prior to analysis.
Recruitment of participants
It was conducted at Baokang hospital,which was an affiliated hospital of Tianjin University of Traditional Chinese Medicine.The participants were free to withdraw at any time during the study.
Inclusion criteria
Participants were eligible if they met items of the inclusion criteria listed below.
(1) Clinical diagnosis of mild to moderate hypertension;
(2) TCM diagnosis of overabundant liver-fire syndrome;
(3) Provision of written informed consent by participants or surrogates.
Exclusion criteria
Patients were excluded for the following reasons.
(1) Diagnosis of secondary hypertension;
(2) Presence of serious cardiovascular,hepatic,hematologic,nephritic diseases;
(3) Pregnancy,lactation or those who are preparing for pregnancy;
(4) History of allergy to NHJY capsule;
(5) Presence of mental illness;
(6) Medication history of Traditional Chinese Medicine in the latest week;
(7) Not return to the state of mild or moderate essential hypertension after the washout period.
Interventions
The eligible patients were given three paired treatment cycles (Figure 1).There were two treatment periods and two washout periods in each cycle.At the beginning of each cycle,patients underwent a one-week washout period.Then,patients were allocated to high-dose or low-dose group using a computer-generated random number scheme.Patients in high-dose group were administered NHJY four capsules two times daily for three weeks.Patients in low-dose group were administered NHJY two capsules and placebo two capsules two times daily for three weeks.Next,patients underwent the secondary washout period.Subsequently,they were assigned to the alternative treatment for three weeks during the secondary treatment period.
Outcome measures
The primary outcome was the Systolic Blood Pressure(SBP) reduction after treatment in each period of three cycles.The secondary outcomes included the Diastolic Blood Pressure (DBP) reduction and TCM symptom score reduction after treatment in each period of three cycles.
Statistical analysis
Bayesian statistics is an alternative statistical analysis approach as important as frequentist statistics [20].Given the hierarchical structure of the data from a series of N-of-1 trials,hierarchical Bayesian models were used to investigate the dose-response relationship between NHJY capsule and the anti-hypertensive effect [21].
Mean difference of SBP,DBP and TCM symptom score reduction between two groups was considered as the effect size.It is important to choose the appropriate prior distribution for parameters.Because no prior information was available from previous studies,non-informative prior distribution was used.According to the literature,we assumed that primary and secondary outcomes followed the normal distribution[21−23]. Hierarchical Bayesian models were constructed by WinBUGs software.We performed 50000 iterations with the burn-in of 5000 updates [23].
The posterior means of population and individual treatment effects were presented.A positive value indicated that the treatment benefit of high-dose NHJY capsule was greater than low-dose.The posterior probability and Bayesian 95% credible intervals (CIs)of posterior means were also provided.Bayesian 95%CIs indicated a 95% chance that the posterior means was between the lower and the upper limit of CIs [23,24].
Results
Characteristics of patients
Five patients completed all of three treatment cycles.The demographic characteristics of patients were presented in Table 1.The age ranged from 50 to 66 years old with a median age of 51.Two of the five patients were male.Duration of hypertension of three patients was more than ten years.
Systolic Blood Pressure (SBP)
Overall population estimate of the posterior mean difference in SBP reduction was 3.18 (mmHg) with 95% CIs (-4.69,9.04) in Table 2.Overall estimate of the posterior probability (>0) was 83.33%.All of individual posterior mean differences were greater than zero and ranged from 1.237 (mmHg) to 5.628(mmHg).The individual posterior probability ranged from 63.63% to 92.95%.
Diastolic Blood Pressure (DBP)
Overall population estimate of the posterior mean difference in DBP reduction was 0.8636 (mmHg) with 95% CIs (-5.19,6.79) in Table 3.Overall estimate of the posterior probability (>0) was 63.38%.The individual posterior mean difference ranged from-0.714 (mmHg) to 3.423 (mmHg).The individual posterior probability ranged from 43.03% to 84.04%.Only one individual posterior mean difference was greater than one (mmHg) with posterior probability of 84.04%.One individual posterior mean difference was less than zero (mmHg) with posterior probability of 43.03%.

Table 3 Overall population and individual estimate of posterior mean difference and posterior probability (>0) in diastolic blood pressure reduction.
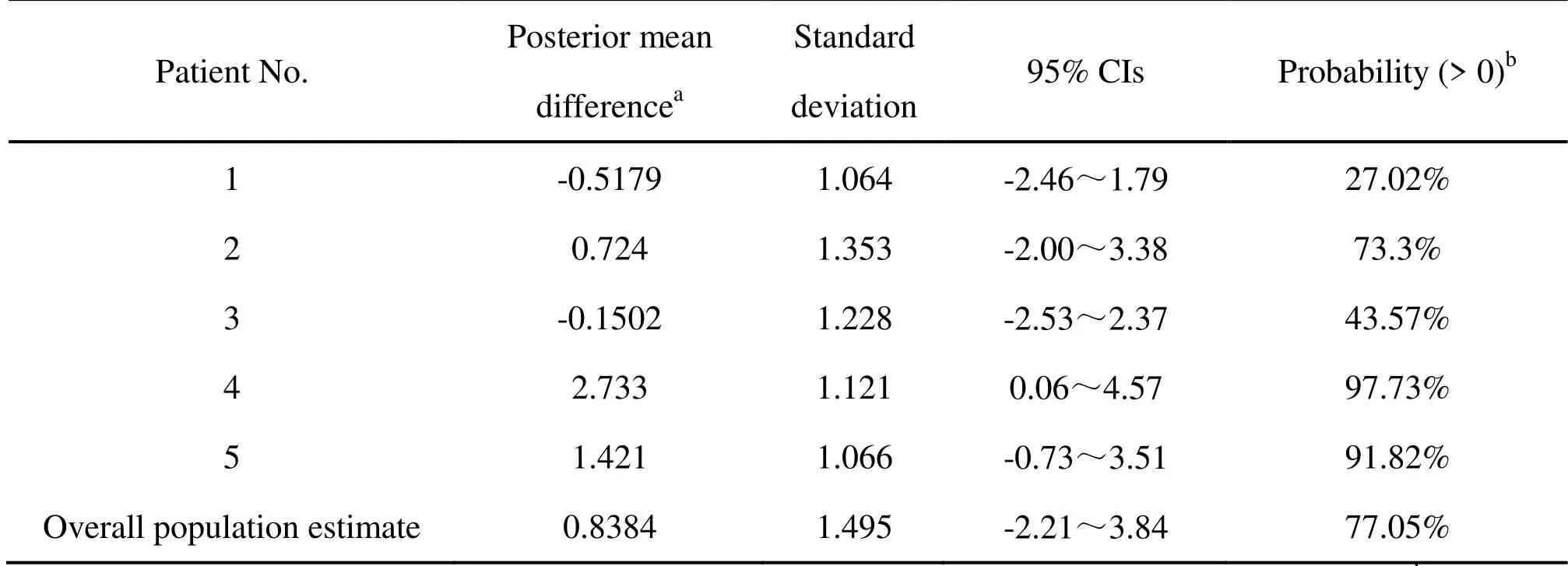
Table 4 Overall population and individual estimate of posterior mean difference and posterior probability (>0) in traditional Chinese medicine symptom score reduction.
TCM symptom score
Overall population estimate of the posterior mean difference in TCM symptom score reduction was 0.8384 (mmHg) with 95% CIs (-2.21,3.84) in Table 4.Overall estimate of the posterior probability (>0) was 77.05%.The individual posterior mean difference ranged from -0.5179 (mmHg) to 2.733 (mmHg).The individual posterior probability ranged from 27.02% to 97.73%.Two individual posterior mean differences were less than zero (mmHg) and posterior probabilities were less than 50%.
Discussion
In the present study,we focused on the dose-response relationship between NHJY capsule and anti-hypertensive effects.The population and individual treatment effects were synchronously estimated using Bayesian methods.The results showed that overall estimates of the posterior mean difference in SBP,DBP and TCM symptom score reduction were greater than zero.As the primary outcome,overall mean difference in SBP reduction was greater than three (mmHg),but not in DBP reduction.
Doctors and patients often show interest in the probability of treating the disease or improving symptoms.In Bayesian settings,the posterior probability can be estimated.It indicates a chance that posterior mean in a patient or population is greater or less than a value [23,25].The interpretation of results from the Bayesian analysis is more natural and easily understood than those from classical methods [21,24,25].In this study,posterior probabilities (>0) of overall mean difference in SBP,DBP and TCM symptom score reduction were more than 60%.In particular,the probability in SBP reduction was more than 80%.The evidence from point estimates of overall treatment effects and posterior probabilities seems to suggest that it is more effective to take high-dose NHJY capsule compared with low-dose NHJY capsule for dropping the blood pressure.However,the value of zero was contained in wide CIs.The wider CIs indicate that the conclusion is less certain [26].The results from the Bayesian analysis should be interpreted cautiously.
The heterogeneity of treatment effects may account in part for the wide CIs.We found that treatment responses were significantly different across patients.The posterior mean difference in patients labeled as 1 and 3 was greater than zero in SBP and DBP but lower than zero in TCM symptom score.The posterior mean difference in patients labeled as 2 and 4 was greater than zero in SBP,DBP and TCM symptom score.The posterior mean difference in the patient labeled as 5 was greater than zero in SBP and TCM symptom score but lower than zero in DBP.The wide range of individual posterior probabilities indicates the large variation in the individual treatment effects.Individual posterior probabilities in SBP,DBP and TCM symptom score ranged from 63.63% to 92.95%,43.03% to 84.04%,and 27.02% to 97.73%,respectively.Bayesian statistics has been widely applied in medicine [27].Compared with frequentist statistics,Bayesian statistics increases the statistical precision by upgrading the prior information into posterior information [13].Furthermore,individual and population effects can be estimated synchronously.It is helpful to identify the heterogeneity of treatment effect [13].The prevention and treatment of hypertension has entered into the new era of precision medicine [28].N-of-1 trial is interesting as an important individualized research design.A comment published in Nature reported that N-of-1 approach could be used to investigate the safety and appropriate dosages of drugs [29].Therefore,Bayesian N-of-1 trial may be helpful to explore the optimal and personalized dosage of anti-hypertensive drugs.
There are several limitations in the study.Firstly,subgroup analysis is important to explore whether the difference of the efficacy depends on certain factors,such as demographic characteristic,medical history,and so forth [30].However,subgroup analysis was not conducted because of the small sample size in this study.Secondly,there was a short treatment period in these N-of-1 trials.The short treatment period can improve the feasibility of N-of-1 trials [31].Nonetheless,the long treatment period may be needed to reach full effect [31].Therefore,the treatment period should be carefully specified to ensure a good balance between the feasibility and full effect.
Conclusion
The efficacy of high-dose versus low-dose NHJY capsule for hypertension may be various across patients.Further studies are warranted to investigate these findings.Moreover,Bayesian N-of-1 trial may be helpful to explore the optimal and personalized dosage of anti-hypertensive drugs.
- Medical Data Mining的其它文章
- Based on Network Pharmacology to Explore the Action Mechanism of Caulis Sinomenii-Caulis Piperis Kadsurae Herb Pair in the Treatment of Rheumatoid Arthritis
- Molecular mechanism of Mimenghua Granules in treating dry eye
- Construction of miRNA-mRNA regulatory network and drug prediction for ulcerative colitis associated colorectal cancer
- Efficacy of acupuncture as an adjuvant to speech and language therapy for aphasia after ischemic stroke:a protocol of systematic review and meta-analysis
- The research on rule of Acupoints and Massage Manipulations selection for Postischemic Stroke Constipation based on association rule and entropy clustering analysis

