Haploidentical hematopoietic stem cell transplantation for pediatric patients with chronic active Epstein-Barr virus infection:a retrospective analysis of a single center
an-Hui Luo · Jun Yang · Ang Wei · Guang-Hua Zhu · Bin Wang · Rui Zhang · Chen-Guang Jia · Yan Yan ·Kai Wang · Sidan Li · Xuan Zhou · Mao-Quan Qin · Tian-You Wang
Abstract
Keywords Chronic active Epstein-Barr virus infection · Haploidentical hematopoietic stem cell transplantation ·Prognosis · Graft versus host diseases · Thrombotic microangiopathy
Introduction
Epstein-Barr virus (EBV) belongs to the gamma-herpesvirus family of double-stranded DNA viruses [ 1]. Chronic active Epstein-Barr virus infection (CAEBV) is an EBVpositive T- or NK-lymphoproliferative disease (EBV-T/NKLPD) [ 2]. Patients often develop a more chronic course with persistent, infectious, mononucleosis-like symptoms, including fever, persistent lymphadenopathy, and splenomegaly.CAEBV is not a simple chronic disease, but its flare-up can result in a rapid, irreversible, and fatal clinical course, such as hemophagocytic lymphohistiocytosis (HLH) and lymphoma [ 3]. At present, the only effective treatment strategy for eradicating EBV-infected T or NK cells is allogeneic hematopoietic stem cell transplantation (allo-HSCT) [ 4- 6].Human leukocyte antigen (HLA)-matched sibling donors or unrelated donors are the first choice for HSCT, but in China, there are usually no matched donors available. Thus,haploidentical donors (HID) are commonly used. Treatment of pediatric CAEBV with haplo-HSCT has rarely been reported. Herein, we report cases with pediatric CAEBV treated with haplo-HSCT with anti-thymocyte globulin(ATG) preparative regimens for partial in vivo T-cell depletion in our center.
Methods
Patients
This is a retrospective observational study. Children suffering from CAEBV who underwent haplo-HSCT between October 2016 and June 2020 were enrolled in this study.None of the patients found an HLA-matched sibling or unrelated donors in the China Bone Marrow Bank. Data were retrospectively reviewed for the source of hematopoietic stem cells, conditioning regimen, adverse effects, and prognosis. The follow-up duration was defined as the number of days between the date of transplantation and the last clinic visit. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Beijing Children’s Hospital, Capital Medical University ([2021]-A-123-R). All patients’ parents or guardians signed informed consent forms.
Diagnostic criteria
CAEBV diagnosis was made based on the previous literature, including persistent fever, lymphadenopathy, hepatomegaly and splenomegaly, generally combined with pathology of hematopoietic, digestive and nervous systems; EBV-DNA load > 500 copies/mL in the peripheral blood; EBV infection of T lymphocytes or NK cells in the affected tissues or peripheral blood; EBV Viral capsid antigen (VCA)-IgG ≥ 1:640, anti-EBV Early antigen (EA)-IgG ≥ 1:160, EBV VCA-IgA and/or EBV EA-IgA ( +) [ 7, 8].Additionally, infectious mononucleosis, immunodeficiency(such as chronic granulomatous disease, X-linked severe combined immune deficiency, etc.), malignancy and HIV infection should be excluded. To determine which population of lymphocytes was infected by EBV, the EBV genome was amplified by real-time quantitative PCR in DNA extracted from each lymphocyte subset in peripheral blood,or EBV-encoded small ribonucleic acid (EBER) and surface markers were stained on thin-sliced tissue from involved organs. EBV load was measured using real-time quantitative PCR, and the lower limit of detection was 500 copies/mL in peripheral blood mononuclear cells (PBMCs) or in plasma. HLH diagnosis was made based on the HLH-04 criteria proposed by the International Histiocyte Society [ 9].Acute and chronic graft versus host diseases (GVHD) were diagnosed and graded by physicians according to defined criteria [ 10, 11]. Thrombotic microangiopathy (TMA) was diagnosed by physicians according to defined overall TMA(O-TMA) criteria by Cho et al. [ 12]. Hepatic veno-occlusive disease (VOD) was diagnosed by physicians according to the defined criteria [ 13].
Evaluation of disease status
Disease status before allo-HSCT was assessed based on clinical features and EBV load and consequently was classified as either active or stable. Active disease was defined by the existence of symptoms and signs, such as fever, persistent hepatitis, significant lymphadenopathy, hepatosplenomegaly,pancytopenia and/or severe hypercytokinemia alongside an elevated EBV load in PBMCs or in plasma.
Disease status after allo-HSCT was divided into two subtypes: overall survival (OS) and event-free survival (EFS).OS was estimated from the date of diagnosis until the date of death due to any reason or last contact with the patients.EFS was estimated from the time of diagnosis to the appearance of any of the following events: progression, relapse or death, or second HSCT (due to engraftment failure or loss of donor chimerism).
Engraftment and chimerism
Engraftment analyses were conducted according to genetic alteration of 19 autosomal STR loci and Amel loci in the sex chromosome to distinguish donor and recipient cells. Full chimerism was defined by the detection of ≥ 95% and mixed chimerism 5-95% of donor hematopoietic stem cells in the recipient bone marrow (BM) or peripheral blood (PB) [ 14].
The date of neutrophil recovery was defined as the first of three consecutive days when the absolute neutrophil count exceeded 500/ml. The date of platelet recovery was defined as the first of 7 consecutive days when the platelet count exceeded 20 × 10 6 /L without infusion of platelets.
Treatment
Treatment before HSCT
Ten patients with EBV-HLH at onset received the HLH-94 protocol to suppress activated T cells, NK cells and macrophages before they were diagnosed with CAEBV [ 15]. Allpatients were treated with 1-4 courses of the L-DEP regimen (PEG-asparaginase, liposomal doxorubicin, etoposide and methylprednisolone) to reduce/eliminate EBV-infected T/NK cells and to suppress the disease activity after they were diagnosed with CAEBV [ 16]. Because PEG-asparaginase could lead to pancreatitis, it was not used in patients with pancreatic insufficiency. Six patients received modified ESCAP regimen followed by L-DEP because they have a high burden of residual disease [ 5]. Planned allo-HSCT was performed in eighteen patients who achieved CR/PR after 2-4 courses of chemotherapy. When the disease condition persisted in active status during chemotherapy, emergency HSCT was planned in seven patients. Before the initiation of conditioning, two of the active patients received the L-DEC regimen to suppress activated T cells [ 17]. Another active patient received 200 mg/kg antilymphocyte globulin (ALG)instead of antithymocyte globulin (ATG) during conditioning because the patient suffered from heart failure and multiple aneurysms (Tables 1 and 2).
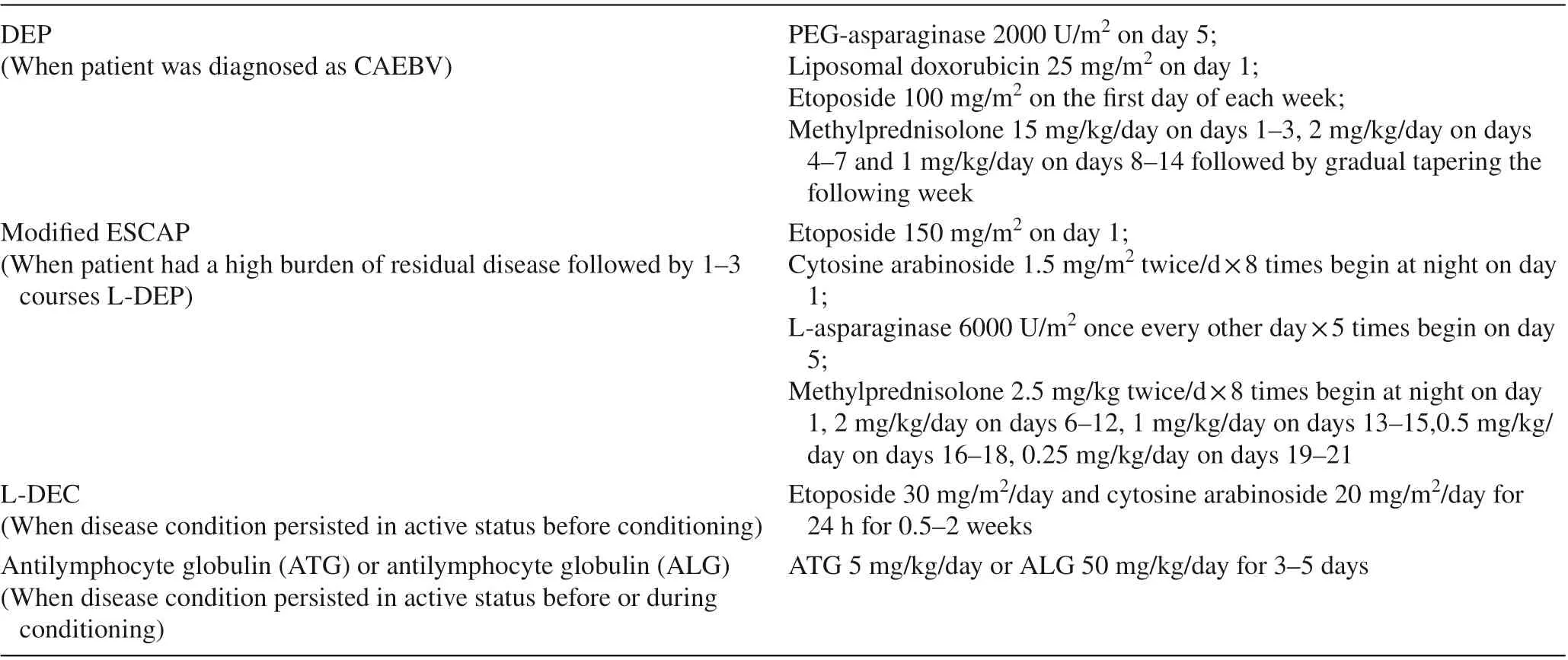
Table 1 Treatment before hematopoietic stem cell transplantation (HSCT)
Haplo-HSCT regimen
Conditioning regimens were classified as modified myeloablative conditioning (MAC) if they contained an alkylating agent (busulfan ≥ 9 mg/kg) with fludarabine (Flu) and cyclophosphamide (Cy). Low-dose total body irradiation(TBI) < 5 Gy or busulfan < 9 mg/kg was classified as reduced intensity conditioning (RIC) [ 18]. More information is provided in Tables 2 and 3. Ursodeoxycholic acid, low molecular weight heparin calcium and alprostadil were used to prevent VOD at the beginning of conditioning. All the transplant recipients received prophylactic CsA, mycophenolate mofetil (MMF), and methotrexate (MTX) for acute GVHD[ 19]. The time of use of immunosuppressants was adjusted according to the GVHD performance and the EBV-DNA copies. If there was no GVHD and EBV-DNA was negative,MMF generally begins to decrease in 3-4 months and stops in 4-6 months after transplantation, while CsA begins to decrease in 9-12 months and stops in 12-18 months. If the child presented with GVHD, the immunosuppressant was temporarily reduced; if the EBV-DNA turned positive, the immunosuppressant was reduced in advance.
To increase the rate of graft chimerism, bone marrow and peripheral blood stem cells were used as a combined graft source for all patients. The rate between PBSC and BM was different in all the patients, but the rate of PBSC fluctuated around 80%. The day of HSC infusion was defined as day 0.
Statistical analysis
The SPSS software package (IBM, Armonk, NY, USA),version 24.0, was used for all statistical analyses. The chisquare test or Fisher’s exact test was used to compare categorical variables. The Mann-Whitney U test or the t test was used to compare quantitative variables. The survival rate was estimated with the Kaplan-Meier method and was assessed with the log-rank test. P < 0.05 was considered statistically significant for all analyses.
Results
General patient information
Twenty-five cases of CAEBV were enrolled in this study,including 16 (64%) males and 9 (36%) females. Amongthese patients, 16 patients were found to have T-cell type disease, and 9 patients were found to have T/NK-cell type disease (Table 2). The mean age at disease onset was 5.0 ± 2.6 years. The mean age at transplantation was 6.9 ± 2.9 years. The median time from diagnosis to transplantation was 3.8 (2.0-40.2) months. Eighteen (72.0%)cases were complicated with HLH at onset. The family history of CAEBV was unremarkable. In the early stages of the disease, the most frequent clinical manifestations were fever (96%), followed by hepatosplenomegaly (92.0%),hemophagocytic phenomenon (88.0%), lymphadenopathy (84.0%) and hemocytopenia (56.0%). The other clinical manifestations included hepatitis (36%), CNS disease(32%), coronary artery dilated (24%), rash (16%) and interstitial pneumonia (5%).
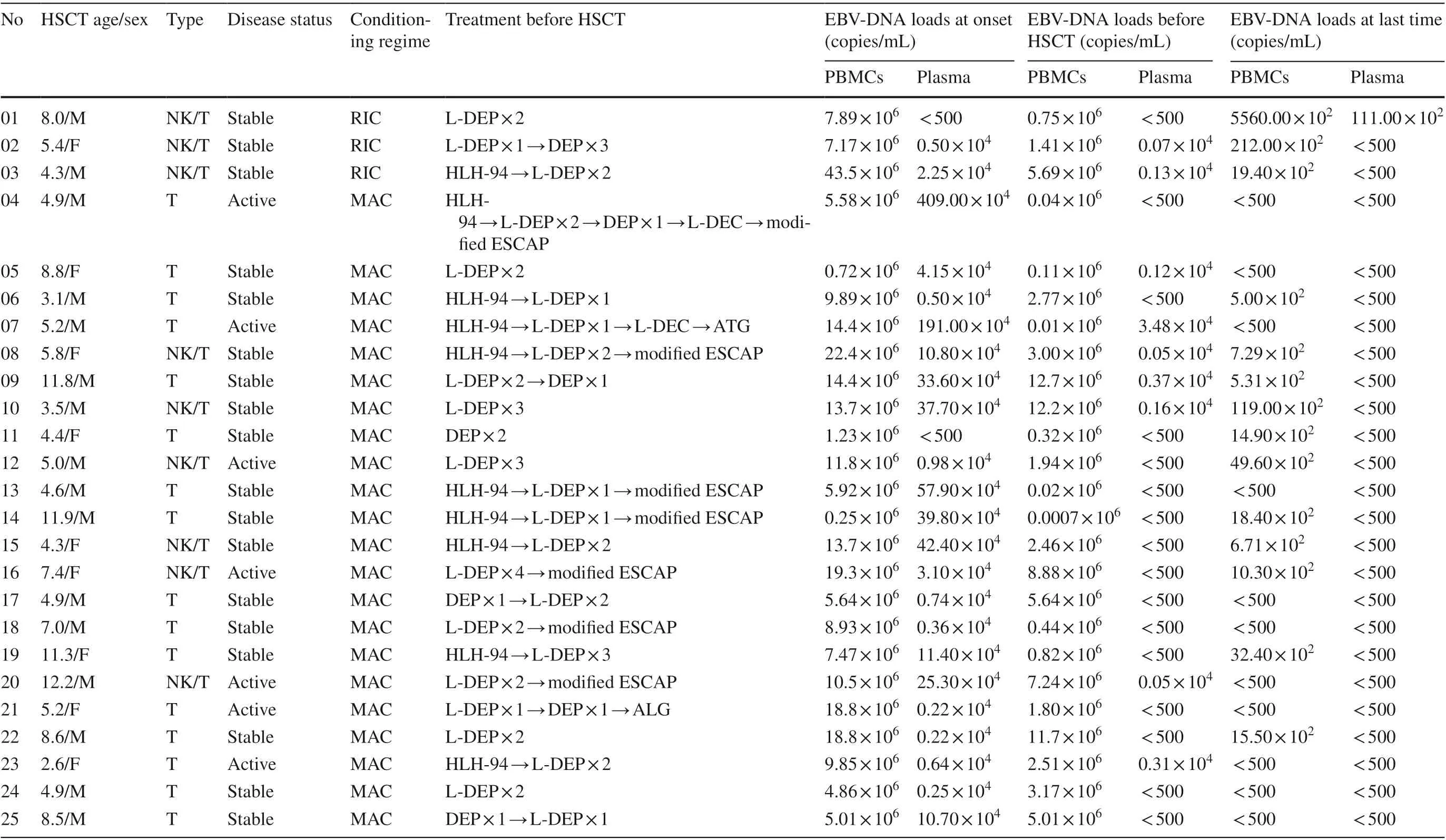
Table 2 Details of the 25 patients with chronic active Epstein-Barr virus infection (CAEBV) treated with haploidentical hematopoietic stem cell transplantation (Haplo-HSCT)

Table 3 Conditioning regimens
Engraftment and chimerism
Three patients (cases 1-3) were treated with the RIC regimen, while the remaining patients received the modified MAC regimen. Stem cells were obtained from G-CSF-mobilized peripheral blood and bone marrow. The mean infused mononuclear cell (MNC) count was 9.2 ± 4.4 × 10 8 /kg, and the mean infused CD34 + cell count was 9.3 ± 2.2 × 10 6 /kg. All twenty-five patients were engrafted successfully,with a mean time of neutrophil recovery at 12.6 ± 2.3 days and with a median time of platelet recovery at 14.5 days(range: 7-135 days), except patient 1, who needed constant platelet transfusion because of TMA. Mixed donor chimerism occurred in three patients after engraftment, ranging from 85.9%-92.4%. Case 2 turned to full donor chimerism at 3 months after transplantation. Case 3 exhibited mixed donor chimerism of maximum 89.9% when his disease recurred at 7 months after transplantation. He then received one dose of rituximab and six donor lymphocyte infusions(DLIs), with immunosuppressant drug reduction. His disease was controlled, and he reached full donor chimerism at 9 months after transplantation. Case 24 changed to mixed donor chimerism to 92.4% without disease recurrence at 4 months after transplantation. After two mesenchymal stem cell infusions and immunosuppressant drug reduction, he achieved full donor chimerism. The other 22 patients showed full donor chimerism without recurrence of disease. Additional information is provided in Table 4.
EBV-DNA loads
The median maximum EBV-DNA loads at onset were 9.9 × 10 6 copies/ml in PBMCs and 3.1 × 10 4 copies/ml in plasma. After chemotherapy, the EBV-DNA loads decreased but did not turn negative in PBMCs, whereas EBV-DNA in plasma turned negative in 16 patients (64.0%) before HSCT.After HSCT, EBV-DNA in PBMCs turned negative in 11 patients (44.0%), and EBV-DNA in plasma turned negative in all patients (100.0%). EBV-DNA in plasma reverted back to positive in cases 1 and 3; case 1 died of TMA, while EBV-DNA in plasma of case 3 turned negative again after treatment. Additional information is provided in Table 2.
GVHD
GVHD is the most common complication after transplantation. In our study, 16 (64.0%) patients suffered acute GVHD (aGVHD), ten of whom were grade I-II (skin stage 1-3 or/and gastrointestinal stage 1) and six of whom were grade IV (skin stage 2-4 or/and gastrointestinal stage 4).Chronic GVHD (cGVHD) was observed in 9 patients(36.0%) (Table 4). Most of them were mild (28.0%), showing local cGVHD manifestations of skin, nails, mouth, and eyes. Two cases (8.0%) showed moderate lung cGVHD. No severe cGVHD was observed except for one patient (case 9), who suffered grade IV aGVHD (gastrointestinal stage 4) and discontinued the treatment for economic reasons and finally died. Other patients who suffered GVHD survived after systemic combination therapies and supportive care.
TA-TMA and VOD
According to the diagnostic criteria of O-TMA, 7 patients(28.0%) fit the criteria for TA-TMA. Case 1 was diagnosed with TA-TMA on day + 6 and treated with defibrotide, low molecular weight heparin and alprostadil. At the same time,cyclosporine was stopped and was replaced with methylprednisolone and basiliximab to prevent aGVHD. However,the patient's condition still progressed, with pulmonary edema, convulsions, multiple organ hemorrhage (including digestive tract, mucous membrane, urinary system and intracranial) and multiple organ failure, and the patient died on day + 35. Case 9 died, and the circumstances of death are described above. The other 5 patients with TA-TMA survived after timely treatment, including defibrotide, reduction or discontinuation of calcineurin inhibitor (CNI) and active control of GVHD with methylprednisolone, ruxolitinib and/or basiliximab instead. Only Case 20 had sequelae of moderate renal insufficiency. In addition, one patient (4.0%)had VOD, and he survived after timely treatment without sequelae.
Follow-up and survival
As of August 1st, 2020, the mean follow-up time was 19.0 ± 12.0 months, and the longest follow-up time was 42.8 months. Among the enrolled patients, 23 patients were characterized by disease-free survival, 22 were characterized by event-free survival, and 2 died. One of the patients died of TMA, and the other died of GVHD; this patient discontinued the treatment for economic reasons. The 3-year OS rate was estimated to be 92.0% ± 5.4%. The 3-year EFS rate was estimated to be 87.4% ± 6.8%.
The 3-year OS of modified MAC-HSCT (95.5% ± 4.4%)was higher than that of RIC-HSCT (66.7% ± 27.2%), but the difference was not statistically significant (P= 0.062).The 3-year EFS of modified MAC-HSCT (95.5% ± 4.4%)was higher than that of RIC-HSCT (33.3% ± 27.2%), with a statistically significant difference (P= 0.001). According to the disease status before HSCT, we divided the patients into an active group and a stable group, and there was no statistically significant difference between them in 3-year OS (100% vs. 88.9% ± 7.4%,P= 0.371) or in 3-year EFS(100% vs. 82.5% ± 9.2%,P= 0.268). We divided the patients into an HLH group and a non-HLH group according to whether they were complicated with HLH at onset, and there was no significant difference between them in 3-year OS(94.4% ± 5.4% vs. 85.7% ± 13.2%,P= 0.451) or in 3-year EFS (88.1% ± 7.9% vs. 85.7% ± 13.2%,P= 0.772) (Fig. 1).
In addition, we analyzed other conditions, such as sex,age at transplantation, time from diagnosis to transplantation, EBV-infected cells, HLA matches, number of MNCs infused, number of CD34 + cells infused, CMV infection,aGVHD, cGVHD, and TMA, and we did not find any statistically significant difference. We also found no statistical difference between L-DEP or L-DEC regimen and intensity/number of courses before HSCT on the outcome/GvHD,TMA afterwards.
Discussion
Without treatment, CAEBV patients might develop hemophagocytic syndrome, multiple organ failure or lymphoma [ 3], and most patients die in the first 10-15 years[ 20]. Conventional therapies in the past for CAEBV have included antiviral therapy, immunotherapy, chemotherapy and so on. All of these strategies have been used without clear benefit. Most of them achieved only temporaryremission. At present, hematopoietic stem cell transplantation is regarded as the only curative therapy [ 4- 6].
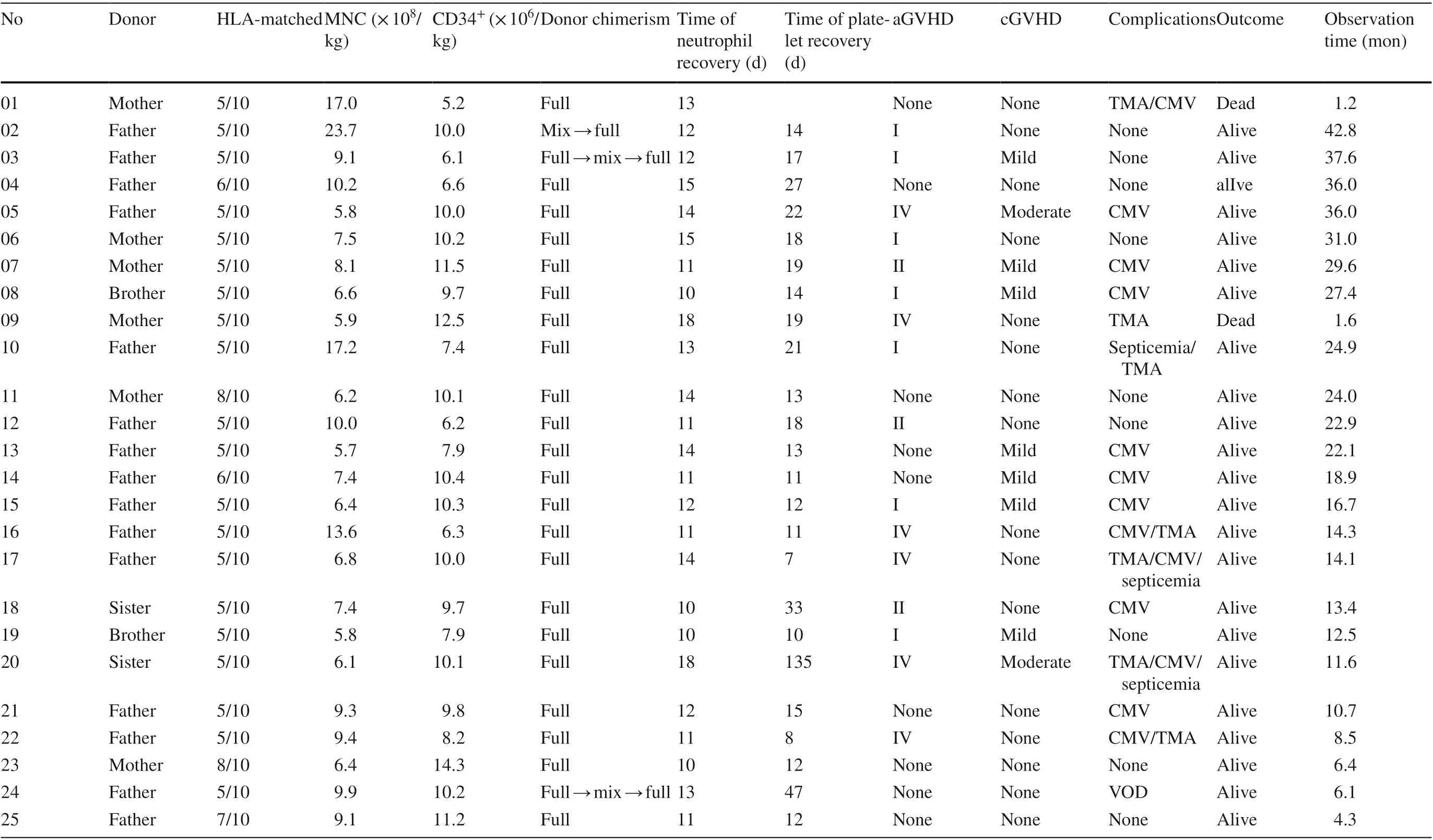
Table 4 Engraftment, chimerism and outcome of the 25 chronic active Epstein-Barr virus infection (CAEBV) patients treated with Haplo-HSCT
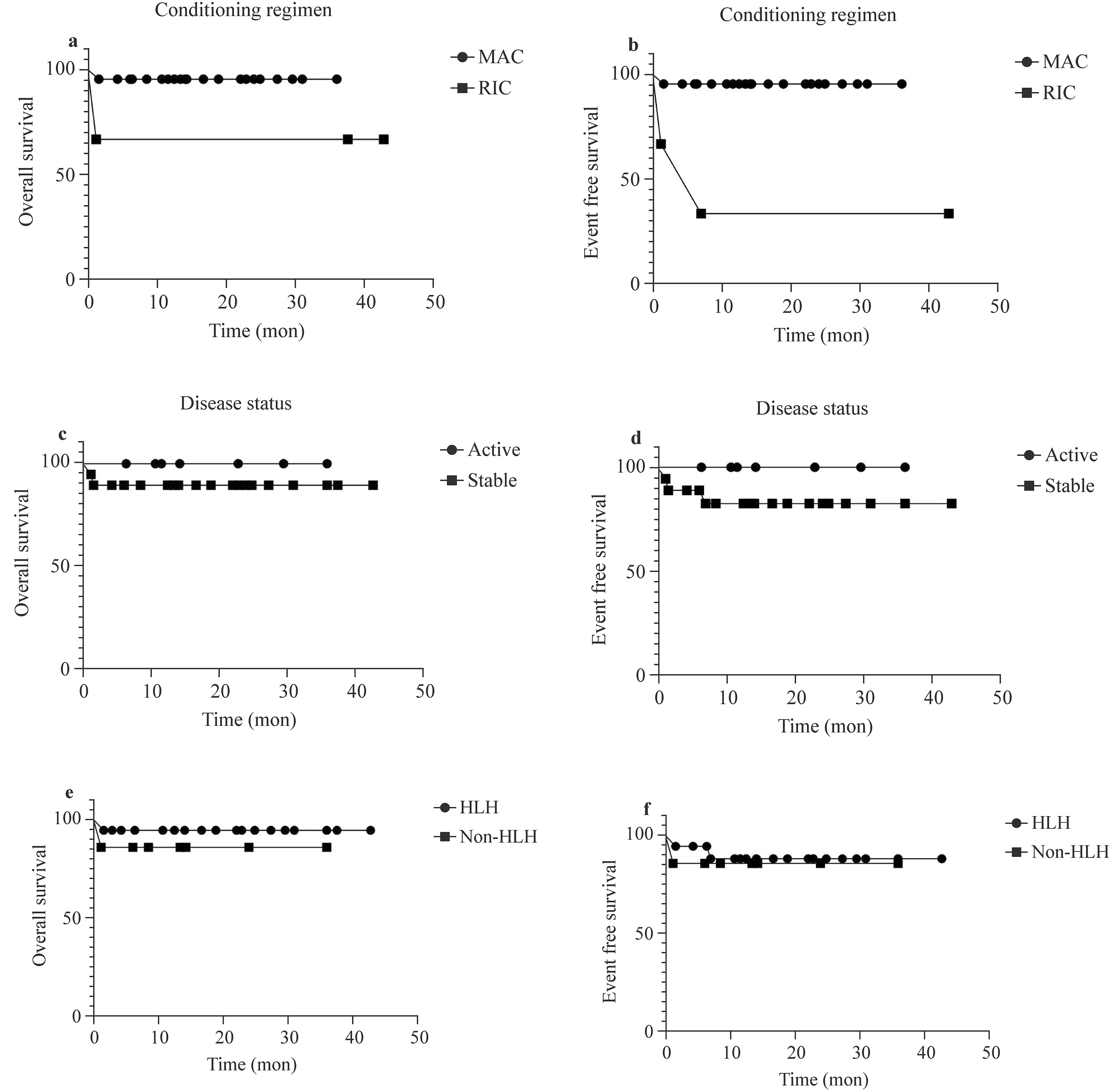
Fig. 1 The 3-year OS of modified MAC-HSCT (95.5% ± 4.4%)was higher than that of RIC-HSCT (66.7% ± 27.2%), but the difference without statistically significant ( P = 0.062) ( a). The 3-year EFS of modified MAC-HSCT (95.5% ± 4.4%) was higher than that of RIC-HSCT (33.3% ± 27.2%), with statistically significant difference ( P = 0.001) ( b). There was no statistically significant between active group and stable group in 3-year OS(100% vs.88.9% ± 7.4%, P = 0.371) ( c). There was no statistically significant between active group and stable group in 3-year EFS (100% vs.82.5% ± 9.2%, P = 0.268) ( d). There was no statistically significant between HLH group and non-HLH group in 3-year OS(94.4% ± 5.4%vs. 85.7% ± 13.2%, P = 0.451) ( e). There was no statistically significant between HLH group and non-HLH group in 3-year EFS(88.1% ± 7.9% vs. 85.7% ± 13.2%, P = 0.772) ( f) Note: MAC myeloablative conditioning, RIC reduced intensity conditioning, HLH hemophagocytic lymphohistiocytosis, mon month
At present, most of the reported cases were matched donor transplantations. A prospective multicenter study showed that the 15-year OS of EBV-associated T/NK-cell lymphoproliferative disease treated with allo-HSCT was 60.6%, whereas that of the non-HSCT group was only 25.7%. Univariable analysis and multivariable analysis of factors associated with mortality revealed that HSCT was an independent factor in reducing the mortality rate [ 21]. Sato et al. [ 22] reported that the EFS of CAEBV patients in Japan who were treated with HSCT was 56.1% ± 8.6%, in which the EFS of MAC-HSCT was 48.8% ± 7.4%, and the EFS of RIC-HSCT was 56.3% ± 12.4%, the figure higher than that of MAC-HSCT; however, the difference was not statistically significant. Sawada et al. [ 5] reported the use of a 3-step strategy for the treatment of CAEBV, including allo-HSCT.The results showed that the 3-year OS of CAEBV patients with stable disease before HSCT was 87.3% ± 4.2%, whereas the 3-year OS of patients with uncontrolled active disease was only 16.7% ± 10.8%.
However, in China, matched donors are rarely available.Haplo-HSCT was performed when patients had no matched sibling donors or unrelated donors or when patients needed emergency transplantation and could not wait for unrelated donors. There have been few reports on the effect of haplo-HSCT in the treatment of CAEBV. In the present study we reported 25 cases of pediatric CAEBV treated with haplo-HSCT with ATG preparative regimens for partial in vivo T-cell depletion in our center. The longest followup period was 42.8 months. The 3-year estimated OS rate was 92.0% ± 5.4%, and the EFS rate was 87.4% ± 6.8%. This proved that haplo-HSCT was an effective method for the treatment of pediatric CAEBV.
Some authors reported that the prognosis of CAEBV was linked to the disease status before HSCT and that patients with uncontrolled active disease had poor prognosis, with a 3-year OS of only 16.7 ± 10.8% [ 5, 23]. However, in our study patients with active disease before HSCT also benefited from haplo-HSCT. All these patients survived eventfree. This might be due to the addition of etoposide and ATG in the conditioning regimen. Etoposide could selectively remove activated T lymphocytes and inhibit the production of cytokines. ATG could not only reduce the occurrence of GVHD but also inhibit T cell proliferation and eliminate T cells. It was reported that ATG was an effective method for the treatment of FHL, and patients who were treated by HSCT shortly after ATG administration had a very good outcome [ 17]. In our study, the active diseases were controlled with ATG and ALG in cases 7 and 21, respectively. Both survived event-freely. In addition, the preparation time of HIDs was shorter than that of unrelated donors, which shortened the course before transplantation and may be advantageous for survival. Clinicopathological investigations have suggested that CAEBV is associated with both malignant neoplasms and immunodeficiency [ 24]. Thus, haplo-HSCT also could reduce recurrence and could improve prognosis through graft versus tumor (GVT) effect. These hypotheses need to be confirmed in larger sample studies.
Some researches have suggested that RIC-HSCT could produce a better outcome in the treatment of CAEBV in studies where the donors were mostly matched donors or where unrelated cord blood was used [ 25, 26]. However,there are little data to demonstrate whether this conclusion can be extended to haploidentical donors. To reduce the toxicity related to the conditioning regimen, the first three patients in our center were treated with RIC-HSCT.The 3-year EFS of modified MAC-HSCT was significantly higher than that of RIC-HSCT. Superior clinical outcomes obtained by modified MAC-HSCT also were observed in the treatment of HLH in our center [ 27]. As reported in the literature, modified MAC was more effective, well tolerated and could achieve adequate engraftment with acceptable toxicity [ 27- 29]. RIC in haploidentical HSCT always results in mixed donor chimerism and in a higher recurrence rate [ 26, 27].
With improvements in the pharmacoprophylaxis of GVHD and supportive care, it has been reported that haplo-HSCT can achieve comparable outcomes to HLA-identical sibling transplantation [ 29, 30]. In our study the incidence of aGVHD was 64.0%, and grades I-II were the most common.Five patients were diagnosed with grade IV aGVHD (gastrointestinal stage 4, with or without skin grade 2-3) based on bloody stool. However, they were also complicated with TMA. It was difficult to distinguish whether the bloody stool was caused by TMA or by gastrointestinal stage 4 aGVHD.Therefore, the incidence of grade IV aGVHD in the present study might be overestimated. The incidence of cGVHD was 36.0%, and most of the cases were mild. The high incidence of GVHD in our center might be related to the large number of infused mononuclear cells. All patients’ GVHD was controlled by systemic combination therapies and supportive care except case 9, who discontinued the treatment for economic reasons.
HID and MAC regimens were associated with a higher risk of VOD/SOS [ 13]. To reduce the toxicity of MAC, the doses of BU and Cy were reduced in the modified MAC in our center. Etoposide was added, and the dose of ATG was increased to 10 mg/kg instead. The incidence of VOD was 4.0%, and the patient survived after timely treatment without sequelae. TMA is also a life-threatening transplant-related complication, with a high mortality (60-90%) in its most severe form [ 31]. Due to the different diagnostic criteria, the incidence of TA-TMA ranges widely (0.5%-76%) between centers [ 32, 33]. In our center a broad definition of overall TMA (O-TMA) by Cho et al. was used so that TA-TMA could be identified early [ 12]. The incidence of O-TMA was 28.0% in our center. It has been suggested that high-dose chemotherapy, radiation, CNI exposure, GVHD and infections may be the causative factors for the development of TA-TMA [ 33]. In addition, CAEBV itself also predisposes the patients to cardiovascular disease. EBV-infected T or NK lymphocytes can infiltrate and damage the myocardium and vascular walls, and high levels of pro- and anti-inflammatory cytokines induced by EBV can lead to an inflammatory response [ 34, 35]. The reason for the high incidence of TMA in our center might be related to haploid transplantation, vascular damage caused by primary disease and broad diagnostic criteria. Early diagnosis and treatment are positively correlated with a survival benefit [ 36]. Through broad diagnostic criteria, TMA could be identified and treated at an early stage. By timely intervention including defibrotide,reduction or discontinuation of CNI and active control of GVHD with methylprednisolone, ruxolitinib and/or basiliximab, only one patient died of TA-TMA in our study.
In conclusion, haploid hematopoietic stem cell transplantation is safe and effective in the treatment of CAEBV and can be used as an alternative therapy for patients who do not have a matched donor or who urgently require transplantation. Patients with active disease before HSCT also benefited from haplo-HSCT. GVHD and TMA/VOD are still the main transplant-related complications. Early detection and timely treatment of complications can reduce transplantrelated mortality and can improve the clinical outcome.We recognize that the number of patients in this study was small and that longer follow-ups are needed to evaluate more accurately the prognosis of CAEBV patients treated with haplo-HSCT.
Acknowledgements We thank all of the patients and their families for their kind cooperation. We thank all of the members of clinical team who provided care for patients. This work was supported by Beijing Municipal Science & Technology Commission (No.Z171100001017050) and National Science and Technology Key Projects (No. 2017ZX09304029001).
Author contributions YHL, JY and AW contributed equally to this paper. YHL and AW designed and performed the literature search and drafted the manuscript. YHL and AW contributed equally to this work. BW, GHZ, RZ, CGJ, YY, KW, XZ and SDL analyzed the data,and verified and discussed the studies. JY, TYW and MQQ amended the paper, designed the research, proofread the manuscript, supervised and approved the study.
Funding No funding.
Data availability statement The data that support the findings of this study are available on request from the corresponding author.
Compliance with ethical standards
Conflict of interest No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethics approval and consent to participate This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Beijing Children’s Hospital, Capital Medical University ([2021]-A-123-R). Informed consent to participate in the study have been obtained from their parent or legal guardian in the case of children under 16.
 World Journal of Pediatrics2021年6期
World Journal of Pediatrics2021年6期
- World Journal of Pediatrics的其它文章
- Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China
- Comparison of clinical outcomes between unrelated single umbilical cord blood and “ex-vivo” T-cell depleted haploidentical transplantation in children with hematological malignancies
- Muscle strength and its association with cardiometabolic variables in adolescents: does the expression of muscle strength values matter?
- Consensus statement on the epidemiology, diagnosis, prevention,and management of cow's milk protein allergy in the Middle East:a modified Delphi-based study
- Evaluation of a new frequency-volume chart for children with primary monosymptomatic nocturnal enuresis: a prospective, comparative study
- Vestibular function of pediatric patients with sudden sensorineural hearing loss: based on vertigo symptom and vestibular function testing
