Comparison of clinical outcomes between unrelated single umbilical cord blood and “ex-vivo” T-cell depleted haploidentical transplantation in children with hematological malignancies
armen Gómez-Santos · Marta González-Vicent · Blanca Molina · Natalia Deltoro · Blanca Herrero · Julia Ruiz ·Antonio Pérez-Martínez ,2 · Miguel A. Diaz
Abstract
Keywords Children; Cord blood transplant · Hematologic malignancies · Immune reconstitution · TCD haploidentical transplant
Introduction
Allogeneic hematopoietic stem cell transplantation is still a necessary and effective therapy for pediatric patients with high-risk hematological malignancies otherwise “non-curable” with current standard treatment protocols. Although a human leukocyte antigen (HLA)-identical sibling donor or a HLA well-matched unrelated donor have been considered as the first option for allogeneic transplantation, such donors are not always available for all patients. Even having one,patients may not have time to wait for donor availability[ 1- 5].
Over the last two decades, umbilical cord blood (UCB)and haploidentical transplantation (HaploHSCT) have emerged as alternative sources of hematopoietic stem cell for allogeneic transplantation. UCB transplant has advantages, such as rapid availability, low risk of infection transmission, absence of donor risk and relatively low risk of severe acute and chronic graft-versus-host disease (GvHD)despite HLA mismatching while maintaining the graft-versus-leukemia (GvL) effect. UCB transplant disadvantages are the low hematopoietic stem cell dose that resulted on graft failures and delayed hematopoietic reconstitution with important clinical repercussion [ 2, 6, 7]. More recently, the use of mismatched/haploidentical-related donors has been considered a suitable approach [ 8- 11]. Nowadays, several HaploHSCT platforms have gained clinical relevance either using “ex-vivo” T-cell depletion with no need of post-transplant pharmacological immunosuppression or T-cell replete HaploHSCT that require the use of post-transplant immunesuppression for GvHD prophylaxis [ 12, 13]. As such, HaploHSCT is the allogeneic transplant modality whose use has grown most in recent years, not only in adults but also in children [ 14].
There are no prospective studies comparing both types of alternative transplantation in pediatric patients. A recently published study comparing outcomes after umbilical cord blood and non-manipulated haploidentical transplantation in children with high-risk acute lymphoblastic leukemia (ALL)suggests that both transplant modalities are valid for highrisk ALL children lacking a HLA matched donor and that both strategies expand the donor pool for children in need of transplantation [ 15].
Herein, we present a retrospective study aimed to analyze and compare transplant outcomes in pediatric patients with hematological malignancies receiving a single UCB transplantation or an “ex-vivo” T-cell depleted (TCD)HaploHSCT.
Methods
Eligibility criteria and patient characteristics
All consecutive pediatric patients who received a UCB transplantation (n= 42) or a TCD transplant from a haploidentical donor (n= 92) at our institution from January 1996 to December 2014 were included in this retrospective study.They were transplanted because of high-risk hematological malignancies and because they lacked a suitable HLAmatched donor. Patients, parents, and/or their legal guardians gave written informed consent in accordance with the Helsinki Declaration.
Patients’ characteristics are provided in Table 1. UCB transplant recipients were younger and smaller than those receiving HaploHSCT. There were more males and more patients with high-risk factors at diagnosis in the haploidentical group. Nevertheless, there were no differences in the proportions of the underlying disease, disease status,antecedent of previous transplants or Lansky scale in each group. The median follow-up was 18 months (range 3-46)for the HaploHSCT group and was 61 months (range 8-85)for the UCB transplant group.
Conditioning regimen, graft-versus-host disease prophylaxis and stem cell infusion
All conditioning regimens in the UCB transplants were myeloablative. Before 2002, conditioning consisted of oral busulfan (16 mg/kg) from day -7 to day -4 or total body irradiation (6 fractions of 2 Gy each) and intravenous cyclophosphamide (60 mg/kg/day) on days -3 and -2.Since 2002, conditioning changed to the Spanish Group of Hematopoietic Transplant’s protocol, which is based on fludarabine, busulfan and cyclophosphamide [ 16]. After 2005, cyclophosphamide was substituted by thiotepa and busulfan was changed to intravenous administration [ 18].Meanwhile, for haploidentical transplants conditioning regimens consisted of intravenous fludarabine at 30 mg/m 2 /day for 5 days (day -6 to day -2), intravenous busulfan administered once daily according to patient body weight for 3 days (day -5 to day -3), only during the first year of the haploidentical transplant program received 2 days of busulfan, intravenous thiotepa at 5 mg/kg/day for 2 days(day -3 to day -2) and intravenous methylprednisolone at 5 mg/kg/day (day -6 to day -2). Busulfan weight-based dosing was as follows: < 9 kg: 1 mg/kg/dose; 9-16 kg:1.2 mg/kg/dose; 16-23 kg: 1.1 mg/kg/dose; 23-34 kg:0.95 mg/kg/dose and > 34 kg: 0.8 mg/kg/dose [ 17].
Cyclosporine A (CsA), thymoglobulin and corticosteroids were used as pharmacological GvHD prophylaxis in the UCB transplant group. Cyclosporine A was administered intravenously at a dose of 3 mg/kg/d starting on day -1, changing to the oral route when the patient was discharged. The thymoglobulin dose was 2 mg/kg/d for 3 days, starting on day -3. Corticosteroids (6-methyl prednisolone) were administered at a dose of 1 mg/kg/d starting on day + 7 until day + 30. Patients underwent haploidentical transplantation received a T-cell depleted graft and CsA as GvHD prophylaxis [ 17].
HLA-A, -B and -C antigens were identified by low-resolution DNA typing, and HLA-DRB1 was defined by highresolution typing for UCB transplant group. The maximum allowable mismatch between the cord blood unit and the recipient was two of six HLA loci. All patients with HaploHSCT received stem cells from a family member who shared one HLA haplotype with the patient. Both parents and all siblings were studied [ 17]. UCB cells were thawed and washed before rapid infusion.
Donor peripheral blood progenitor cells for HaploHSCT were mobilized and collected as previously described [ 18,19]. The manipulation procedure was performed using the automated large clinical scale CliniMACS device (MiltenyiBiotec, BergischGladbach, Germany), and the grafts were analyzed by fluorescence-activated cell sorting(FACS) on the FACS CANTO II flow cytometer (Becton
Dickinson Bioscience, Franklin Lakes, NJ, USA). All grafts were freshly infused [ 17].
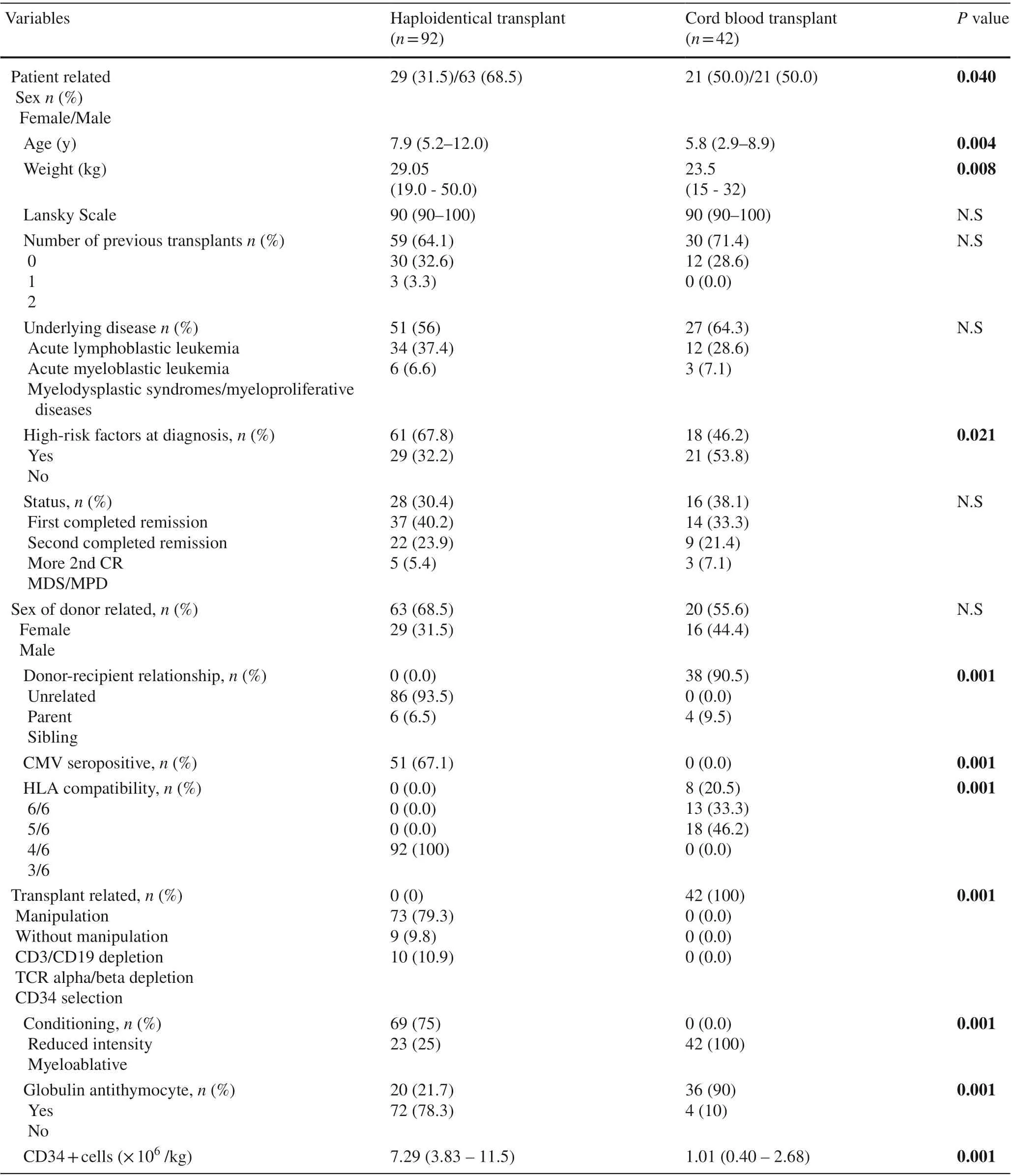
Table 1 Patient, donor and transplant characteristics
Supportive care
All the patients received antiviral, antifungal and anti-Pneumocystisprophylaxis. If febrile neutropenia appeared, it was treated empirically with antibiotics and/or antifungal drugs as appropriate. In patients with GvHD, fluconazole was substituted by posaconazole as antifungal prophylaxis.
Polymerase chain reaction screening was performed weekly for cytomegalovirus and Epstein-Barr virus in the blood and for adenovirus in the stool. If it was positive, ganciclovir, rituximab or cidofovir was begun, respectively. Ganciclovir was used at 5 mg/kg/12 h for at least 14 days; cidofovir at 5 mg/kg/weekly for 2 weeks, and then once every 2 weeks; and rituximab at 375 mg/m 2 /weekly for 4 weeks. In the UCB transplant setting, granulocyte colony stimulating factor (G-CSF) was used at 10 g/kg/day starting on day + 7 until neutrophil engraftment.
Study design, endpoints, and definitions
This study was designed to retrospectively comparing transplant outcomes between two transplant cohorts. Primary endpoints were disease-free survival (DFS), relapse incidence (RI) and transplant-related mortality (TRM). Secondary endpoints were engraftment kinetics, immune reconstitution and morbidity, such as infections or GvHD.
Neutrophil recovery was defined as the first day of 3 consecutive days of absolute neutrophil count above 500/μL.Platelet recovery was defined as the first day of 3 consecutive days of an absolute platelet count of 20,000/μL without transfusions. Primary graft failure was considered when a patient did not achieve neutrophil recovery by day + 30 after transplantation in the absence of bone marrow disease.Secondary graft failure was considered to occur when, after initial hematopoietic recovery, at least two hematopoietic cell lines were lost. Donor chimerism was analyzed by STR-PCR method at time of engraftment and at 3, 6,12 months and annually after transplant. Supportive care,including antibiotics, transfusions, parenteral nutrition and hospitalization days, was analyzed. Immune reconstitution was also analyzed using multi-parametric flow cytometry with an EPIC Elitec (Beckman Coulter) cytometer until 2007 and using a FACS CANTO II (Becton Dickinson)cytometer since then. Immune reconstitution was analyzed at day + 15, + 30, + 60, + 180 and + 270 post-infusion and 1,2, 3 and 5 years after transplantation.
GvHD was diagnosed and graded according to classic definitions: acute if it appeared before day + 100 post-infusion or chronic if it appeared after day + 100 post-infusion[ 20- 22]. DFS was defined as time from transplantation to either relapse or death of any cause. Relapse was defined as morphological or clinical evidence of recurrence in peripheral blood, bone marrow or extramedullary sites. TRM was defined as any cause of death other than relapse.
Statistical analysis
Data were summarized by standard descriptive statistical methods. Median values were used for continuous variables and percentages for categorical variables. Characteristics among groups were compared using the Chi-squared test or Fisher’s exact test for categorical variables, whereas the distribution of continuous variables was compared using the Mann-Whitney test. The Kaplan-Meier method was used to estimate the probabilities of survival, and the log-rank test was used for univariate comparisons. A Cox proportional hazard regression model was used to estimate the adjusted survival curves. Factors that were clinically meaningful or with a univariateP< 0.05 were considered in the multivariate analysis. Prognostic factor models for all clinical outcomes were built by the backward selection method(P< 0.05 was considered significant for remaining in the model). All the statistical analyses were implemented using SPSS (SPSS Inc, Chicago, IL, USA).
Results
Hematological recovery, toxicity and supportive care
The median times to neutrophil and platelet recovery were 13 days (range: 11-15) and 10 days (range: 9-13)after HaploHSCT and 16 days (range: 14-20) and 57 days(range: 30-79) after UCB transplant (P< 0.001).
Graft failure occurred in 25 out of 92 patients (27%) in the HaploHSCT group: 13 with primary failure and 12 with secondary failure. Five patients had autologous reconstitution, 4 had disease recurrence, and 16 had no recovery and needed another transplant. Five patients in the HaploHSCT group died due to graft failure. In the UCB group, 10 out of 42 (24%) developed graft failure: seven with primary failure and three with secondary failure. One had autologous reconstitution, two had disease recurrence and seven had no hematologic recovery. Two patients in the UCB group died due to graft failure. The differences between both groups did not reach statistical significance.
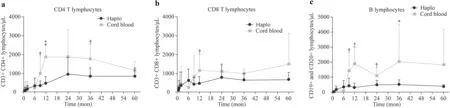
Fig. 1 Comparison of immune reconstitution kinetics. a CD4 + T-cells subpopulation; b CD8 + T-cells subpopulation; c B-cell subpopulation.Haplo: haploidentical transplant group, UCB umbilical cord blood
One patient had respiratory distress, and 4 out of 92 patients (4.3%) suffered hemodynamic instability during infusion in the HaploHSCT group, whereas in the UCB group, 1 patient suffered respiratory distress and 10 out of 42 patients (24%) had hemodynamic instability during stem cell infusion despite antihistamine pre-treatment support (P< 0.001). In all, 55 of 92 patients (60%) in the HaploHSCT had conditioning-related complications whereas in UCB group 34 of 42 patients (81%), had this type of complications (P< 0.001). The median length of hospitalization was 25 days in the HaploHSCT group(range 22-43) and was 46 days (range 31-56) in the UCB group (P< 0.001). Patients in HaploHSCT received a median of 2 red blood transfusions (range 1-4) and 3 platelet transfusions (range 1-8), whereas those UCB transplant patients received a median of 6 red blood transfusions (range 4-15) and 15 platelet transfusions (range 11-27) (P< 0.001). The median antibiotic day was 13(range 10-22) in the HaploHSCT group and was 30 days(range 21-41) in the UCB group (P< 0.001). In the HaploHSCT group, 51 of 92 patients (55%) needed parenteral nutrition, compared with 34 of 42 patients (81%) in the UCB group (P< 0.001).
Immune reconstitution and infections
Immune reconstitution was characterized in all patients by a rapid increase in NK cells in the early phase after transplant. There was a significant rise in the natural killer (NK)cell count on day + 15 in the HaploHSCT group (median:142/μL; range: 23 - 231.5/μL) compared with the UCB group (median: 47/μL; range; 10-80/μL) (P= 0.04). This difference was more evident on day + 30 (median: 328/μL;range: 182 - 540) in the HaploHSCT group vs. the UCB group (median: 180/μL; range; 97 - 293 /μL) (P= 0.03)with no significant differences thereafter. HaploHSCT recipients had significantly higher median numbers of CD3 + T-cells by post-transplant 6 months (median: 620/μL; range: 392-1426) compared with the UCB group(median: 225/μL; range: 143 - 621/μL) (P= 0.04) and higher numbers of CD8 + T-cells (median: 330/μL; range:129 - 969/μL vs. (median; 55/μL, range; 30 - 211), respectively (P= 0.02).
However, 1-year post-transplant, both CD8 + T-cells and CD4 + T-cells became significantly higher in the UCB group. This superiority remained significant 2 and 3 posttransplant years. The number of B cells (CD19 +) showed similar behavior and was significantly higher in the UCB recipients after first year post-transplantation. Detailed data of lymphoid subpopulations recovery and kinetics are shown in Fig. 1.
Sixty-two of 92 patients (67%) in the HaploHSCT group and 41 of 42 (97.6%) in the UCB group suffered any kind of infection during transplant admission (P< 0.001)(HR: 1.42; 95% CI: 1.22-1.64).PolyomavirusandPneumocystis jirovecciwere found to occur significantly more frequently in the UCB group before day + 100 post-transplant. In the HaploHSCT group, 16 of 92 patients (17%)hadPolyomavirusinfection and there was noPneumocystisinfection, whereas in the UCB group there were 16 of 42 patients (38%) withPolyomavirusinfection and three (7%) withPneumocystisinfection (P< 0.05). There were no differences in other kind of infections (data not shown).
Graft versus host disease and transplant-related mortality
Thirty-eight patients (41%) transplanted from a haploidentical-related donor developed acute GvHD: 8 grade I, 10 grade II, 13 grade III and 7 grade IV. Twenty-seven patients(64%) with UCB transplantation developed acute GvHD:four grade I, seven grade II, six grade III and ten grade IV.These differences were statistically significant (P= 0.016).If only those patients who suffered GvHD greater than grade 1 are taken into account, the number of patients suffering GvHD in the UCB group continues to be greater than in HaploHSCT setting (54% vs. 32%,P= 0.02). With respect to the affected organ, there were significantly more patients with cutaneous and gastrointestinal GvHD in the UCB group. Hepatic involvement was not significantly different. The median time to the diagnosis of GvHD in the HaploHSCT group was 29 days (range 20-63), whereas it was 19 days (range 11-39) in the UCB group (P= 0.025).Among patients who survived longer than 100 days, the incidence of chronic GvHD was higher in the UCB group:22 patients (37%) in the HaploHSCT group and 17 patients(65%) in the UCB group (P= 0.027). There were no statistically significant differences between groups in the extension of chronic GvHD.
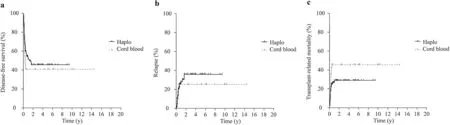
Fig. 2 Transplant outcomes curves. a Disease-free survival: Probability of DFS was 58±8% in HaploHSCT versus 40±9% in UCB group( P = 0.051). b Relapse incidence: the cumulative incidence of relapse was 21±7% in the HaploHSCT group versus 19±8%) in the UCB group ( P > 0.05); c Transplant-related mortality: the cumulative incidence of TRM was 29±8% in the HaploHSCT group versus 40±5%in UCB group ( P > 0.05), Haplo haploidentical transplant group,Cord blood UCB umbilical cord blood
The cumulative incidence of TRM was 29±8% in the HaploHSCT group versus 40±5% in the UCB group (Fig. 2).The median time until death was 217 days (range; 71-636)in the HaploHSCT group and was 146 days (range: 92-1556)in the UCB group. Infection and GvHD represented 28.6%of transplant-related causes of death in the UCB group,whereas it was 13.0% in the HaploHSCT group. These differences were not statistically significant. Eventually, 41 and 23 patients died in the HaploHSCT and UCB transplant groups, respectively. The leading cause of death was relapse of disease in both (21% and 19%, respectively) followed by infection (9% and 14%, respectively). These differences were not statistically significant.
Relapse incidence and disease-free survival
The cumulative incidence of relapse was not significantly different between the two groups: 20 patients (21±7%)relapsed in the HaploHSCT group and 8 (19±8%) in the UCB group. Probability of DFS was 58±8% in the HaploHSCT versus 40±9% in the UCB group (P= 0.051)(Fig. 2 ). In the multivariate analysis, the only factors that impact on DFS were disease phase at time of transplantation and presence of chronic GvHD. Patients transplanted in more advance phase of disease had an increased risk of death (HR: 3.616; 95% CI: 1.322-4.362;P< 0.001). Conversely, patients developing chronic GvHD had a lower risk of death in both groups (HR: 0.103; 95% CI: 0.025-0.430;P< 0.002).
Discussion
Allogeneic hematopoietic stem cell transplantation from haploidentical donors or from UCB are suitable options for those patients lacking an HLA-identical donor. Both options have advantages and disadvantages. As there are no prospective studies comparing both types of alternative donors for transplantation in pediatric patients, it is not clear which donor would be the better option. Herein, we reported the analysis of risk factors and a detailed analysis of immune reconstitution of pediatric patients with malignant hematological diseases undergoing allogeneic stem cell transplantation using “ex-vivo” T-cell depleted haploidentical family donor or unrelated UCB grafts. The main findings of the present study are commented below.
First, as expected, we observed a delayed neutrophil and platelet engraftment in children undergoing UCB transplant,as previously reported [ 15, 23- 27]. A lower cellularity in the inoculum and the use of thymoglobulin may have contributed to these findings [ 7, 28, 29]. Although higher graft failure incidence was found in UCB transplant than in HaploHSCT [ 4, 28, 30, 31], in our study engraftment probability at the end of follow-up was similar between the two groups.Beyond HLA disparity, there are other potential risk factors for graft failure in T-cell depleted haploidentical transplant. Recently, patient age and the pre-transplant number of CD3 + CD8 + cells have been reported as risk factors in the T-cell depleted haploidentical transplant setting [ 50]. For those patients with high risk of graft failure in haploidentical transplant, the use of serotherapy as part of conditioning regimen might increase the probability of engraftment despite high risk of delayed immune reconstitution [ 50- 52].
The pattern of immune reconstitution was different between both groups. An early onset of immune reconstitution was observed in HaploHSCT. NK cells, lymphocytes and particularly CD8 + T cells showed better values in the first 6 months after transplant. This increase was delayed in the UCB transplant, but after the first year post-transplantation, T and B lymphocyte numbers in the UCB group surpassed that of those transplanted from haploidentical-related donor; and differences remained by two and three years after transplantation. Previous studies on the immune reconstitution of UCB transplants and HaploHSCT showed similar results [ 8, 9, 13, 16, 33- 41]. However, few studies have compared the immune reconstitution of both types of transplant.In adults, better lymphocyte reconstitution was found in the UCB transplant recipients compared with HaploHSCT,although during the first year of follow-up, both alternative sources had delayed immune reconstitution with respect to an identical transplant [ 25]. Mo et al. compared immune reconstitution in children transplanted with UCB to that of unmanipulated HaploHSCT. The absolute numbers of CD4 + and CD8 + T cells were comparable in both sources on day + 30, whereas on day + 90 the absolute number of CD8 + T lymphocytes was higher in the HaploHSCT, similar to that observed in our study [ 15].
In our study, children transplanted with UCB showed more infectious processes during admission for the procedure than those transplanted from HaploHSCT. There were also significantly more patients with infection byPolyomavirusandPneumocystis jiroveciibefore day + 100 in the UCB group. Eventually, there were no differences in the number of infections between both groups at the end of follow-up.Other authors also found an increased infectious risk in the UCB transplant [ 9, 42]. Mo et al. found a higher cumulative incidence ofCytomegalovirusinterstitial pneumonia in UCB transplantation than in the HaploHSCT setting [ 15],but they did not find any differences in infections between the two sources in a later study [ 27]. Ruggeri et al. found that infectious diseases were the second leading cause of death in both transplants [ 43]. Although Raiola et al. observed greaterCytomegalovirusand Epstein-Barr virus reactivation in patients transplanted from haploidentical family donors,there was higher mortality due to an infectious cause in the UCB group [ 25]. In the UCB transplant, the delay in engraftment and immune reconstitution during the first 6 months after infusion appears to play a fundamental role in the risk of infection, with the risk decreasing as the patient achieves an adequate immune system. The early recovery of NK cells, monocytes and CD8 + T lymphocytes could explain the low incidence of infections during the first months of HaploHSCT, whereas the delay in the reconstitution of CD4 + T and B lymphocytes is likely responsible for late infections [ 52].
Second, greater toxicity was observed during the infusion in patients transplanted with UCB. This would be related to the presence of dimethyl sulfoxide, which is necessary for the cryopreservation of cord blood. Other authors have found similar differences [ 32]. Complications derived from conditioning were more frequent among those transplanted with UCB because of the use of myeloablative conditioning. Myeloablative conditioning in the UCB transplant was the norm in the past. Currently, researchers suggest that reduced intensity conditioning would be an alternative in both HaploHSCT and UCB transplant [ 6, 23]. Supportive care measures were more needed in the cord blood group,including red blood cell and platelet transfusions, parenteral nutrition, antibiotics and hospitalization days. Other studies have found similar results [ 18, 26].
Third, acute GvHD rates were unexpectedly increased after UCB transplantation, appearing earlier and more frequently than in the HaploHSCT group. There was also more chronic GvHD in the UCB group. The risk of GvHD in UCB transplantation could be influenced by the degree of cord disparity. Rocha et al. found more acute GvHD in children with hematological malignancies transplanted with UCB with disparities than in those using identical cord blood [ 28].In our sample, only 20.5% of unrelated UCB transplants were identical. Having so many HLA disparities, it could contribute to the presence of a higher percentage of GvHD.On the other hand, the few T lymphocytes found in umbilical cord blood are naïve. Several lines of researches have associated this type of lymphocyte with more GvHD than that triggered by mature lymphocytes. This also could explain the higher incidence of GvHD observed in our study in the UCB group, even more when compared with lymphocytedepleted peripheral blood [ 44- 47]. On the other hand, high rates of GVHD were found in the haplo group. Peripheral blood as source could be the reason why higher GvHD than other series are observed. However, if patients who suffered grade I aGvHD are excluded, the percentage of grade≥2 decreased, being these data consistent with recent report[ 53]. Heterogeneous results have been published, probably because the incidence and degree of GvHD depends not only on the source but also on the conditioning, the inoculum manipulation and the post-transplant prophylaxis [ 4, 15,18, 23, 25- 28, 32, 42, 43]. With adequate prevention, transplantation from haploidentical family donors has an acceptable risk of GvHD, even lower than in UCB transplantation[ 25- 27, 42].
Finally, although there is a trend to a better DFS for patients in the haploidentical group, overall, there are no clear differences in transplant outcomes in our study.However, the multivariate analysis of transplant outcomes showed that patients transplanted in advance phase of disease had worse DFS due to an increased risk of relapse and mortality. Conversely, those patients that developed chronic GvHD did better in terms of DFS due to lower relapse incidence, no matter transplant group. Clinical events is likely to occur during the first 6 months in the UCB group, whereas they would occur progressively during the first 2 years in HaploHSCT. Other studies found no significant differences in overall survival or disease-free survival neither [ 3, 4, 15,23, 25- 28, 30, 32, 42, 43]. Although some authors have found higher mortality related to transplantation between those transplanted with UCB and higher mortality due to relapse among those transplanted from haploidenticalrelated donors [ 3, 4, 15, 18, 26, 28, 30, 32]. In our study, no differences were found regarding the cause of death. Relapse was the first cause of death in both groups, being relapse incidence not significantly different between the two sources.Other studies have published similar findings [ 15, 18, 23,25, 27, 32, 43]. However, recently some retrospective studies have shown a clear advantage in terms of survival of haploidentical transplant in adults [ 54, 55].
The study limitations are mainly those derived from its retrospective nature. It covers a broad time range during which there have been several major changes in this field. The follow-up in HaploHSCT group was significantly shorter than UCB transplant group; thus, late consequences of the transplant might have been lost. Moreover, the sample size is small, especially in the UCB group. Because some of the differences can be attributed to the type of conditioning received, patients who received myeloablative conditioning were studied separately. However, the multivariate analysis did not show any survival advantages of type of conditioning used.
New strategies are being studied to improve engraftment,such as double cord transplant, intrabone infusion of stem cells, co-infusion of mesenchymal stem cells, co-infusion of haploidentical peripheral blood cells and cord blood cells and expansion culture of cord blood progenitor cells ex vivo. With respect to the cord and haploidentical progenitor cell co-transplant, there are already several studies that have exposed an early leukocyte graft with the first transient phase of mixed chimerism and engraftment of the haploidentical stem cells, followed by a second phase in which they are replaced by the umbilical cord blood stem cells.This procedure has the potential benefit of mitigating the consequences of slow hematological recovery in the early stages of UCB transplantation and of a later immunodeficiency phase associated with haploidentical transplantation[ 35, 48, 49].
Few studies have compared alternative mismatched donors in children with acute leukemia. Thus, it is difficult to establish guidelines for choosing the best donor, and the criteria should be based on donor characteristics, type of disease, urgency of transplant and the center’s experience.
In conclusion, TCD haploidentical transplant is associated with advantages in terms of engraftment and early immune reconstitution kinetics. TCD haploidentical transplant was associated with lower incidence of infectious and non-infectious complications, especially in the early phases of the transplant compared with UCB transplant recipients.However, there are no advantages in transplant outcomes compared with UCB transplant.
Acknowledgements Acknowledgments are deserved by all members of the clinical laboratory, clinicians and nurses of Hematology/Oncology department at Hospital Infantil Universitario “Niño Jesús" (Madrid,Spain) involved in patients care as well as the contributions made by the patients themselves and their families.
Author contributions C.G-S & A.P-M. developed the first draft and edited and wrote the first manuscript; BM, ND, BH, JR, AP-M.,MG-V., & MAD. designed the study, analyzed data, and participated in writing the manuscript; All authors participated in data interpretation.M.A.D. revised and edited the final version of manuscript. All authors approved the final version of manuscript.
Funding This study was not funded.
Data availability statement The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Compliance with ethical standards
Ethical approval All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest Authors declare no financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Informed consent Informed consent was obtained from all individual or their legal guardians and tutors included in the study.
 World Journal of Pediatrics2021年6期
World Journal of Pediatrics2021年6期
- World Journal of Pediatrics的其它文章
- Consensus statement on the epidemiology, diagnosis, prevention,and management of cow's milk protein allergy in the Middle East:a modified Delphi-based study
- Challenges and suggestions for precise diagnosis and treatment of Wilson’s disease
- Vestibular function of pediatric patients with sudden sensorineural hearing loss: based on vertigo symptom and vestibular function testing
- Evaluation of a new frequency-volume chart for children with primary monosymptomatic nocturnal enuresis: a prospective, comparative study
- Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China
- Haploidentical hematopoietic stem cell transplantation for pediatric patients with chronic active Epstein-Barr virus infection:a retrospective analysis of a single center
