Viral hepatitis in 2021 : The challenges remaining and how we should tackle them
Rebecca Dunn,Aaron Wetten,Stuart McPherson,Mhairi C Donnelly
Abstract Viral hepatitis results in 1 .4 million deaths annually. The World Health Organization (WHO) set an ambitious target to eliminate viral hepatitis by 2030 , but significant challenges remain. These include inequalities in access to healthcare,reaching at risk populations and providing access to screening and effective treatment. Stigma around viral hepatitis persists and must be addressed. The WHO goal of global elimination by 2030 is a worthy aim, but remains ambitious and the coronavirus 2019 pandemic undoubtedly has set back progress. This review article will focus on hepatitis A to E, highlighting problems that have been resolved in the field over the past decade, those that remain to be resolved and suggest directions for future problem solving and research.
Key Words: Hepatitis A; Hepatitis B; Hepatitis C; Hepatitis D; Hepatitis E; COVID-19
INTRODUCTION
Our understanding of the epidemiology of viral hepatitis and associated treatment strategies has advanced significantly over the past decade. Arguably, the most significant advances have occurred in the treatment of chronic hepatitis C, which is now curable with a short course of all oral antiviral therapy. Despite this, viral hepatitis still kills more than 1 .4 million people a year[1 ]. As such, viral hepatitis has become a global health priority and a number of large-scale public health policies have been implemented. The World Health Organization (WHO) has set out an ambitious global elimination strategy for viral hepatitis, aiming to eliminate viral hepatitis as a public health threat by 2030 [2 ]. Key interventions for viral elimination have been identified and include hepatitis B vaccination, facilitation of safe injection practices and safe blood transfusions, promotion of safe sex, hepatitis B treatment and hepatitis C cure. However, modelling studies suggest that up to 80 % of high-income countries will not meet the WHO target[3 ].
This review article will focus on hepatitis A-E, highlighting problems that have been resolved in the field over the past decade, those that remain to be resolved and suggest directions for future problem solving and research. We will also discuss the impact of the coronavirus 2019 (COVID-19 ) pandemic on viral elimination.
METHODS
A PubMed search was performed using the following terms: “hepatitis A”; “hepatitis B”; “hepatitis C”; “hepatitis D”; “delta agent”; “hepatitis E”; “cirrhosis”; “direct acting antivirals”; “chronic kidney disease”; “chronic liver disease”; “functional cure”;“hepatocellular carcinoma”; “liver transplant”; “reinfection”; “ribavirin”; “viral elimination”; “viral resistance”; “virologic cure”. Only English-language articles were included in this review. Reference lists of selected articles were reviewed for relevant studies. Published abstracts were included.
HEPATITIS A VIRUS
Worldwide, the incidence of hepatitis A virus (HAV) is decreasing[4 ,5 ], but with increasing globalization there are significant shifts in the epidemiology of HAV infection[6 ]. Due to a large number of cases being asymptomatic and an estimated under-reporting of up to 80 % of cases, it is acknowledged that the true incidence is difficult to quantify[7 ]. The incidence rate of HAV infection is strongly correlated with socioeconomic indicators; the incidence decreases with increasing access to clean water and sanitation. HAV infection is commonly reported in countries where conflict leads to the displacement of people, resulting in poor sanitation and overcrowding[8 ].
Advances in the past decade and problems now solved
Recent studies have expanded our understanding of the molecular virology and pathobiology of HAV. It is likely that multiple immune mechanisms contribute to the development of acute liver injury due to HAV infection, including decreased frequency of regulatory T-cells due to Fas-mediated apoptosis[9 ] and a polymorphism in TIM1 [10 ]. Factors now recognized to influence the clinical course of HAV infection include variations in the viral nucleotide sequence within the 5 ’UTR[10 ].
The WHO estimated that HAV infection caused approximately 7134 deaths in 2016 [11 ]. In the United States, case-fatality estimates range from 0 .3 % to 0 .6 % for all age groups, rising to 1 .8 % amongst patients aged > 50 years[12 ]. A safe and effective inactivated vaccine has been in use for almost 30 years[13 ]. It was initially developed for individual prophylaxis, but now is used to control endemics[13 ]. A live attenuated vaccine has been developed and licensed in China and it is used in the Chinese national vaccination program. Use of this vaccine in children has reportedly reduced the incidence of HAV infection by 80 %[14 ]. There are now 34 countries that use or are planning to introduce HAV vaccination into routine immunization of children in specific risk groups[11 ]. Within the United Kingdom, persons who are considered high-risk for HAV infection and should be offered vaccination include those in close contact with someone with HAV infection, travelers who plan to travel to parts of the world where HAV is highly endemic, persons with chronic liver disease, men who have sex with other men (MSM), people who inject drugs (PWIDs) and those who are likely to be exposed to HAV from their employment, for example workers who are exposed to raw sewage such as within the construction industry.
Another advance in the past decade has been in the area of post-exposure prophylaxis (PEP) against HAV. PEP is recommended for persons who are immunocompromised and those who have chronic liver disease[15 ]. Immunoglobulin was previously the only recommended PEP however due to a number of factors including declining anti-HAV IgG titres in donor pools, new strategies were sought. Recent data support post-exposure immunization with an inactivated HAV vaccine as being effective in preventing infection when given within 14 d of exposure[13 ].
Problems remaining to be solved
Prevention of infection in high-risk populations (including targeted vaccination):With increasing numbers of forcibly displaced persons in certain parts of the world[16 ], endemic HAV infection will continue to be an ongoing but preventable issue that requires a global response to provide public health infrastructure, sanitation and free HAV vaccination programmes. This approach requires significant input from public health agencies and politicians alike.
Person to person transmission is described, with infection reported amongst PWIDs and homeless populations. These populations can be difficult to engage, and vaccinating these high-risk individuals needs to be a public health priority (at least in developed countries). MSM have been linked to outbreaks of cases in developed countries, with epidemiological and laboratory investigations linking genotypes between countries[17 ]. It is important that high-risk groups such as MSM are identified and offered vaccination to prevent outbreaks in susceptible communities where there is lack of herd immunity[18 ]. Improving uptake of HAV vaccination in the MSM population is a remaining challenge. Targeting these at risk populations by methods such as social media and dating apps have been shown to improve vaccination uptake[17 ]. Patients with chronic liver disease should also be offered HAV vaccination due to their risk of more severe infection, however doing so has not entered widespread clinical practice[19 ]. In one American study of HAV vaccination in patients with chronic liver disease, 28 % of patients seen in specialist centres underwent vaccination compared with 5 % of patients managed in primary care[20 ]. In another American study of patients with hepatitis C, 7 .9 % of patients underwent HAV vaccination[21 ]. As HAV is a vaccine-preventable disease, universal vaccination of infants would be an effective method for controlling the infection going forwards.
Treatment of severe liver injury due to HAV infection:Although rare, patients with acute HAV infection can progress to acute liver failure (ALF)[7 ]. Whilst these patients can recover with supportive management, a small number of patients may require transplantation. Patients progressing to ALF are typically older and may not be suitable candidates for liver transplantation, and therefore other specific treatment strategies are required. Furthermore, liver transplantation is not accessible to those most at risk in displaced communities. Ribavirin has successfully been used in treatment of acute hepatitis E infection; it has been shown to have an inhibitory effect on HAVin vitrobut has not been assessedin vivofor therapeutic activity[22 ].
HEPATITIS B + D
Hepatitis B virus
Chronic hepatitis B infection is a global problem, but the burden of disease is mostly in low to middle income countries, with 248 million of the estimated 292 million people affected residing in Asia, Africa, the Pacific and Latin America. Chronic hepatitis B virus (HBV) accounts for approximately 47 % of all viral hepatitis related deaths, the vast majority of which are secondary to complications of chronic liver disease[23 ,24 ].
Advances in the past decade and problems now solved
In 2017 the nomenclature to describe the different phases of chronic HBV changed within the updated European Association for the Study of the Liver (EASL) hepatitis B guidelines[25 ]. This was to better reflect and highlight the two main pathological processes of chronic infection and chronic hepatitis, in particular taking into account the presence of hepatitis B e antigen (HBeAg), HBV DNA levels, alanine aminotransferase (ALT) values and the presence or absence of liver inflammation. The new definition of phases highlights the increased risk of advancing liver disease in both chronic hepatitis phases - even in HBeAg negative patients - where there is elevated HBV DNA levels and/or elevated ALT, removing the somewhat misleading term“inactive carrier”. These changes in nomenclature have now been widely adopted[24 ].
Multiple societies now provide guidance on when to initiate treatment. Viral resistance to treatment is a problem which has now been largely overcome. The nucleos(t)ide analogues (NAs) entecavir (ETV), tenofovir disoproxil fumarate (TDF)and tenofovir alafenamide are recommended as first-line treatment in both American and European HBV guidelines[25 ,26 ]. These agents show high rates of viral suppression and high genetic barriers to resistance[27 ,28 ] and have largely replaced lamivudine (LAM) with which resistance was problematic and common. Following treatment with LAM for 1 year, 14 %-32 % of patients developed resistance, increasing to over 80 % after 4 years[27 ]. Those who develop resistance to LAM and are switched to ETV are more likely to subsequently develop resistance to ETV, with resistance rates of up to 50 % after 5 years of treatment compared to only 1 .2 % of patients developing resistance with ETV where LAM has not been previously used[27 ]. TDF monotherapy has been shown to be effective in patients who have previously experienced treatment failure due to LAM resistance[29 ] and although there have been cases reported of reduced efficacy of tenofovir, there have been very few reported cases of resistance.
Problems remaining to be solved
There remain a number of challenges in the diagnosis and management of patients with chronic hepatitis B infection - Figure 1 .
Diagnosing and linking infected patients to care programmes:A significant proportion of infected persons have not been identified; current estimates suggests that only 10 .5 % of infected individuals have been diagnosed and only 5 % of those eligible for treatment for chronic HBV infection are receiving treatment[30 ]. A large systemic review found that 10 % of people (26 million) with HBV infection might need urgent treatment due to cirrhosis and 12 %-25 % of patients would also be eligible for treatment according to different international guidelines[30 ]. Many countries do not have the infrastructure to deliver widespread testing, vaccination or treatment; this is particularly true in low-middle income countries where resources are limited. Detailed discussion on the challenges of such health inequalities are beyond the scope of this review. The approach to up-scaling diagnostic testing needs to vary according to the target population. In the United Kingdom and other developed countries, the majority of individuals with undiagnosed hepatitis B infection are born in countries with intermediate or high prevalence rates. Identifying these individuals may increase diagnosis rates. Case finding in high-risk groups is effective; in North-East England,individuals from the British-Chinese and South Asian communities were invited to education and screening (viadry blood spot testing) sessions in local community centres[31 ]. The prevalence of hepatitis B surface antigen (HBsAg) positivity was 4 .6 %,which is above the 2 % screening threshold recommended by the Centers for Disease Control and Prevention[31 ]. Another study looked at the cost-effectiveness of a onetime opt out case-finding approach in a primary care setting in the United Kingdom migrant population. This approach was deemed very likely to be cost effective amongst migrant populations with HBsAg prevalence ≥ 1 %[32 ].
New point of care (POC) tests are also becoming available, making diagnosing infection easier and quicker. For example, the Determine HBsAg 2 test provides a HBsAg result in 15 min with high sensitivity and specificity[33 ]. POC tests allow testing and diagnosis to move out of established health care settings and may be of particular utility in resource poor settings and high-risk communities.
Increasing testing and subsequent diagnosis rates relies on public engagement to break down stereotypes and address stigma, improved interactions with health care services and addressing health inequalities arising from poverty and language barriers[34 ,35 ]. Collaboration and integration with other successful public health programs such as human immunodeficiency virus (HIV) services is also likely to be effective.

Figure 1 Remaining challenges in hepatitis B virus infection. HBV: Hepatitis B virus; cccDNA: Closed circular DNA; HCC: Hepatocellular carcinoma;pgRNA: Pregenomic RNA.
Defining cure:A ‘cure’ for HBV might be considered as one where the virus is completely eliminated [undetectable HBsAg, HBeAg, HBV DNA and hepatic covalently closed circular DNA (cccDNA)] and where any (risk of) associated liver disease is also removed[36 ]. Consensus on definitions of cure remain contentious and as there is no current or upcoming treatment to achieve the ‘holy grail’ described above, there is reticence in how the word ‘cure’ is used. However, this is a key aspect of clinical care and research, therefore a globally accepted definition of cure needs to be obtained.
The term ‘sterilising cure’ (complete eradication of the virus) has been replaced with’functional cure’. Functional cure is currently defined as sustained HBsAg loss,undetectable HBV DNA, with or without seroconversion to hepatitis B surface antibody, following a finite course of treatment[25 ] and it occurs in 1 % of chronically infected patients annually[37 ]. However, HBV genomes can persist in the liver even if HBsAg is undetectable questioning the true value of achieving a functional cure. A‘partial functional cure’ is considered an intermediate goal of therapy and signifies detectable HBsAg but persistent undetectable HBV DNA 6 mo post-treatment.Virologic cure is essentially ‘halting’ all forms of HBV replication, however difficulties with obtaining virologic cure remain due to the persistence of cccDNA in hepatocytes.To obtain virologic cure, treatments inhibiting both cccDNA and viral replication are required[38 ].
An agreed definition of cure remains elusive, however with clearly defining treatment endpoints and new therapies targeting different aspects of the HBV life cycle, virologic cure may be achievable in the future.
Striving for prevention rather than cure:To prevent HBV infection, there needs to be a focus on improving vaccination strategies. Barriers to HBV vaccination, particularly in resource limited or remote regions, can be attributed to inadequate resources to acquire vaccinations, current dosing regimens, insufficient trained health staff for administration of the vaccine and lack of facilities to keep vaccinations between 2 -8oC.One study of a two-dose regime of HBsAg-1018 (containing HBsAg plus a toll-like receptor 9 agonist adjuvant) demonstrated a higher seroprotection rate at one year compared with the standard three dose regimen[39 ]. Simplified regimens with fewer doses over a shorter time period (HBsAg-1018 given at 0 and 4 wk) are likely to be associated with increased uptake[39 ]. Many countries have now instituted effective COVID-19 vaccination programmes, and similar systems could be used to roll out simplified HBV vaccination regimens.
Preventing mother to child (vertical) transmission of hepatitis B is vital if global elimination is to be achieved[40 ,41 ]. High maternal viral load is the greatest risk factor for mother to child transmission; HBeAg positivity also increases risk[41 ]. In resource poor settings the WHO-recommended vaccine strategy may be difficult to deliver, and diagnostic assays for HBV testing may not be readily available. A potential strategy in these settings is POC testing to establish HBeAg status, followed by empirical treatment with tenofovir in the 3rdtrimester in those who are HBeAg positive to reduce viral load and the risk of perinatal transmission[42 ], however such diagnostic assays are not readily available and remain costly.
Defining ‘stopping rules’ for HBeAg negative patients treated with NAs:Where seroconversion of HBeAg occurs, 67 %-85 % of patients have a sustained inactive state(HBeAg negative chronic infection); this is particularly the case where seroconversion occurs below the age of 30 years and where a low or undetectable HBV DNA level has been maintained[43 ]. However, given significant relapse rates it remains controversial as to whether NA treatment can be stopped after HBeAg loss. A HBeAg negative state is associated with higher rates of regression of fibrosis but some patients will develop HBeAg negative hepatitis, the risk of which increases with time (22 % at 10 years) and increases the risk of progression to advanced liver disease[44 ].
Given the low rate of clearance of HBsAg, HBeAg seroconversion is considered as a potential endpoint of treatment, where undetectable HBV DNA is achieved on three separate occasions in a 6 [25 ] or 12 -mo[26 ] period. If treatment is stopped at this endpoint, 50 % will undergo HBeAg reversion requiring treatment with NAs to restart;close biochemical monitoring is therefore required. There is evidence to suggest that longer treatment with NAs results in a higher chance of persistent remission, with viral remission for 24 -mo on NAs offering the most likely chance of sustained remission[45 ].
Therefore, currently there is no universal stopping rule. In real-world practice,many different factors are taken in to consideration when making the decision to stop treatment with NAs, including the stage of fibrosis and family history of hepatocellular carcinoma (HCC). Further studies are needed to more clearly define the predictors of sustained remission and/or relapse to guide stopping decisions.
Establishing treatment endpoints - aiming for viral suppressionvscure:Currently,long-term suppression of HBV DNA levels is the main endpoint of treatment (+/-HBeAg loss in HBeAg positive patients). It remains a subject of debate as to whether the endpoint of treatment should be viral suppression, functional cure, partial functional cure or virologic cure. The ideal goal however would be virologic cure. In 2019 the joint EASL-American Association for the Study of Liver Diseases HBV treatment endpoints conference agreed that a “functional cure” should be the primary endpoint of phase III trials; sustained HBsAg loss in more than 30 % of patients was accepted as an acceptable rate of response in phase III trials[38 ]. The endpoint for trials may not be the same as the endpoint for real world clinical practice however.
Biomarkers continue to be developed and may prove useful in defining future treatment endpoints. These biomarkers are likely to be used in conjunction with currently utilised clinical markers. The development of hepatitis B core-related antigen(HBcrAg) as a potential serological marker for cccDNA levels may identify patients who could discontinue NA therapy, those at risk of HCC development or of recurrence following treatment[28 ,46 ]. Pregenomic RNA may be a novel marker of viral replication; evidence is emerging that this may provide an earlier predictor for HBeAg seroconversion for those patients on NAs (an important indicator for partial immune response) and may help guide future treatment in those not achieving HBeAg seroconversion[47 ].
Establishing a universally accepted endpoint of treatment along with biomarkers to help predict or confirm the achievement of this endpoint would be an important advance in the treatment of chronic HBV infection.
Risk of HCC and surveillance in patients on long term NAs:Chronic HBV infection is a leading cause of HCC; it is responsible for around 25 % of liver cancer cases in developed countries and up to 60 % of cases in developing countries[48 ]. NA therapy has been reported to decrease incidence of HCC[49 ,50 ]. While HBsAg loss after the development of advanced fibrosis minimizes the risk of the development of HCC, it does not negate it completely[49 ]. A number of factors are taken into consideration when deciding which patient to survey for HCC including disease phase, age,ethnicity and family history of HCC[49 ]; international guidelines do not agree on the populations for surveillance however, promoting inequalities in care.
In those on NA therapy, risk scores such as the REACH-B score[51 ] or PAGE-B score[52 ] are used to identify patients who would benefit from HCC surveillance. The REACH-B scoring system was developed in a cohort of Asian patients with chronic HBV infection who were treatment naïve; no patients with cirrhosis were included in the development of this score[51 ]. This score does not offer good predictability in Caucasian patients with chronic HBV infection[53 ]. The modified REACH-B score substituted HBV DNA levels for the liver stiffness value which increased its accuracy[54 ]. The PAGE-B score was developed for use in Caucasian populations receiving tenofovir or ETV. A modified PAGE-B score (addition of serum albumin) has recently been tested in Asian patients on NA therapy, with an area under the receiver operating characteristic curve of 0 .82 [53 ]. The PAGE-B score is also predictive of HCC development in untreated patients[52 ].
Quantitative HBsAg and HBcrAg have been proposed as new biomarkers for HCC risk which might influence patient selection for HCC surveillance[55 ]. Risk models incorporating these biomarkers would be an advance in the field of HBV. New models could also incorporate other novel markers such as specific HBV mutations, presence of the metabolic syndrome and HBV genotype.
Identifying new treatments with finite duration and high cure rates:Most patients with chronic HBV currently require lifelong therapy, achieving viral suppression rather than cure[25 ,26 ]. To achieve cure, combinations of therapy targeting different aspects of the HBV lifecycle are likely to be required including inhibition of cccDNA and viral replication[38 ].
A number of new treatments are being investigated for HBV and these are aiming to achieve clearance of HBsAg rather than just suppressing HBV DNA[36 ]. A detailed description of these treatments is beyond the scope of this review, but these include the development of new NAs (besifovir and metacavir), cccDNA silencers (e.g.,lymphotoxin beta receptor agonist) and HBV entry inhibitors (Myrcludex B)[28 ,38 ,56 ].There may also be a role for immunomodulatory therapies such as toll-like receptor agonists (actingviaactivating the innate immune response), check point inhibitors(helping to restore T-cell dysfunction) or therapeutic vaccines such as TherVacB[56 ,57 ]. Gene editing strategies and RNA interference may be other potential treatment strategies[56 ]. Where eligible, patients should be considered for entry into clinical trials of novel therapies.
Hepatitis D virus
The current burden of hepatitis D virus (HDV) infection is unknown; estimates from a recent meta-analysis vary considerably, ranging from 12 million to 72 million individuals infected with HDV worldwide[58 ]. There is geographical variation in the prevalence of HDV infection. A recent systematic review and meta-analysis estimated anti-HDV prevalence to be 4 .5 % amongst HBsAg positive individuals globally with rates lower in Europe (3 .0 %) compared with Africa (5 .97 %)[58 -60 ]. However, other meta-analysis estimates differ, demonstrating higher seroprevalence amongst HBsAg positive individuals worldwide (10 .58 %-13 .02 %) and within Europe (13 .81 %). Such differences are likely due to variation in modelling strategies and highlight the difficulties in truly identifying the burden of HDV[58 -60 ]. Issues and challenges remaining in the field of HDV infection include identification of infected individuals,effective treatments, treatment endpoints and prevention.
Problems remaining to be solved
Identification of infected patients:A positive HDV antibody should be accompanied by detectable serum HDV RNA to detect active infection. However, some guidelines do not explicitly make recommendations for HDV testing and therefore many patients who are HBsAg positive are not tested for HDV. One study looking at clinic-led anti-HDV testing identified that only 40 % of HBV patients were tested[61 ]. The same study looked at a different centre offering reflex laboratory testing and found that 99 .4 % of first HBsAg positive samples were tested for anti-HDV. This is a potentially reliable approach to increasing detection of patients with HDV infection, as all patients who are newly diagnosed with HBsAg positivity should be tested for serological evidence of HDV infection.
There is an epidemiological association between anti-HDV seroprevalence and PWIDs, commercial sex workers, MSM and recipients of haemodialysis[58 ,62 ].Suggested patient groups who should be prioritised for screening for HDV include:Patients who are HBsAg positive, patients with HIV, PWIDs, MSM and migrants from highly endemic regions.
Treatment for HDV infection:Pegylated-interferon(PEG-IFN) is the only treatment proven to have antiviral efficacy against chronic HDV infection, however viral suppression rates with PEG-IFN are poor in HDV infection and the adverse effects of PEGIFN therapy are well described[62 ,63 ]. Extended duration of treatment has not been associated with a consistent or significant increase in efficacy, and the addition of NAs does not improve efficacy. New treatments are urgently required; therapies currently being evaluated include HBV/HDV entry inhibitors (Myrcludex B), virion secretion inhibitors (REP 2139 ) and inhibitors of the prenylation of the large HDV antigen(lonafarnib)[63 ]. Patients with HBV/HDV co-infection should be considered for entry into clinical trials. Ultimately, global prevention of HBV infection would be the most effective means of treating HDV infection.
Establishing treatment endpoints:Unfortunately, endpoints for HDV treatment and indicators of response to treatment have not been well established[38 ]. Cure may not be feasible. ALT normalization, changes in HDV RNA and qHBsAg are markers of response to treatment. Barriers to establishing treatment endpoints include lack of widespread availability of HDV diagnostics and lack of standardization of HDV RNA assays. Composite endpoints are likely to be more useful than singular end-points.
HEPATITIS C VIRUS
Perhaps the greatest advances in our understanding of virology and development of treatment strategies over the past decade have occurred in relation to hepatitis C virus(HCV) infection. Despite these advances a number of challenges remain, including targeting difficult to reach populations and expanding HCV testing and treatment programmes in resource poor countries. Addressing these areas will be critical if global elimination of HCV is to be achieved by 2030 .
Advances in past decade and problems now solved
Treatment and cure:HCV treatment has evolved rapidly in the last 10 years, with the emergence of direct acting antiviral (DAA) regimens. These drugs are very well tolerated and highly effective in achieving sustained virologic response (SVR), even in patients who were previously considered ‘hard to treat’ or in whom interferon-based treatment was contraindicated. As a result, antiviral treatment with DAAs is recommended in all patients with active HCV infection[64 ] and elimination of HCV is an achievable goal if these drugs can be made widely available worldwide.
In 2011 the first protease-inhibitors (telaprevir and boceprevir) were approved for use in HCV infected individuals in combination with pegylated-interferon and ribavirin, but whilst SVR rates improved so did the frequency of side effects[65 ]. This was quickly followed by the approval of the first interferon-free regimens for the treatment of genotype 1 HCV infection in 2014 , followed by the first pangenotypic regimen, sofosbuvir-velpatasvir, in 2016 [66 ]. Pangenotypic regimens are advantageous because they remove the need for genotype testing prior to the commencement of treatment which simplifies treatment regimens, thus reducing the frequency of patients dropping out before they start antiviral treatment.
Presently, the availability of safe and highly effective DAA regimens supports a strategy of treating all individuals with chronic HCV infection over the age of 12 ,irrespective of the stage of disease[67 ]. Current regimens offer a number of advantages over previous interferon-containing regimens including much greater efficacy, few side-effects, oral once daily dosing and shorter duration of treatment. For current DAA regimes, SVR rates (undetectable HCV RNA at 12 or 24 wk after treatment) well exceed 90 % for most patient cohorts, compared with approximately 50 % of patients treated with PEG-interferon and ribavirin. Patients with chronic kidney disease(including dialysis-dependent patients) and cirrhosis were previously considered difficult to treat but now have similar SVRs when treated with DAAs to those without chronic kidney disease and cirrhosis[68 ,69 ].
Significant improvements in SVR rates with DAAs has translated into a reduction in morbidity and mortality rates in patients with HCV. A systemic review and metaanalysis concluded that there was an 87 % reduction in the incidence of HCC and a 75 % reduction in all-cause mortality in those who achieved SVR when compared with those who did not[70 ]. By 2019 in the United Kingdom, the incidence of HCV-related end stage liver disease and HCC had fallen by 24 % following the introduction of DAAs and the associated increase in the number of patients completing treatment. In Scotland, new presentations of HCV-related decompensated cirrhosis decreased by 51 % in the DAA area with an estimated avoidance of 330 cases of decompensated cirrhosis[73 ].
Problems remaining to be solved
Prevention:The ideal preventative treatment for HCV would be a vaccine. However,development of an HCV vaccine has been challenging due to the genetic diversity of the virus, the virus’ ability to avoid the host immune response and a lack ofin vitroandin vivomodels of infection[71 ]. Some progress has been made, and a recent trial of a vaccine regimen to prevent chronic HCV infection was safe and induced HCVspecific T-cell responses but it did not prevent chronic HCV infection in a cohort of patients with a recent history of intravenous drug use[72 ]. It is therefore unlikely that an available efficacious vaccine will be available in the short-term. Work to develop a vaccine is ongoing.
In the absence of a vaccine, improving harm reduction approaches for PWIDs is vital. Existing strategies include promotion of sterile injection equipment use through needle exchange programmes and opioid substitution therapy. These services are often poorly provided and under-utilized, but they have been shown to be highly costeffective[73 ]. It is been estimated that eliminating non-sterile injection techniques could prevent 43 % of incident HCV infections between 2018 and 2030 [74 ].
Difficult to reach populations:Despite advances in the medical treatment of hepatitis C, global elimination is unlikely to be achieved unless all infected patients are identified and then complete their treatment regimen. A significant proportion of people with HCV infection are unaware of their diagnosis, and our ability to find these patients is becoming increasingly challenging. Previous work has shown that HCV testing is concentrated in areas with lower risk of infection[75 ], commonly settings where patients are either in recovery from previous drug use or ongoing drug use is more ‘controlled’. Testing needs to be expanded among ‘difficult to reach’ populations, especially those who may be in a more ‘chaotic’ phase of their drug use and are not in contact with addiction or other medical services. This group can be challenging to find and engage, but approaches such as testing and treatment in homeless hostels and food kitchens can be effective[76 ]. Moreover, testing delivered by peers is an approach that can increase diagnosis and subsequent treatment in patients considered hard to reach. In the United Kingdom, the hepatitis C trust run a peer-to-peer education programme, in which peer educators with personal experience of HCV deliver workshops sharing the importance of testing and treatment[77 ]. This has increased testing numbers and reduced attrition, whilst providing valuable education.
One important area to target to increase testing and treatment is in the prison population. Prison populations have a high prevalence of HCV infection with many studies reporting an incidence > 10 times that of the general population[78 ]. Drug use prior to or during imprisonment is common, yet harm reduction methods such as access to clean injecting equipment is non-existent or inadequate in the majority of prisons. Opt-out screening for blood borne viruses (BBVs) is recommended in the EASL HCV guidelines for all prison inmates[79 ], but even where this is practiced rates of testing are suboptimal[78 ]. Opt-in testing is more commonly practiced but is a far less effective approach. BBV testing can be challenging, particularly in reception prisons (prisoners awaiting sentencing) because these typically have a very large throughput of inmates and periods of incarceration can be short. However, these challenges can be effectively overcome with investment and an organized approach to testing. Effective approaches to increasing testing for HCV and scaling up of treatment with DAAs can also be used as ‘treatment as prevention’. This approach was practiced in an Australian prison population and led to a significant reduction in incidence of new HCV infections[80 ].
Another approach that could be considered to identify undiagnosed patients with HCV is a ‘track and trace’ approach by mapping the social networks of individuals with a history of injecting drug use and offering HCV testing to those in a group who may not have been tested. Whilst this may sound like a practical solution, one study showed that this was ineffective in real world clinical practice with only one participant coming forward for testing[81 ]. Further work is needed to determine whether this approach could be refined to increase its efficacy, particularly since people are now more aware of ‘track and trace’ programmes as a result of the COVID-19 pandemic.
Attrition:Increasing detection rates will only help in the strive for global elimination if these are translated into increased treatment rates. An analysis of two large national laboratory databases from 2013 to 2016 found that 89 .4 % of patients diagnosed with chronic HCV infection did not receive a prescription for antiviral therapy[82 ]. In Spain,49 .8 % of those with a positive anti-HCV result were not then linked into specialist care[75 ]. One reason for this is that care pathways have been unnecessarily complex including multiple investigations prior to treatment, which leads to patients frequently being lost to follow up and never completing treatment. Attrition appears to occur early in the treatment cascade; in one study 57 .3 % of patients dropped off prior to having liver enzymes checked[83 ]. With the advent of pangenotypic regimens and simple non-invasive fibrosis scores [e.g.,fibrosis-4 (FIB-4 ) and aminotransferaseplatelet ratio index] pathways can effectively be simplified, which is likely to reduce attrition. An ideal pathway is shown in Figure 2 .
Moving care delivery out of hospital settings may improve attrition rates. One study from the North-East of England found that distance from a HCV treatment service was a major predictor of patients not commencing antivirals[84 ]. DAAs can be effectively delivered in non-hospital settings, increasing access to treatment. A cluster-randomized trial showed that pharmacist delivered treatment in patients on opiate substitution therapy was more effective than conventionally delivered HCV therapy with more patients initiating and completing treatment, and achieving SVR[85 ]. Other examples of successful non-traditional HCV services have been delivered in primary care, nurse led community clinics, addiction services and homeless hostels[86 ].Empowering addiction workers and those working with the homeless to become involved in the care cascade is also likely to improve attrition rates.
Reinfections:Re-infection with HCV after SVR, detected by the presence of HCV RNA rather than HCV antibodies, is largely related to an individuals’ ongoing high risk behavior, inadequate harm reduction knowledge and/or lack of availability of clean injecting equipment. The true rate of reinfection is not known and is likely to vary significantly depending on the population studied. Individuals who continue to actively inject drugs after treatment have the highest rates. Very high rates of reinfection (up to 40 %) have been seen in some high-risk groups, but other studies have reported lower rates[87 ]. Better access to harm reduction methods is vital to reduce reinfection rates. In addition, PWIDs should be tested at least annually for HCV RNA if they have ongoing high-risk behavior to identify reinfections. It is critical that they are offered re-treatment to try and reduce the risk of onward transmission of the infection[79 ].
Long term impact of hepatitis C infection:HCV infection is associated with multiple extrahepatic complications including increased risk of autoimmune disorders,cryoglobulinaemia and lymphoma. In addition, there is increased risk of type II diabetes, cardiovascular disease, chronic fatigue and psychological morbidity. Many of these comorbidities persist following SVR and one study found that nearly all patients have at least one co-morbidity that remains long-term[88 ].
Despite individuals with HCV having a significantly increased risk of cardiovascular disease, few are actively treated to reduce their cardiovascular risk[89 ].Moreover, even though quality of life improves following successful antiviral treatment, this remains significantly worse than the general population[89 ]. This probably relates to high rates of mental health disorders, unhealthy alcohol consumption,ongoing drug use, deprivation, type II diabetes and the metabolic syndrome.Participation in physical activity in individuals with HCV is associated with improved quality of life[90 ]. Taking a more holistic approach to the care of individuals with HCV rather than just focusing on treating the infection may help improve long-term outcomes and improve quality of life. Use of a holistic care bundle may help achieve this[89 ].
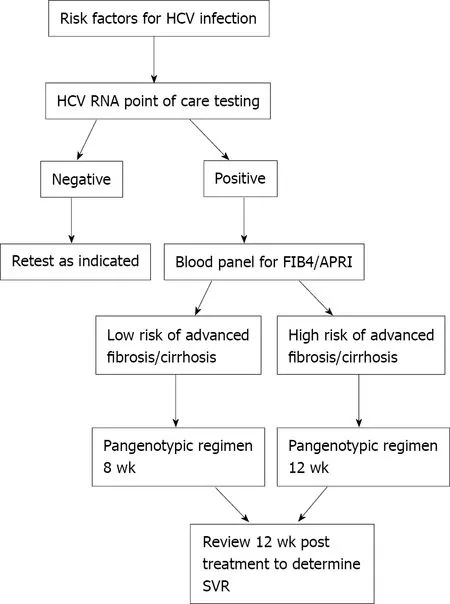
Figure 2 Proposed simplified pathway for hepatitis C virus diagnosis, staging and treatment. HCV: Hepatitis C virus; FIB-4 : Fibrosis-4 ; APRI:Aminotransferase-platelet ratio index; SVR: Sustained virologic response.
HCC surveillance in non-cirrhotic patients:The risk of development of HCC in individuals with HCV-related cirrhosis falls following SVR, but remains approximately 2 % per year and as a result, surveillance is recommended for these individuals[91 ]. HCC may also occur in patients with advanced fibrosis, but at a lower rate than in those with cirrhosis and it remains uncertain whether HCC surveillance is clinically effective and cost effective in this group. This is further complicated by the fact that many patients are staged using transient elastography and relevant cut-offs to identify those who are likely to benefit from HCC surveillance have not been defined. There is a clear cut need to develop better models to predict the development of HCC in individuals following SVR. International societies have different recommendations regarding HCC surveillance in those achieving SVR which reflects the overall uncertainty - Table 1 .
There have been some recent studies that have attempted to more clearly define patients who would benefit from HCC surveillance post SVR[90 ,91 ]. One study developed a model to predict patients with advanced fibrosis who have a low risk of HCC and may therefore not benefit from surveillance. The model used a combination of baseline and dynamic changes in liver stiffness measurement, FIB-4 score and serum albumin after SVR and identified that nearly 20 % of their cohort of patients with compensated advanced fibrosis had a very low risk of developing HCC[90 ].Dynamic assessment of the FIB-4 score in isolation may also predict the risk of development of HCC after SVR[98 ]. In one study, no patients with a FIB-4 < 1 .45 after SVR developed HCC[92 ]. A number of studies are underway with the aim of developing better predictive models for HCC using clinical parameters and novel biomarkers.
Increased use of hepatitis C positive donor organs:The advent of safe and highly effective DAAs for HCV infection has increased the potential to use HCV-positive organs even when the donor is viraemic, expanding the donor pool[93 ]. HCV positive(HCV RNA +) donor organs universally transmit HCV to the recipient[94 ] so prior to the widespread availability of DAAs use of these organs was restricted to those who already had HCV viraemia. However, given the efficacy of DAAs it is now possible to transplant HCV RNA + organs in to HCV negative recipients and then treat the HCV infection in the recipient.
In 2019 , Kwong et al[95 ] assessed the outcomes from HCV treatment with DAAs in 10 non-viraemic patients who received HCV RNA + livers. Short-term outcomes wereexcellent with 100 % achieving SVR at 12 wk post treatment. The practice of using HCV RNA + organs with subsequent DAA treatment is now routine in some countries around the world.

Table 1 Recommendations for hepatocellular carcinoma surveillance in patients with hepatitis C virus achieving sustained virologic response
HEPATITIS E VIRUS
Hepatitis E virus (HEV) is the most common cause of acute hepatitis worldwide and carries a significant global burden of disease. HEV genotypes 1 and 2 account for approximately 20 .1 million HEV infections, 3 .4 million symptomatic cases, 70000 deaths, and 3000 stillbirths annually[96 ]. Our understanding of the impact of hepatitis E infection has advanced significantly over the past decade, with the recognition of chronic infection, risk of progression to cirrhosis, risk factors for transmission and treatment strategies. Despite these advances, there are problems that remain to be resolved.
Advances in past decades and problems now solved
There are now eight recognized genotypes of HEV. Genotypes 1 -4 and 7 cause human infection. Genotypes 1 and 2 are obligate human pathogens transmitted by the faecooral route and cause both sporadic infection and large outbreaks. In the developed world, sporadic infections are mainly caused by genotype 3 infection.
Transmission of HEV infection
Ingestion of raw or under-cooked meat (particularly pork products), shellfish and contaminated fruits is a significant risk factor for locally-acquired infection in the Western world. Genotype 3 and 4 HEV infection can be transmittedviatransfusion of infected blood products and solid organ transplantation, and may have a significant clinical impact upon immunosuppressed individuals. A French study looked at 23 cases of reported transfusion related HEV infections in France between 2006 -2016 . It reported that 14 of these cases, all of whom were immunosuppressed, went on to develop chronic HEV infection[97 ]. The United Kingdom introduced a universal screening policy for blood products in 2017 and also screens deceased and live organ donors for HEV RNA[98 ]. Other countries have a more selective strategy and only screen blood products intended for high-risk patients[99 ]. Universal screening has been shown to be more cost effective than selective screening if the incidence of HEV infection is above 1 in 10000 blood donations[100 ]. Sexual transmission in MSM has also been reported more recently[101 ].
Chronic infection and risk of cirrhosis
Prior to 2008 , HEV was recognized to cause an acute, self-limiting illness. Genotype 3 HEV was first reported to cause chronic infection in 2008 and chronic infection has now been reported in immunocompromised individuals including solid organ transplant (SOT) recipients, patients receiving chemotherapy for haematological malignancies, HIV-1 infected patients and patients receiving immunomodulating drugs. In immunocompromised patients, the detection of HEV RNA in plasma or stool after 3 mo is defined as chronic infection[102 ]. Progression to cirrhosis in those with chronic hepatitis E infection occurs in 10 %-15 % and can occur rapidly, within 2 -3 years[103 ]. In a study of 85 patients with chronic HEV infection in 17 transplant centres across Europe and North America, almost 66 % of transplant recipients who contracted HEV developed chronic infection and 10 % progressed to cirrhosis[103 ,104 ]. Chronic infection and the risk of cirrhosis is not seen with genotype 1 or 2 infection.
Treatment of chronic infection
Most published data regarding treatment of chronic HEV infection are from case series and reports in SOT recipients[105 ]. Reducing immunosuppression dose by around 30 % has been shown to be effective in clearing HEV in around one third of patients[106 ]. Both PEGylated interferon and ribavirin are effective in treating chronic HEV infection. Interferon increases the risk of organ rejection in transplant recipients and therefore ribavirin monotherapy is the preferred option[107 ]. A systematic review has shown that 64 % of patients were HEV RNA negative at 6 mo after the end of treatment with ribavirin monotherapy[108 ]. The optimal dose and duration of treatment is still to be determined but 3 mo courses have been used most commonly[107 ]. A multi-centre case series of 59 transplant recipients infected with HEV showed that ribavirin monotherapy, at a median dose of 600 mg/d for 3 mo achieved SVR in 78 % of cases[107 ].
Problems remaining to be solved
Non-response to ribavirin:The main problem to be solved in relation to chronic HEV infection is how to manage non-response to ribavirin. Sofosbuvir has been proposed as an alternative agent to treat chronic HEV infection. It has shown promise in inhibiting HEV replicationin vitro[109 ] but it had a negligible effect on improving viraemia in a case report[110 ]. A later study of sofosbuvir monotherapy in nine patients demonstrated a modest reduction in viral load but viral elimination was not achieved[111 ].Convalescent plasma has also been trialed in a patient with persistent hepatitis E infection, and showed no effect on HEV RNA levels.
We also need to understand the relevance of HEV mutations and their effect on ribavirin resistance. Mutations have been identified in ribavirin non-responders but their impact on the treatment of these and other individuals has yet to be established.For example, the G1634 R mutation does not lead to absolute ribavirin resistance and does not appear to compromise the response to a second course of treatment with ribavirin[112 ]. New treatments are ultimately required for those who fail treatment with ribavirin.
IMPACT OF COVID-19 ON VIRAL ELIMINATION
The COVID-19 pandemic has compromised efforts to progress towards the WHO goal of elimination of viral hepatitis. This impact of the pandemic is likely to be felt for years to come and during the initial peaks has resulted in delays in diagnosis and treatment, and reduced access to harm reduction services. In April 2020 in the United Kingdom, new diagnoses of HCV were down 85 % and new treatment initiations had also fallen by 63 % compared with the year prior[113 ]. Although there has been some recovery, pre-COVID 19 levels of testing and treatment have not yet been reached.Funding and resources have also been re-allocated to fighting the COVID-19 pandemic. In addition to the impact on global elimination, the COVID-19 pandemic has significantly impacted upon the provision of HCC surveillance programmes for patients with viral hepatitis.
However, during the pandemic many new ways of working (such as telemedicine)and care cascades have been adopted, which may in fact positively impact upon the delivery of viral hepatitis services in the years to come. For example, in some centres patients have been commenced on HCV treatment remotely using telemedicine(personal communication). The vaccination programmes and ‘track and trace’ systems set up during the COVID-19 pandemic could be extrapolated to viral hepatitis to improve service delivery.
SUGGESTED PUBLIC HEALTH AND RESEARCH PRIORITIES FOR THE NEXT DECADE
The global hepatology community is well placed to set public health and research priorities in viral hepatitis for the forthcoming decade, striving towards global elimination and reduced health care burden. Potential priorities for each individual virus are proposed in Table 2 .
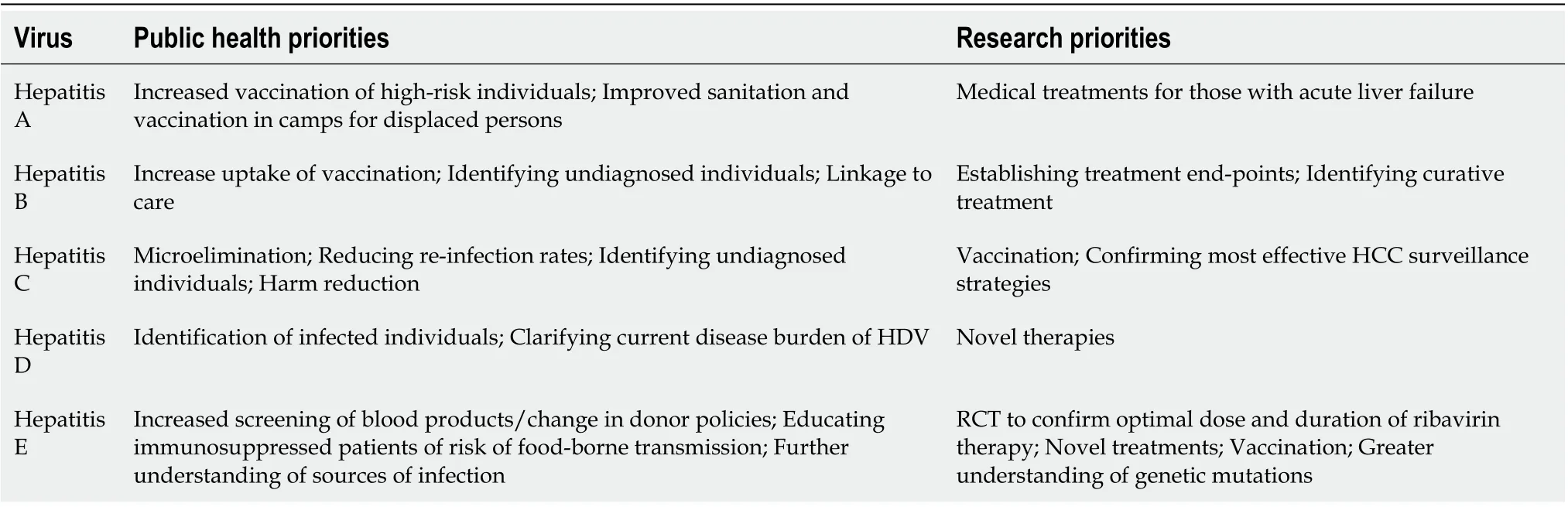
Table 2 Public health and research priorities for the next decade
CONCLUSION
Significant advances have occurred in the field of viral hepatitis over the past decade,particularly in relation to the treatment and cure of hepatitis C. Over the next decade -as we strive towards global elimination of viral hepatitis - the gastroenterology and hepatology community must focus on identifying the undiagnosed and engaging these individuals in to treatment programmes whilst continuing to develop novel treatments with the ultimate aim of cure.
 World Journal of Gastroenterology2022年1期
World Journal of Gastroenterology2022年1期
- World Journal of Gastroenterology的其它文章
- Risk of hepatocellular carcinoma after hepatitis C virus cure
- Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases
- Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction?
- Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway
- Gastric pentadecapeptide BPC 157 in cytoprotection to resolve major vessel occlusion disturbances,ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome
- Transfusion-transmitted hepatitis E: What we know so far?
