Transfusion-transmitted hepatitis E: What we know so far?
Carmen Ka Man Cheung, Sunny Hei Wong, Alvin Wing Hin Law,Man Fai Law
Abstract Hepatitis E virus (HEV) is a major cause of viral hepatitis globally. There is growing concern about transfusion-transmitted HEV (TT-HEV) as an emerging global health problem. HEV can potentially result in chronic infection in immunocompromised patients, leading to a higher risk of liver cirrhosis and even death.Between 0 .0013 % and 0 .281 % of asymptomatic blood donors around the world have HEV viremia, and 0 .27 % to 60 .5 % have anti-HEV immunoglobulin G. HEV is infectious even at very low blood concentrations of the virus. Immunosuppressed patients who develop persistent hepatitis E infection should have their immunosuppressant regimen reduced; ribavirin may be considered as treatment.Pegylated interferon can be considered in those who are refractory or intolerant to ribavirin. Sofosbuvir, a nucleotide analog, showed modest antiviral activity in some clinical studies but sustained viral response was not achieved. Therefore,rescue treatment remains an unmet need. The need for HEV screening of all blood donations remains controversial. Universal screening has been adopted in some countries after consideration of risk and resource availability. Various pathogen reduction methods have also been proposed to reduce the risk of TT-HEV. Future studies are needed to define the incidence of transmission through transfusion,their clinical features, outcomes and prognosis.
Key Words: Hepatitis E virus; Acute and chronic hepatitis; Immunosuppression; Blood transfusion; Transplantation
INTRODUCTION
Hepatitis E virus (HEV) was first discovered as an epidemic of non-A, non-B hepatitis in the 1980 s[1 ], and has since become one of the major global causes of viral hepatitis.The World Health Organization estimated that HEV caused approximately 44000 deaths in 2015 , and accounted for 3 .3 % of global deaths related to viral hepatitis[2 ]. A recent meta-analysis concluded that approximately 939 million of the global population have ever experienced HEV infection, and 15 to 110 million individuals have recent or ongoing infection[3 ]. The infection is generally self-limiting; however, it poses a threat to some vulnerable patients resulting in a significant burden of inpatient admissions, chronic infection, organ failure, and death[4 ]. The mortality rate can be greater than 20 % in patients with chronic liver disease, cirrhosis, or pregnancy[4 ,5 ]. With a high HEV serological prevalence among the global population, the safety of blood products has become a public health concern. Herein, we review existing evidence on transfusion-transmitted HEV (TT-HEV), and the implications for screening of blood donations.
VIROLOGY
HEV is a positive-sense, single-stranded RNA icosahedral virus belonging to the genusOrthohepeviruswithin the Hepeviridae family[6 ].Orthohepevirus Ahas eight distinct genotypes, of which HEV-1 , -2 , -3 and -4 infect humans[7 ]. HEV genotype C1 , belonging to the speciesOrthohepevirus C, circulates in rats and can cause cross-species infection and sporadic zoonotic transmission to humans[8 ].
HEV exists in urine or feces as non-enveloped virions encased by a capsid. It circulates in blood in a membrane-associated, quasi-enveloped form (eHEV) which is considered to be less contagious[9 ]. The entry mechanisms for HEV are not well characterized, but once the genomic RNA is uncoated and delivered to the cytosol, the replication cycle is initiated[10 ]. The viral release that initiates subsequent infection requires multivesicular bodies through endosomal sorting complexes required for transport[11 ].
EPIDEMIOLOGY
The prevalence rates of HEV antibody are higher in developing countries than in developed countries[12 ]. The highest anti-HEV immunoglobulin G (IgG) seropositivity rate has been reported in Africa with a mean of 21 .76 %, followed by Asia (15 .80 %),Europe (9 .31 %), North America (8 .05 %), South America (7 .28 %), and Oceania (5 .99 %).In addition, the reported anti-HEV immunoglobulin M (IgM) seroprevalence rate was 3 .09 %, 1 .86 %, 0 .79 %, 0 .22 % and 2 .43 % in Africa, Asia, Europe, North America, and South America, respectively[3 ].
Among the four major genotypes that can infect humans, HEV-1 and -2 are mostly found in developing countries including Asia, Africa, Latin America, and Mexico.Infection is mainly transmittedviafecally contaminated water, but occasionally also by person-to-person and vertical transmission[13 ]. Hepatitis E occurs as outbreaks as well as sporadic cases of acute hepatitis, with the preponderance of cases among adolescents and young adults. When stratified by age, the estimated incidence of HEV-1 and -2 infection is roughly between 0 .5 % and 1 .0 % for ages 0 to 15 years, with rates increasing to between 1 .0 % and 1 .4 % for ages 15 years to 20 years, then falling rapidly to a lower rate of 0 .2 % and below in individuals older than 30 years[14 ].
HEV-3 accounts for most of the autochthonous infection in developed countries while HEV-4 is mainly found in Asia and sporadically in Europe[15 ,16 ]. The reported seroprevalence of HEV-3 ranged from 0 .6 % to 52 .5 % in Europe, 6 % in United States, 3 to 16 % in United Kingdom and up to 52 % in some regions of France[17 ]. HEV-3 and HEV-4 are zoonotic viruses which are frequently transmittedviafood, close contact with animals, or transfusion of viremic blood units[18 ].
CLINICAL FEATURES AND EXTRAHEPATIC MANIFESTATIONS
The incubation period following exposure to HEV ranges from 2 to 6 wks. HEV infection commonly takes a clinically silent, asymptomatic course with around 5 % to 30 % of infected individuals developing acute hepatitis[19 ]. Symptoms of acute hepatitis include fever, malaise, anorexia, vomiting, followed by jaundice, tea-colored urine, and hepatomegaly[20 ]. It is then followed by a convalescent phase with gradual recovery within a few weeks in immunocompetent patients[21 ]. Acute liver failure is rare and occurs more frequently in middle-aged/elderly patients[22 ]. Fulminant hepatitis with fatal outcome is uncommon, but has been observed in pregnant women or in patients with pre-existing liver disease. The development of fulminant hepatitis appears to be related to host-specific factors rather than virus genotype, variants, or specific substitutions[23 ]. HEV superinfection may trigger liver decompensation in patients with chronic liver disease or cirrhosis, resulting in acute-on-chronic liver failure, which is associated with significant short-term mortality[24 ,25 ]. Further research is needed to clarify the clinical features, course of illness, and prognosis of patients with decompensated cirrhosis who develop HEV infection.
HEV-3 and HEV-4 can persist in immunocompromised patients resulting in chronic infection, defined as viral replication lasting for more than 3 to 6 mo[26 ]. It has been well described in patients after solid organ or stem cell transplant, hematology patients receiving chemotherapy, or HIV-infected patients[27 -32 ]. The prevalence of anti-HEV IgG was about 11 .6 % and viral RNA was 2 % in solid organ transplant recipients[33 ]. In solid organ transplant recipients who were positive for HEV RNA,more than 60 % developed chronic hepatitis[33 ].
The natural history of chronic hepatitis E infection is not well understood[34 ]. In liver transplant recipients infected by HEV, histological analyses of liver biopsy revealed atypical morphology that is distinct from those in immunocompetent patients during early phases of infection[35 ]. Proliferation of, and cytokine production by,CD4 + and CD8 + T-cells were impaired in patients with persistent HEV viremia[36 ].Chronic hepatitis E leads to liver fibrosis and cirrhosis. Cases of HEV-related hepatocellular carcinoma have been reported[37 ].
Although HEV predominantly infects hepatocytes, it may also affect other organs and present as extrahepatic manifestations. The mechanisms by which HEV can induce extrahepatic manifestations are not fully understood, but hypotheses include direct cytopathic tissue damage by extrahepatic replication, or immunological processes induced by an overwhelming host immune response[38 ]. Details of extrahepatic manifestations are shown in Table 1 [39 -44 ].
PREVALENCE IN BLOOD DONORS
Viremia
The prevalence of HEV RNA in blood donors varies around the world. (Table 2 )[45 -78 ]. Most countries have a low prevalence of HEV viremia, ranging from 0 .0013 % to 0 .086 %. A relatively higher rate of viremia was reported in Germany (0 .12 %) and China (0 .281 %)[49 ,70 ]. A meta-analysis of 10 studies from China showed a pooled prevalence of HEV RNA of 0 .1 %[79 ]. The actual prevalence might have been underes-timated as some studies included in the meta-analysis conducted RNA detection only in those donors who were positive for anti-HEV IgM or antigen[79 ].

Table 1 Extrahepatic manifestations associated with hepatitis E virus infection
The prevalence of HEV-3 and -4 is affected by dietary habits[80 ]. Consumption of raw pork tartare and undercooked pork liver may represent a relevant risk factor for HEV infection in Germany[49 ]. Regular consumption of pork meat and shellfish were also reported in the viremic donors in China[70 ].
Since 70 % of infections with HEV-3 and -4 are asymptomatic[81 ], it can be difficult to identify infected blood donors, as viremia occurs primarily during the pre-icteric phase[82 ]. Katiyar et al[72 ] described anti-HEV IgG positivity in 60 .5 % of the tested donors in India and yet none of them were positive for HEV RNA. In India, human HEV is caused exclusively by the HEV-1 genotype, which causes brief hepatitis and seldom results in chronic infection[83 ,84 ]. The difference in endemicity between HEV genotypes may affect the propensity to cause symptomatic disease and viral persistence, which in turn influences the likelihood of viremia among blood donors.
Other factors influencing the reported prevalence of HEV viremia are the sensitivity and plasma pool size of the various nucleic acid test screening platforms used[85 ]. For example, 33 of 90 donations with a viral load of 20 -750 IU/mL were positive when tested individually but missed in the pooled screening in a study by Hogemaet al[57 ].Delageet al[66 ] revealed a low prevalence (n = 11 /50765 ) and viral loads of HEV-RNA in Canadian blood donors based on individual nucleic acid amplification techniques(NAT). They postulated that if pooled NAT was used, only two positive donations with viral loads > 1000 IU/mL would have been detected. The true frequency of viremia in blood donors in studies using pooled NAT could be underestimated due to a dilution effect. Vollmeret al[86 ] found that screening using individual NAT yielded an approximately 50 % higher detection frequency compared with NAT of a mini-pool of 96 samples; nevertheless, samples exclusively positive for individual NAT had acorresponding viral load of < 25 IU/mL. High-sensitivity individual NAT can yield false-positive results[55 ]. Whether the identification of low-level HEV-positive donors translates into clinical significance and whether a single individual NAT is adequate remain undefined.

Table 2 Hepatitis E virus ribonucleic acid prevalence in donor, only studies include more than 1000 study subjects are included
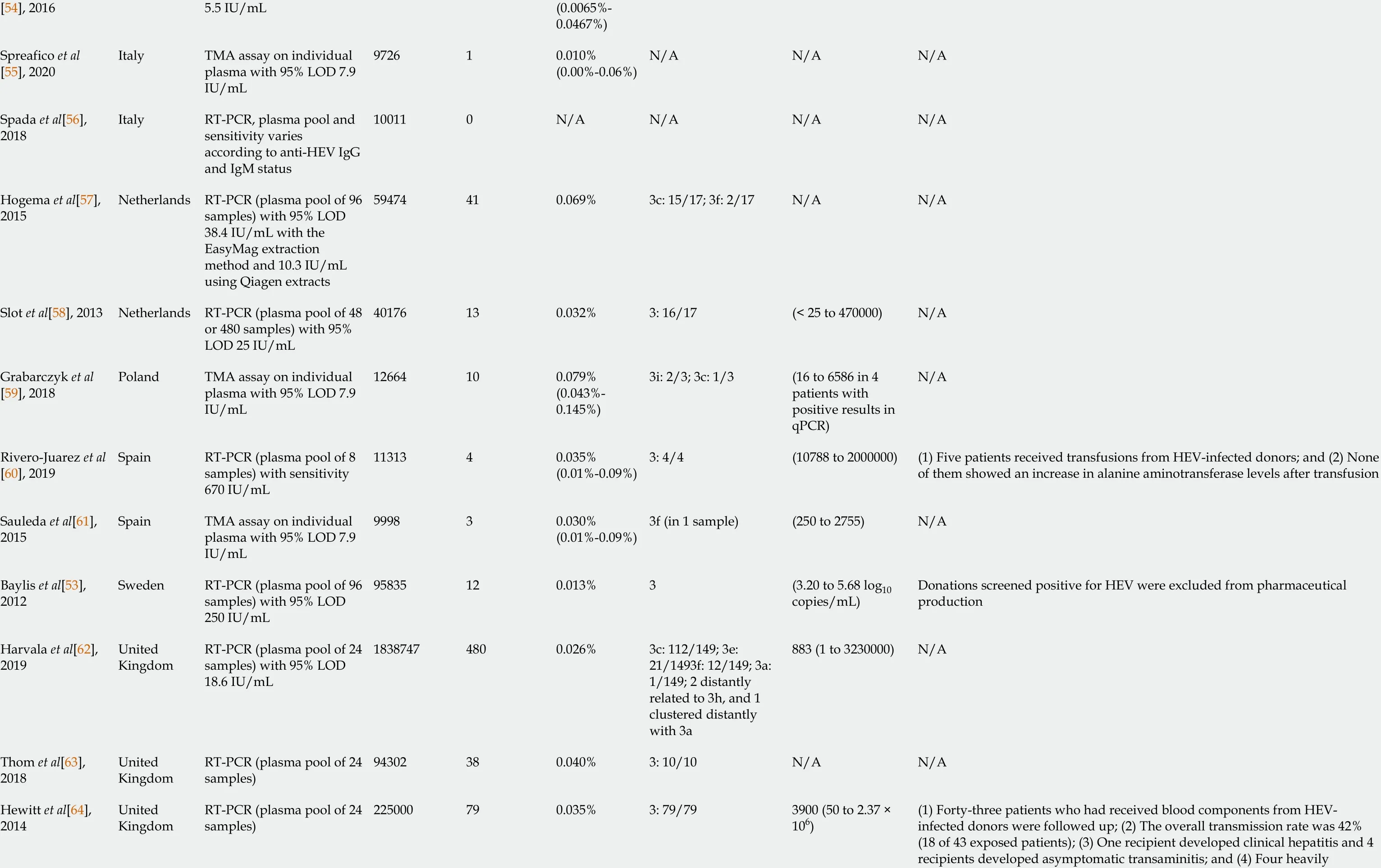
[54 ], 20165 .5 IU/mL (0 .0065 %-0 .0467 %)Spreafico et al[55 ], 2020 Italy TMA assay on individual plasma with 95 % LOD 7 .9 IU/mL 972610 .010 %(0 .00 %-0 .06 %)N/A N/A N/A Spada et al[56 ],2018 Italy RT-PCR, plasma pool and sensitivity varies according to anti-HEV IgG and IgM status 100110 N/A N/A N/A N/A Hogema et al[57 ],2015 Netherlands RT-PCR (plasma pool of 96 samples) with 95 % LOD 38 .4 IU/mL with the EasyMag extraction method and 10 .3 IU/mL using Qiagen extracts 59474410 .069 %3 c: 15 /17 ; 3 f: 2 /17 N/A N/A Slot et al[58 ], 2013 Netherlands RT-PCR (plasma pool of 48 or 480 samples) with 95 %LOD 25 IU/mL 40176130 .032 %3 : 16 /17 (< 25 to 470000 )N/A Grabarczyk et al[59 ], 2018 Poland TMA assay on individual plasma with 95 % LOD 7 .9 IU/mL 12664100 .079 %(0 .043 %-0 .145 %)3 i: 2 /3 ; 3 c: 1 /3 (16 to 6586 in 4 patients with positive results in qPCR)N/A Rivero-Juarez et al[60 ], 2019 Spain RT-PCR (plasma pool of 8 samples) with sensitivity 670 IU/mL 1131340 .035 %(0 .01 %-0 .09 %)3 : 4 /4 (10788 to 2000000 )(1 ) Five patients received transfusions from HEV-infected donors; and (2 ) None of them showed an increase in alanine aminotransferase levels after transfusion Sauleda et al[61 ],2015 Spain TMA assay on individual plasma with 95 % LOD 7 .9 IU/mL 999830 .030 %(0 .01 %-0 .09 %)3 f (in 1 sample)(250 to 2755 )N/A Baylis et al[53 ],2012 Sweden RT-PCR (plasma pool of 96 samples) with 95 % LOD 250 IU/mL 95835120 .013 %3 (3 .20 to 5 .68 log10 copies/mL)Donations screened positive for HEV were excluded from pharmaceutical production Harvala et al[62 ],2019 United Kingdom RT-PCR (plasma pool of 24 samples) with 95 % LOD 18 .6 IU/mL 18387474800 .026 %3 c: 112 /149 ; 3 e:21 /1493 f: 12 /149 ; 3 a:1 /149 ; 2 distantly related to 3 h, and 1 clustered distantly with 3 a 883 (1 to 3230000 )N/A Thom et al[63 ],2018 United Kingdom RT-PCR (plasma pool of 24 samples)94302380 .040 %3 : 10 /10 N/A N/A(1 ) Forty-three patients who had received blood components from HEVinfected donors were followed up; (2 ) The overall transmission rate was 42 %(18 of 43 exposed patients); (3 ) One recipient developed clinical hepatitis and 4 recipients developed asymptomatic transaminitis; and (4 ) Four heavily Hewitt et al[64 ],2014 United Kingdom RT-PCR (plasma pool of 24 samples)225000790 .035 %3 : 79 /793900 (50 to 2 .37 ×106 )

immunosuppressed patients had delayed (37 -38 wk) seroconversion or no antibodies detected Cleland et al[65 ],2013 United Kingdom Nested PCR (plasma pool of 24 samples) with 95 %LOD 201 IU/mL 4356030 .0069 %3 : 3 /3 N/A N/A North America United States 5072430 .0059 %3 : 2 /3 ; genotyping was unsuccessful in 1 patient(23 to 1420 )Delage et al[66 ],2019 Canada RT-PCR on individual samples with 95 % LOD 18 .6 IU/mL 50765110 .022 %3 (in 1 sample)(< 10 to 3080 )N/A Roth et al[67 ],2017 United States RT-PCR (plasma pool of 96 samples) with 95 % LOD 18 .6 IU/mL 12802140 .003 %3 a: 3 /3 (3 .0 to 3 .8 log IU/mL)N/A Stramer et al[68 ],2016 United States TMA assay on individual plasma with 95 % LOD 7 .9 IU/mL 1882920 .011 %(0 .0018 %-0 .351 %)N/A 14 IU/mL in one sample N/A Xu et al[69 ], 2013 United States RT-PCR (plasma pool of 7 to 8 samples) with 95 %LOD 400 IU/mL and nested PCR with 95 % LOD 200 IU/mL 19390 N/A N/A N/A N/A Baylis et al[53 ],2012 United States RT-PCR (plasma pool of 96 samples) with 95 % LOD 250 IU/mL 510750 N/A N/A N/A N/A Asia Wen et al[70 ],2018 China RT-PCR on individual plasma 5345150 .281 %One 4 h, another one clustered between genotype 2 and 4 i N/A N/A Tsoi et al[71 ], 2019 Hong Kong RT-PCR with 95 % LOD 7 .89 IU/mL 1000020 .02 %4 (in 1 sample)N/A N/A Katiyar H et al[72 ], 2018 India RT-PCR (plasma pool of 3 samples) with LOD 100 IU/mL 17990 N/A N/A N/A N/A Minagi T et al[73 ], 2016 Japan RT-PCR (plasma pool of 50 or 500 samples) with 95 %LOD 152 IU/mL 620140360 .0058 %3 : 36 /36 (< 1 .69 to 7 .22 log10 copies/mL)N/A Intharasongkroh et al[74 ], 2019 Thailand RT-PCR (plasma pool of 6 samples) with 95 % LOD 53 .5 IU/mL 30115260 .086 %3 : 6 /6 N/A N/A
CI: Confidence interval; FFP: Fresh frozen plasma; HEV: Hepatitis E virus; Ig: Immunoglobulin; LOD: Limit of detection; RBC: Red blood cells; RNA: Ribonucleic acid; RT-PCR: Real time polymerase chain reaction; TMA: Transcription mediated amplification.
Antibodies
In addition to direct detection of HEV RNA, another important indirect assessment of HEV burden is the prevalence of anti-HEV IgM and IgG in blood donors (Table 3 )[45 ,46 ,54 -56 ,58 ,59 ,61 ,63 ,65 ,68 ,69 ,71 ,72 ,77 ,87 -124 ]. HEV IgG prevalence increases with age which likely represents the cumulative effect of HEV exposure over a lifetime,especially as IgG antibodies can persist for decades[81 ]. The absence of detectable antibodies in donors was related to an increased risk of transfusion transmission of HEV[64 ]. However, the presence of anti-HEV IgG may not always be protective as multiple HEV reinfections could occur despite pre-existing antibodies[125 ]. Various HEV strains in serum are capable of replication in cell culture and generate infectious particles in the culture supernatant despite the coexistence of antibodies[126 ]. Anti-HEV IgM could be used to detect recent infection yet it failed to identify infected donors during the window period. For example, a meta-analysis of data from 28 countries found that only 26 .6 % of viremic blood units had positive anti-HEV antibodies[127 ]. In another study by Tedder et[128 ]al, a significant portion of viremic individuals (n= 57 /79 ) were seronegative at the time of donation. Anti-HEV IgM sometimes exhibits unexpectedly long persistence for up to 3 years after a self-limiting acute hepatitis E episode[129 ]. Only a minority of anti-HEV IgM-positive donors have detectable RNA[58 ,93 ,103 ,109 ]. All these findings suggest that detection of anti-HEV IgG or IgM alone may not provide effective screening of HEV in blood donors.

Table 3 Seroprevalence of hepatitis E in blood donors

Hogema et al[95 ], 2014 Netherlands 513 Wantai 5811 .31 %N/A N/A N/A N/A N/A Slot et al[58 ],2013 Netherlands 5239 Wantai 140126 .7 % (25 .6 %-28 .0 %)490 .94 %4 Range: < 25 to 37003 Grabarczyk et al[59 ], 2018 Poland 3079 Wantai 134043 .52 % (41 .78 %-45 .28 %)391 .27 % (0 .93 %-1 .73 %)N/A N/A N/A Wantai 21619 .96 % (17 .60 %-22 .32 %)Sauleda et al[61 ], 2015 Spain 1082 Mikrogen 11610 .72 % (8 .90 %-12 .60 %)131 .20 %0 N/A N/A Mateos et al[96 ],1999 Spain 863 Abbott assay and Western blot 343 .9 %0 N/A N/A N/A N/A Niederhauser et al[97 ], 2018 Switzerland 3609 Wantai 73720 .4 % (19 .1 %-21 .8 %)N/A N/A N/A N/A N/A Kaufmann et al[98 ], 2011 Switzerland 550 MP Biomedicals 274 .9 %N/A N/A N/A N/A N/A Thom et al[63 ],2018 United Kingdom 1714 Wantai 1046 .1 % (5 .0 %-7 .3 %)N/A N/A N/A N/A N/A Cleland et al[65 ],2013 United Kingdom 1559 Wantai 734 .7 % (3 .6 %-5 .8 %)0 N/A N/A N/A N/A Beale et al[99 ],2011 United Kingdom 262 Wantai 3111 .8 %41 .5 %0 N/A N/A North America DSI 56911 .29 %1462 .90 %MP Biomedicals 53710 .65 %931 .85 %Zafrullah et al[100 ], 2018 United States 5040 (from HEV RNA negative donor)Wantai 61912 .28 %340 .67 %0 N/A N/A Stramer et al[68 ], 2016 United States 4499 MP Biomedicals 3297 .3 % (6 .6 %-8 .1 %)260 .58 % (0 .39 %-0 .85 %)N/A N/A N/A Xu et al[69 ], 2013 United States 1939 Wantai 36418 .8 % (17 .0 %-20 .5 %)80 .4 % (0 .1 %-0 .7 %)0 N/A N/A South America Di Lello et al[101 ], 2020 Argentina 391 DiaPro 4411 .3 %82 .0 %0 N/A N/A Bangueses et al[102 ], 2020 Uruguay 400 DiaPro 4010 %194 .75 %3 N/A 3

Asia Nouhin et al[103 ], 2016 Cambodia 301 Wantai 8528 .2 % (23 .4 %-33 .5 %)31 .0 % (0 .01 %-1 .8 %)19563 Chen et al[104 ],2019 China 4044 Wantai 79919 .8 % (18 .6 %-21 .0 %)431 .1 % (0 .8 %-1 .4 %)2 N/A 4 Wen et al[70 ],2018 China 5345 Wantai 122722 .96 %380 .71 %15 N/A N/A Wang et al[105 ],2017 China 9069 Wantai 268229 .57 %1311 .44 %5 N/A N/A Ma et al[106 ],2015 China 816 Wantai 17221 .1 %40 .5 %0 N/A N/A Ren et al[107 ],2014 China 10741 Wantai 294527 .42 %1091 .01 %0 N/A N/A Zhuang et al[108 ], 2014 China 486 ELISA based on antigen protein pB166 and MPII 11323 .3 %N/A N/A N/A N/A N/A Tsoi et al[71 ],2019 Hong Kong 2000 Wantai 31515 .8 % (14 .2 %-17 .4 %)160 .8 %0 N/A N/A Tripathy et al[109 ], 2019 India 2447 Wantai 43317 .70 % (16 .23 %-19 .26 %)50 .20 %2 Ranged from 3 .5 ×104 to 4 .6 × 105 copies/mL 1 Katiyar et al[72 ],2018 India 633 Wantai 38360 .5 %N/A N/A 0 N/A N/A Gajjar et al[110 ],2014 India 460 DiaPro N/A N/A 224 .78 %N/A N/A N/A Parsa et al[111 ],2016 Iran 700 DiaPro 426 .0 %50 .71 %5 (only 50 seropositive blood donors were tested)N/A 1 Hesamizadeh et al[112 ], 2016 Iran 559 DiaPro 458 .05 %N/A N/A N/A N/A N/A Naeimi et al[113 ], 2015 Iran 628 HEV IgG, Pasto, Iran 10516 .72 %N/A N/A N/A N/A N/A Ehteram et al[114 ], 2013 Iran 530 DiaPro 7614 .3 %N/A N/A N/A N/A N/A Taremi et al[115 ], 2007 Iran 399 DiaPro 317 .8 %N/A N/A N/A N/A N/A Takeda et al[116 ], 2010 Japan 12600 in-house ELISA 4313 .42 %N/A N/A N/A N/A N/A Shrestha et al 41 .9 % (39 .7 %-Nepal 1845 Wantai 773553 .0 % (2 .2 %-3 .8 %)N/A N/A N/A

ALT: Alanine aminotransferase; CI: Confidence interval; DSI: Diagnostic Systems Incorporated; ELISA: Enzyme-linked immunosorbent assay; HEV: Hepatitis E virus; NIH: National Institutes of Health.
Geographical variation, racial differences, and diverse study methodology and laboratory techniques all contribute to differences in HEV seroprevalence. More than one-third of donors had evidence of past HEV infection in Poland, India, Nepal and Burkina Faso[59 ,72 ,117 ,120 ]. Lucarelli et al[93 ] reported an unexpectedly high prevalence (48 .9 %) of anti-HEV IgG among 313 donors in central Italy. Eating raw dried pig liver sausage was the only independent risk factor for HEV IgG in their study, but the authors speculated that the uncontrolled expansion of the wild boar population had resulted in contamination of the soil and watercourses for people living in rural areas, and this may also have also contributed to the high prevalence of HEV[93 ].
Caution is needed when interpreting the HEV serology results because commercial kits for serological detection show marked variation in sensitivity and specificity.Despite the relatively high sensitivity of the IgM assay, the sensitivity of IgG detection kits is highly dependent on a patient’s immune status, being 80 % to 90 % in immunocompetent individuals, but falling dramatically to 15 % to 45 % in immunocompromised patients[130 ]. In a meta-analysis conducted in Europe, the pooled anti-HEV IgG seroprevalence rates determined by different commercial assays showed large variability with reported seroprevalence rates ranging from 2 % to 17 %[131 ]. Poor concordance of test results between the Wantai, Dia.Pro and MP Diagnostics HEV enzyme-linked immunosorbent assays (ELISA) were observed[132 ,133 ]. This may partly explain the broad ranges of anti-HEV IgG prevalence (5 .3 % to 48 .9 %) reported in Italy[55 ,56 ,92 -94 ]. In contrast, most studies conducted in China used the Wantai assay and revealed a similar seroprevalence of around 20 % to 30 %. This assay is believed to be more sensitive than other commercial assays in detecting anti-HEV IgG[134 ,135 ].
TRANSFUSION-TRANSMITTED HEPATITIS E
HEV transmissionviatransfusion has been reported since 2004 [136 ] and there has been increasing recognition of the risk of transmitting HEV by transfusion in recent years.Cases of TT-HEV are shown in Table 4 [137 -150 ]. Identical genomic sequences were identified in most infected patients and blood donors. Table 4 Likely only represents the tip of the iceberg as other probable or possible cases have been reported in the literature[151 ,152 ]. At the same time, patients with mild symptoms of hepatitis E may have gone undiagnosed. Physicians should stay vigilant for HEV infection in patients who have received a blood transfusion.
Although blood components that contain larger plasma volumes, principally fresh frozen plasma and platelet components, are believed to transmit HEV more readily[64 ], a number of TT-HEV cases associated with red blood cell transfusion have also been described[138 ,140 ,141 ,143 ,144 ,148 -150 ]. Red blood cell transfusion was a significant risk factor for HEV seropositivity in patients on hemodialysis in Croatia[153 ].Twenty percent (n= 8 /40 ) of multiply transfused thalassemia patients were anti-HEV IgG positive compared with 11 .0 % (n = 10 /91 ) in blood donors[154 ]. In contrast, a study in Iran found anti-HEV antibodies in only 1 .67 % of patients with thalassemia,suggesting a low rate of TT-HEV in that country[155 ]. Results from these two studies in thalassemia patients were limited by the small sample size. Ankcornet al[156 ]analyzed 1591 patients with hematologic malignancy and found that the more transfusions of non-HEV screened blood products the patients had received, the higher their likelihood of being IgG seroreactive was, suggesting HEV acquisitionviatransfusion in these patients.
A study by Hewittet al[64 ] indicated that a viral concentration of between 407 and 257039 IU/mL in blood products was associated with TT-HEV, and that a high viral load in donors rendered infection more likely (P< 0 .0001 ). However, this may not be true in immunocompromised patients. In a systematic review, Dreieret al[50 ]calculated the median transfused viral load in HEV-infected and non-infected immunocompromised patients. Although the transfused viral load was higher in the infected than the non-infected individuals (4 .80 × 105 IU vs 1 .55 × 104 IU), the betweengroup difference was not statistically significant (P= 0 .1006 )[50 ]. A potential reason for this finding is that a low viral concentration (150 IU/mL) of the blood component could already be infectious[140 ].
Most cases of TT-HEV occur in immunocompromised recipients, such as patients with hematologic malignancies, or recipients of solid organ or hematopoietic stem cell transplants. However, patients on simple immunosuppressants like corticosteroids and cyclosporine or even immunocompetent individuals are also at risk[157 ]. Massive transfusion increased the risk of HEV transmission in an immunocompetent trauma patient[158 ]. Spontaneous resolution, viral eradication by immunosuppressant reduction and/or ribavirin are possible[159 ] but occasionally there are cases which have progressed into chronic hepatitis, liver cirrhosis or multi-organ failure.Transfusion recipients are more vulnerable to chronic liver injury than the general population as a result of foodborne infection[140 ]. More than 60 % (n = 56 /85 ) of solid organ transplant recipients infected with HEV developed chronic hepatitis, with tacrolimus use as an independent predictive factor[160 ]. Pas et al[161 ] screened 1200 solid-organ transplant recipients in the Netherlands for HEV RNA and identified 12 patients with HEV infection. Nine of these 12 patients had been treated with a tacrolimus-based regimen postoperatively. In liver transplant recipients, graft hepatitis with rapid histological disease progression and requirement of re-transplantation due to liver cirrhosis has been reported[162 ,163 ]. The rapid progression of HEV infection to advanced fibrosis and cirrhosis has also been observed in individuals receiving kidney or heart transplants[33 ]. In 50 patients with hematologic malignancy and clinicallyovert hepatitis E, the mortality rate was 16 % (n = 8 ), with liver-related death occurring in 4 patients[164 ]. HEV could actively suppress the cellular immune response and increase levels of immunosuppressive interleukin-10 that may perpetuate chronic infection and subsequent liver damage[165 ,166 ].

Table 4 Reported cases of transfusion transmitted hepatitis E

aTwo cases were not confirmed by sequence identity and should only be considered as probable TT-HEV.ALT: Alanine aminotransferase; AMI: Acute myocardial infarction; AML: Acute myeloid leukemia; BMT: Bone marrow transplant; CABG: Coronary artery bypass graft; FFP: Fresh-frozen plasma; HEV: Hepatitis E virus; Ig:Immunoglobulin; Plt: Platelet concentrates; RBC: Red blood cell.
TREATMENT
The management strategy for HEV infection should be determined by the clinical presentation. Currently, there is limited information in the published literature that describes the clinical features of TT-HEV, or the optimal approach to management.Acute TT-HEV infections are usually subclinical or mild, with no severe or fulminant cases reported[140 ]. Therefore, most acute HEV infections should be treated conservatively, while waiting for spontaneous clearance, although a short course of ribavirin may also be considered. In 21 patients with acute HEV infection who were at high risk of liver failure, receiving immunosuppressive therapy for an autoimmune disease or undergoing chemotherapy, a short course of ribavirin for up to 3 mo was associated with rapid virological response and normalization of liver enzymes[167 ].
The current practice for management of chronic HEV infection is mainly based on observational data[18 ]; Figure 1 shows a proposed algorithm for management. In patients who are on immunosuppressants, the first-line intervention should be a dose reduction or discontinuation of the immunosuppressive drug[168 ,169 ]. In solid organ transplant recipients, reducing the dose of immunosuppressive therapies that principally target T-cells can achieve HEV clearance in nearly one third of patients[160 ]. Most immunosuppressive drugs such as cyclosporine and tacrolimus increase HEV replication in vitro; mycophenolate mofetil is the only immunosuppressant agent demonstrated to have an anti-viral effect[170 ].
If modification of the immunosuppressant regimen is not possible or is unsuccessful, pharmacological agents such as ribavirin and/or pegylated interferon-alpha(peg-IFN) can be used[171 ]. In a meta-analysis that included 395 patients with chronic hepatitis E, ribavirin monotherapy for a median of 3 mo achieved sustained virological response (SVR) in 76 % of patients[172 ]. The reported dose of ribavirin in the literature ranged from 29 to 1200 mg/d, and the duration from 1 to 18 mo. Data on the optimal treatment regimen are needed[173 ]. HEV RNA should be assessed in the serum and in the stool before treatment discontinuation[169 ]. A second course of ribavirin for 6 mo can be attempted in cases of treatment failure[172 ]. HEV RNA concentrations decrease within the first week of initiating ribavirin therapy, and a greater reduction in viral load on day 7 is an independent predictor of SVR[174 ]. Ribavirin failure has been linked to the presence of certain single nucleotide variants (SNVs) and in-frame insertions in the hypervariable region of open reading frame (ORF) 1 in the HEV genome[175 ].

Figure 1 Recommended algorithm for management of transfusion-transmitted hepatitis E.
For those who are refractory to, or intolerant of, ribavirin, peg-IFN can be considered. Its efficacy has been documented in patients with hematologic disorders,patients receiving hemodialysis, and in combination with ribavirin in patients with HIV[176 -178 ]. Close monitoring is needed if it is used in transplant recipients because of an increased risk of acute humoral and cellular rejection[179 ,180 ]. Peg-IFN was thought to be safe only in liver transplant recipients until recent case reports described its successful use in a kidney transplant recipient[181 -183 ].
Sofosbuvir is a nucleotide analog shown to decrease replication of HEV-3in vitro[184 ]. However, in clinical studies, only modest antiviral activity was observed and SVR was not achieved[185 -187 ]. Rescue treatment for patients who are not eligible for,or not responding to, ribavirin and/or peg-IFN remains an unmet need.
HOW TO REDUCE TRANSFUSION-TRANSMITTED HEPATITIS E
The background risk of foodborne HEV transmission to both donors and recipients of blood products is not negligible. The transfusion-related risk of infection only exceeds the annual dietary risk when more than 13 individual donor components are transfused[188 ]. Strategies to reducede novoinfection, such as modifying eating habits and eliminating HEV from pigs and other animals that are used for food production are essential[189 ]. The one available vaccine (HEV 239 , Hecolin, Xiamen, China) is licensed only in China, and has yet to play a fundamental role in global outbreaks or pandemic control[190 ]. Nonetheless, the transmissibility and disease phenotype may not be the same for a person who acquires the virus orally and a person who gets infected intravenously, as there may be some protection provided by the acidic environment of the stomach and the mucosal barrier in the gut[191 ]. The infectivity of the non-enveloped form is different to that of enveloped HEV[9 ]. Data reporting outcomes of recipients of HEV-infected blood products are sparse[47 ,49 ,50 ,60 ,64 ].
Policies on screening HEV in blood products differ between countries. Universal screening was adopted in the United Kingdom, Ireland, and the Netherlands.Germany and France implemented targeted screening of donated plasma intended for use in high-risk patients[192 ]. In Japan, the use of nucleic acid-based screening is limited to Hokkaido[193 ]. Blood donors are not routinely tested for HEV infection in China including Hong Kong[70 ,71 ,194 ]. There has been much debate on mandatory HEV screening in blood donations[195 ]. Key questions, such as whether or not to screen, which laboratory assay to use, which donors to screen (universal or selective screening), and which types of blood components to screen should be assessed based on risk assessment, resource availability, health economics, and political or other influences. The answers may vary considerably by geographical location[169 ,196 ]. In areas where HEV is highly endemic, most donors and/or recipients have probably been exposed to HEV previously and would have positive IgG antibodies. Therefore,the decision on serological screening should also take into consideration the prevalence of HEV infection in that particular region.
All donors should answer a questionnaire about symptoms of clinical hepatitis and potential exposure to HEV prior to blood donation. Donation should be deferred in any donors with a history of clinical hepatitis[197 ]. Neither alanine aminotransferase(ALT) nor anti-HEV IgM testing correlate with the presence of HEV RNA, supporting the use of NAT for screening of blood donations[60 ,61 ,105 ]. A simulation study by Kampet al[198 ] reported that testing for HEV RNA by NAT with a pool size of 96 , and a 95 % limit of detection of 20 IU/mL will result in an 80 % reduction in expected HEV transmissions as well as of consequent chronic infections with severe complications.The risk of transmission could be reduced by 90 % in NAT using a mini-pool of 24 samples[198 ].
If opting for selective screening instead of universal screening, a clear definition of at-risk patients is warranted[199 ]. Targeted screening should be contemplated for blood components that will be supplied to transplant recipients, or patients with hematologic malignancies or chronic liver disease, as these individuals are at high risk of developing fulminant hepatitis, acute on chronic liver failure, or chronic hepatitis.However, it is not yet clear whether patients with rheumatologic diseases, those on low-intensity immunosuppression, or elderly individuals should only receive HEVnegative blood products. A multicenter retrospective study in Europe including 21 rheumatology and internal medicine patients found that patients with rheumatoid arthritis who were receiving methotrexate or biologics were at risk of chronic hepatitis E infection[200 ]. However, another study in France did not find worse hepatitis E severity or increased risk of chronicity in 23 patients with inflammatory arthritis treated with immunosuppressants[201 ].
Patients co-infected with HIV with CD4 + count < 200 /mm3 are at risk for persistent HEV infection[29 ]. In HIV patients with low CD4 + count, anti-HEV IgG seroconversion was delayed until immune reconstitution occurred[202 ]. A recent metaanalysis found that the HEV RNA positivity rate was significantly higher in transplant recipients than in HIV-positive patients [1 .2 % (95 %CI: 0 .9 -1 .6 ) vs 0 .39 % (95 %CI: 0 .2 -0 .7 ); P = 0 .0011 ], possibly due to better immune status in the HIV-positive individuals using anti-retroviral therapy[203 ].
HEV-1 and -2 infections can take a fulminant course in pregnancy, resulting in liver failure, membrane rupture, spontaneous abortions, and stillbirths[204 ]. HEV-3 infection in pregnancy appears to be less virulent without significant maternal, fetal, or neonatal complications[205 -207 ]. During pregnancy, a reduced cellular immunity and a high level of steroid hormones, in particular estrogen, progesterone, and human chorionic gonadotropin, influence viral replication/expression and possibly explain the disease severity[208 ]. The immune response could be influenced by HEV genotype, translating into different outcomes[209 ]. Ribavirin and peg-IFN are contraindicated in pregnancy due to concerns of teratogenicity[210 ]. Further studies are needed to clarify the risk of transmission of HEV to pregnant womenviablood transfusion; however, in view of the potentially serious disease course and absence of a safe treatment, pregnant women are a priority group for HEV-negative blood products.
Rothet al[67 ] evaluated the safety of plasma-derived medicinal products (PDMP)and found a very low prevalence of HEV RNA (0 .002 %) in plasma donors. Since viral reduction methods are used in the manufacturing processes of PDMP, these data do not support routine screening of all plasma pools intended for producing PDMP.Currently there is a lack of evidence to suggest that human serum albumin or coagulation factor concentrates are a major source of HEV infection[211 ,212 ].
The cost effectiveness of HEV screening of blood donations was analyzed in the Netherlands. Screening of whole blood donations in pools of 24 would prevent 4 .52 of the 4 .94 TT-HEV infections annually at a cost of approximately €310000 (Euro) per prevented chronic case. The estimated cost per incurable case prevented was 10 -fold higher. Costs could potentially be reduced by 85 % if only the blood products intended for use by immunocompromised patients were screened. Additional costs for selective screening may arise for logistic reasons and a possible increase in the number of blood products that expire before use. They concluded that preventing HEV transmission by screening of blood donations appears not excessively expensive compared with other blood-screening measures but the impact on disease burden may be small as only a minority of all HEV cases are transmitted by blood transfusion[213 ]. Another economic analysis performed in North America found a very low estimated risk of TTHEV infection risk leading to severe liver disease. When compared with no screening,the costs were $2 .68 (USD) per component for a selective screening approach, and$6 .68 per component for universal screening. The respective costs per quality-adjusted life-year gained were $225546 and $561810 , respectively, which exceeded the threshold for what is considered as “cost-effective”[66 ].
In addition to screening, various pathogen reduction methods have been proposed to reduce risk of TT-HEV. Solvent/detergent treatment could not eliminate nonenveloped HEV in plasma[214 ]. Non-enveloped HEV is also resistant to the Intercept method, which combines a synthetic psoralen amotosalen HCl treatment with ultraviolet A light illumination to block the replication of DNA and RNA[215 ].However, substantial viral reduction has been demonstrated during the manufacturing process of plasma products using immunoaffinity chromatography, nanofiltration, cold ethanol fractionation and heat treatment[216 ]. Anti-HEV antibodies enhanced HEV removal by nanofiltration[217 ]. Furthermore, ultraviolet C light provided effective inactivation of HEV in platelet concentrates[218 ].
CONCLUSION
To conclude, TT-HEV is gaining attention worldwide. Although the overall prevalence of viremic blood donations is low, HEV can cause sinister consequences in immunocompromised recipients. Future studies are needed to define the incidence of transmission through transfusion, clinical features, outcomes, and prognosis. The decision on a screening policy in asymptomatic blood donors should be based on local risk assessment and health economics.
 World Journal of Gastroenterology2022年1期
World Journal of Gastroenterology2022年1期
- World Journal of Gastroenterology的其它文章
- Viral hepatitis in 2021 : The challenges remaining and how we should tackle them
- Risk of hepatocellular carcinoma after hepatitis C virus cure
- Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases
- Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction?
- Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway
- Gastric pentadecapeptide BPC 157 in cytoprotection to resolve major vessel occlusion disturbances,ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome
