Dysbiosis of the duodenal microbiota as a diagnostic marker for pancreaticobiliary cancer
Mitsuru Sugimoto,Kazumichi Abe,Tadayuki Takagi,Rei Suzuki,Naoki Konno,Hiroyuki Asama,Yuki Sato,Hiroki Irie,Ko Watanabe,Jun Nakamura,Hitomi Kikuchi,Mika Takasumi,Minami Hashimoto,Tsunetaka Kato,Ryoichiro Kobashi,Takuto Hikichi,Hiromasa Ohira
Mitsuru Sugimoto,Kazumichi Abe,Tadayuki Takagi,Rei Suzuki,Naoki Konno,Hiroyuki Asama,Yuki Sato,Hiroki Irie,Ko Watanabe,Jun Nakamura,Hitomi Kikuchi,Mika Takasumi,Minami Hashimoto,Tsunetaka Kato,Ryoichiro Kobashi,Hiromasa Ohira,Department of Gastroenterology,Fukushima Medical University School of Medicine,Fukushima 960-1295,Japan
Ko Watanabe,Jun Nakamura,Hitomi Kikuchi,Minami Hashimoto,Tsunetaka Kato,Ryoichiro Kobashi,Takuto Hikichi,Department of Endoscopy,Fukushima Medical University Hospital,Fukushima 960-1295,Japan
Abstract BACKGROUND Pancreaticobiliary cancer (PB Ca) is a lethal disease,and a useful diagnostic marker is urgently needed.A correlation between the human microbiota and malignant gastrointestinal diseases was recently reported.AIM To investigate the efficacy of the duodenal microbiota for diagnosing PB Ca.METHODS We recruited 22 patients with benign pancreaticobiliary diseases (benign group)and 12 patients with PB Ca (malignant group).The duodenal microbiota of each patient was analyzed by the 16S rDNA terminal restriction fragment length polymorphism method.Patient characteristics,tumor markers,and relative abundances of the duodenal microbiota were compared between the benign and malignant groups.RESULTS Cancer antigen 19-9 (CA19-9),Bifidobacterium,Clostridium cluster XVIII,and Prevotella levels differed significantly between the benign and malignant groups.Clostridium cluster XVIII had the greatest area under the receiver operating characteristic curve (AUC) among the four factors with respect to diagnosing PB Ca (cutoff value:3.038%;sensitivity:58.3%;specificity:95.2%;AUC:0.81).The combination of Clostridium cluster XVIII (cutoff value:3.038%) and CA19-9 Levels(cutoff value:18.8 U/mL) showed 91.7% sensitivity and 71.4% specificity for diagnosing PB Ca.CONCLUSION The duodenal microbiota may be useful for PB Ca screening.
Key Words:Pancreaticobiliary cancer;Diagnostic marker;Duodenal microbiota;Clostridium cluster XVIII;Cancer antigen 19-9
INTRODUCTION
Pancreaticobiliary cancer (PB Ca) is a lethal disease[1].Surgery is the only radical treatment for pancreatic cancer,but unfortunately,many pancreatic cancer patients have advanced-stage lesions or other organ metastases,and they thus are not candidates for surgery[2].For those who can undergo surgical treatment,the 5-year survival rate is reported to be 20%-30%[3,4].
In general,biliary tract cancer is difficult to diagnose.Conventional diagnostic methods include evaluating tumor markers [cancer antigen 19-9 (CA19-9) or carcinoembryonic antigen (CEA)],biliary biopsy,biliary juice cytology,and brush cytology,but the diagnostic power of these methods is not sufficient[5-19].It has been reported that serum CA19-9 elevation is observed in 85% of patients with cholangiocarcinoma,though elevation of this marker can also be found in benign obstructive jaundice.Similarly,elevated serum CEA,which is not seen in obstructive jaundice,occurs in only 30% of patients with cholangiocarcinoma[20].Therefore,effective diagnostic methods for the early diagnosis of PB Ca are urgently needed.Recently,a correlation between the human microbiota and malignant gastrointestinal diseases was reported[21-26].In addition,oral and salivary microbiota communities have been reported to be effective in diagnosing pancreatic cancer or predicting the onset of pancreatic cancer[27-29],and the risk of pancreatic cancer is reportedly increased in patients with a history of periodontal disease[30].Furthermore,serum antibodies against oral microbiota are reported to be a risk factor for the onset of pancreatic cancer[31].However,the mechanism by which this dysbiosis leads to pancreatic cancer is unknown,especially as the pancreas is relatively distant from the mouth.
Thus,we hypothesized that the duodenal microbiota would be more efficient than the oral microbiota for diagnosing PB Ca because the duodenum is closer to the bile duct and pancreas than the oral cavity.The aim of this study was to determine the efficacy of the duodenal microbiota for diagnosing PB Ca.
MATERIALS AND METHODS
Ethical approval
This study was approved by the Institutional Review Board of Fukushima Medical University.
Patients
We assessed 34 patients with pancreaticobiliary disease who visited our hospital over two years.Twenty-two patients were diagnosed with benign pancreaticobiliary diseases (benign group) [chronic pancreatitis:6;intraductal papillary mucinous neoplasm (IPMN):5;gallbladder adenomyomatosis:3;autoimmune pancreatitis:3;benign common bile duct (CBD) stricture of unknown origin:2;serous cystic neoplasm:2;and CBD stone:1] (Table 1).The other 12 patients were diagnosed with PB Ca (malignant group) (pancreatic cancer:9;bile duct cancer:3).The patients provided written informed consent to participate in this study.For all pancreatic cancer cases,the lesion was located in the head.Eight pancreatic cancer patients were diagnosed by endoscopic ultrasound-guided fine needle aspiration.One pancreatic cancer patient was diagnosed with intraductal papillary mucinous carcinoma with evident worsening of the lesion by imaging.Benign diseases were diagnosed by no histological malignancy or unchanging lesions after a clinical course of at least six months.Furthermore,the IPMN patients in the benign group did not have high-risk stigmata or worrisome features[32].The cases of bile duct cancer were diagnosed by biliary biopsy or surgery.According to the cytology grade,classes IV and V were diagnosed as malignancies.The stage of PB Ca was determined based on the UICC classification,ver.8.
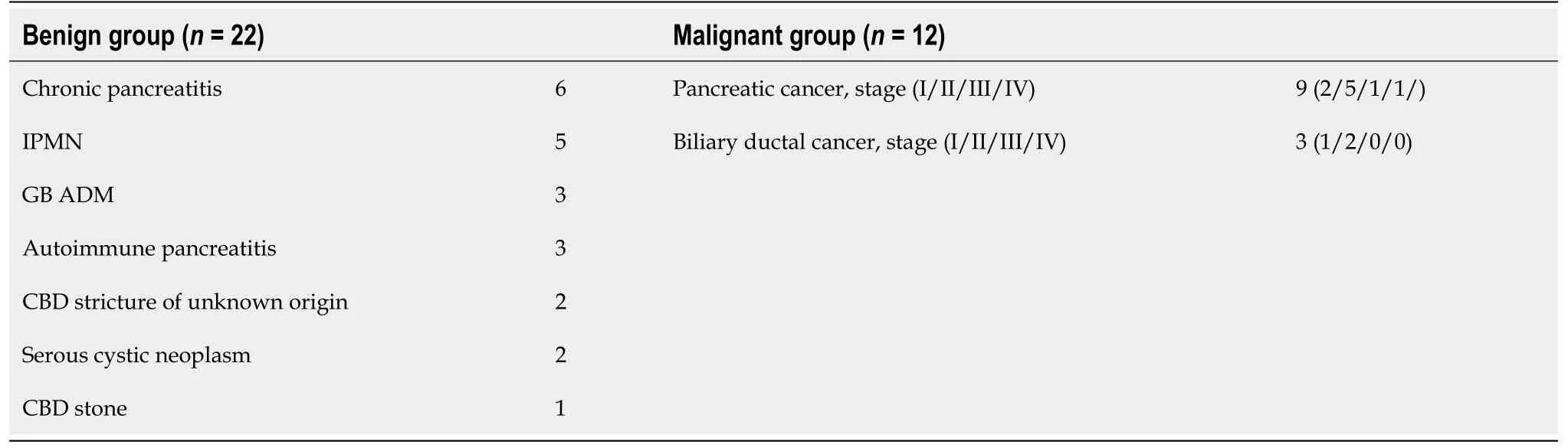
Table 1 Final patient diagnoses
The patients did not receive antibiotic agents for at least a week prior to duodenal juice collection,and they did not receive steroids at all.
Sample collection and DNA extraction
An endoscope was used under sedation with midazolam.The endoscope was advanced to the duodenum,and 0.5-1.0 mL of duodenal juice was collected through a catheter and stored at -20°C.The endoscope used was Q260 and Q260H,and the catheter was a PR-109Q-1 or PR-104Q-1 (Olympus,Tokyo,Japan).
Bacterial DNA was extracted from duodenal juice samples in accordance with a previous report by Takahashiet al[33].
Terminal restriction fragment length polymorphism
Terminal restriction fragment (T-RF) length polymorphism (T-RFLP) was performed by TechnoSuruga Laboratory (Shizuoka,Japan) according to Nagashima’s methods[34,35].The 16S rRNA gene was amplified from the extracted DNA using the primers 5’FAM-labeled 516F (5’-TGCCAGCAGCCGCGGTA-3’) and 1510R (5’- GGTTACCTTGTTACGACTT-3’) and HotStarTaq DNA Polymerase (Qiagen,Hilden,Germany) with a Thermal Cycler Dice (Takara,Shiga,Japan).The amplification program used was as follows:preheating at 94°C for 15 min;35 cycles of denaturation at 94°C for 30 s,annealing at 50°C for 30 s,and extension at 72°C for 2 min;and a terminal extension at 72°C for 10 min.DNA amplification was verified by electrophoresis of the polymerase chain reaction (PCR) products (2 μL) through a 1.0% agarose gel with Tris-acetate-EDTA buffer.The amplified DNA was purified by a MultiScreen PCR96 Filter Plate(Millipore,Billerica,MA,United States).
The purified PCR product (3 μL) was digested with 10 U of Fast DigestBseLI (BslI)(Thermo Fisher Scientific) in a total volume of 15 μL at 37°C for 10 min.The restriction digestion products (0.5 μL) were mixed with 0.1 μL of a DNA fragment-length standard size marker and 10 μL of deionized formamide.The standard size marker was MapMarker X-Rhodamine Labeled 50-1000 bp (Bio Ventures,Murfreesboro,TN,United States).The samples were denatured at 95°C for 2 min and then placed immediately on ice.The T-RF length was established using an ABI PRISM 3130xl genetic analyzer (Thermo Fisher Scientific),and the length and peak area were determined using the genotyping software GeneMapper (Thermo Fisher Scientific).The fragment sizes were estimated using the Local Southern method in GeneMapper software (Thermo Fisher Scientific).If the peak height was less than 50 fluorescence units,the T-RF was excluded from the analysis.The fragments were resolved to one base pair by manual alignment of the size standard peaks from different electropherograms,and the predicted T-RFLP patterns of the 16S rDNA of known bacterial species were obtained using publicly available sequences.T-RFs were divided by operational taxonomic units (OTUs),and bacterial classification was performed according to the ratio of each OTU per total OTU area.The OTUs were identified by correspondence to a database of human intestinal flora (https://www.tecsrg.co.jp/trflp/index.html).
Analyzed traits
Patient characteristics and tumor markers (age,sex,reduction in body weight ≥ 5 kg within 6 mo prior to duodenal juice sampling,intake of proton pump inhibitors,CA19-9) were compared between the two groups.The body weight marker was selected for the following reasons.The composition ratio of the microbiota has been reported to be different between subjects with obesity and those with a normal body mass index[36].Because the intake of high-fat foods influences the quantity and composition of bile acid,the intestinal bacterial flora might change[37].The relative abundances of duodenal microbiota members (Bacteroides,Bifidobacterium,Lactobacillales,Prevotella,Clostridiumcluster IV,Clostridiumsubcluster XIVa,Clostridiumcluster IX,Clostridiumcluster XI,Clostridiumcluster XVIII,and others) were compared between the benign and malignant groups.
Statistical analysis
Normally distributed continuous variables were compared using Student’sttest and nonnormally distributed continuous variables using the Mann-WhitneyUtest.Nominal variables were compared with Fisher’s exact test.A receiver operating characteristic (ROC) curve was employed to compare the accuracy of the biomarkers.ThePvalue<0.05 was considered statistically significant.
These statistical analyses were performed using the EZR platform (Saitama Medical Center,Jichi Medical University,Saitama,Japan),which is a graphical user interface for R (The R Foundation for Statistical Computing,Vienna,Austria).EZR is a modified version of R-commander that was designed to perform functions that are frequently used in biostatistics[38].
RESULTS
Among the patient characteristics and tumor markers,age and CA19-9 Levels were significantly different between the benign and malignant groups (mean ± SD,age:63.3± 12.2vs73.0 ± 8.3 years,Pvalue=0.016;median (range),CA19-9:5.4 (2.0-54.8)vs22.8(2.0-9893.2),Pvalue=0.03) (Table 2).
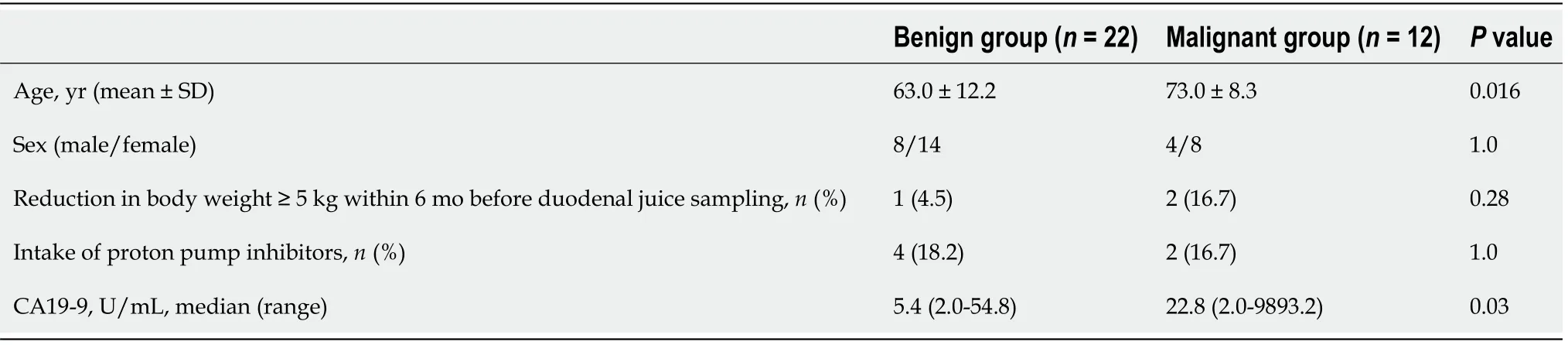
Table 2 Comparison of patient characteristics,tumor markers,and microbiomes
Comparison of microbiome components revealedBifidobacterium,Clostridiumcluster XVIII,andPrevotellato be significantly different between the benign and malignant groups (median (range),Bifidobacterium:0 (0-0.5)%vs0.3 (0-4.5)%,Pvalue<0.05;Clostridiumcluster XVIII:1.3 (0-3.9)%vs3.6 (0.8-14.9)%,Pvalue=0.006;Prevotella:2.2(0-25.9)%vs0.1 (0-10.9)%,Pvalue=0.04) (Figure 1 and Table 3).
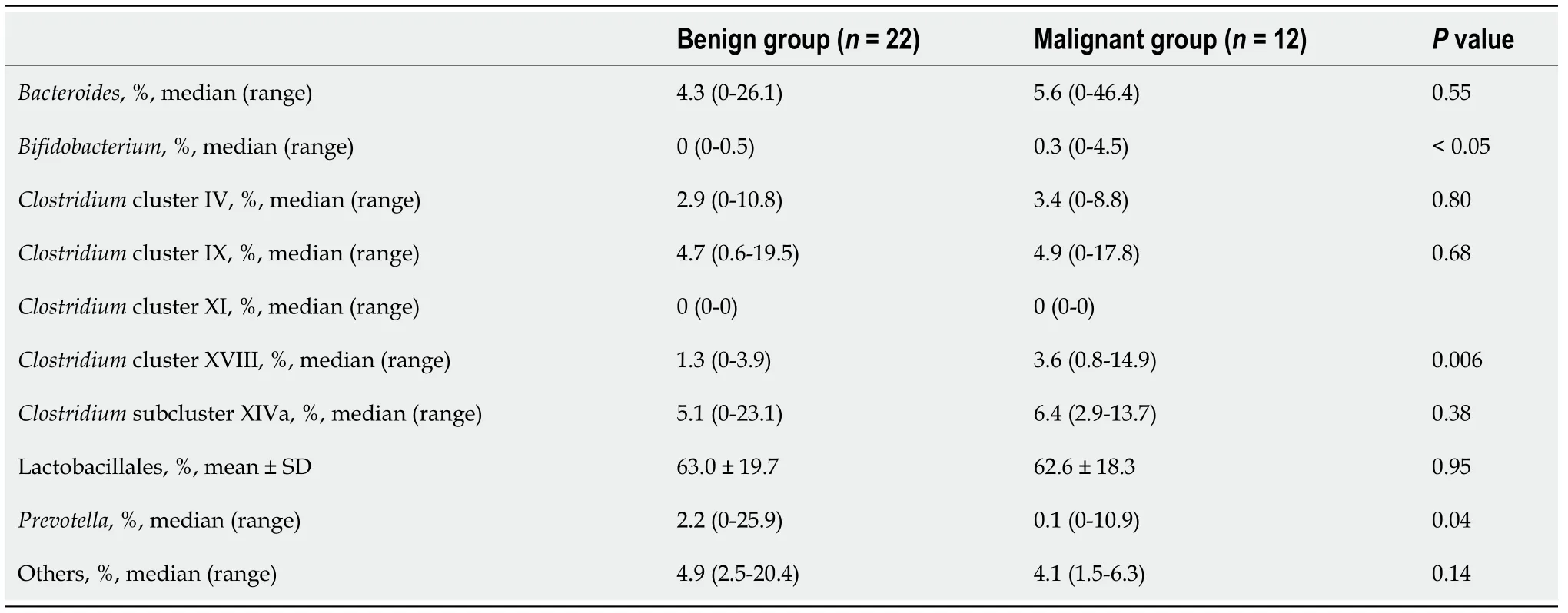
Table 3 Microbiome comparison
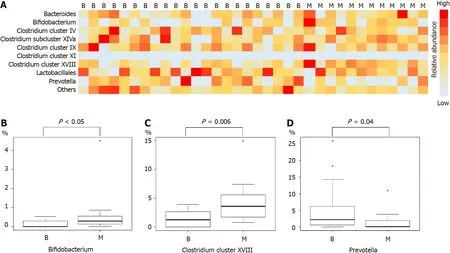
Figure 1 Analysis of the duodenal microbiota.
To determine the influence of age on CA19-9 Levels and microbiome composition,these factors were compared between the subgroup of patients<69 years and the subgroup of those ≥ 69 years (the median age of all patients was 69 years).According to the results,CA19-9 Levels,Bifidobacterium,Clostridiumcluster XVIII,andPrevotellawere not influenced by age (Table 4).

Table 4 Effects of age on cancer antigen 19-9 levels and the human microbiome
We assessed the ability of the microbiota to diagnose PB Ca by calculating the area under the ROC curve (AUC) and found thatClostridiumcluster XVIII had the highest AUC (cutoff value:3.038%,sensitivity:58.3%,specificity:95.2%,AUC:0.81) among the three microbiome components (Bifidobacterium,Clostridiumcluster XVIII,Prevotella)and CA19-9 Levels (Figure 2).
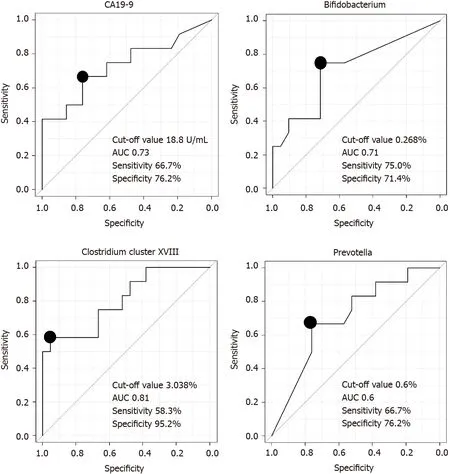
Figure 2 Comparison of the ability of microbiome components and cancer antigen 19-9 Levels to diagnose pancreaticobiliary cancer.
The combination ofClostridiumcluster XVIII (cutoff value:3.038%) and CA19-9 Levels (cutoff value:18.8 U/mL) was also examined as a marker to diagnose PB Ca;the sensitivity of this combination was 91.7% (11/12),and the specificity was 71.4%(15/21) (Table 5).CA19-9 data were missing for one patient in the benign group.
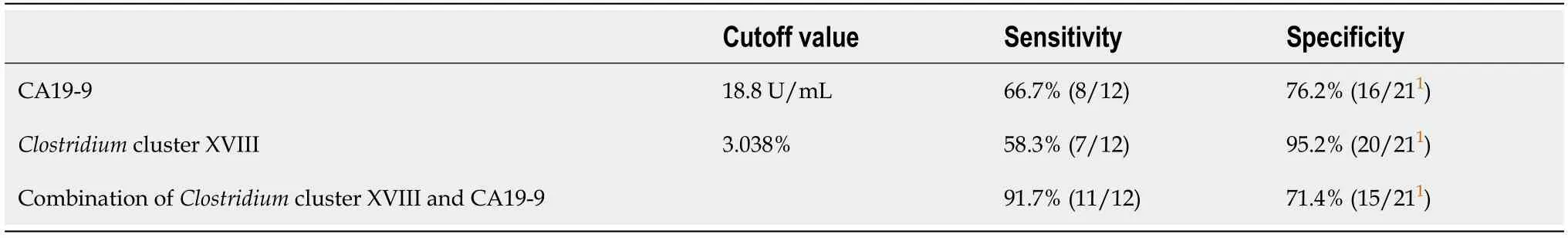
Table 5 Diagnosis of pancreaticobiliary cancer by the combination of Clostridium cluster XVIII and cancer antigen 19-9 levels
DlSCUSSlON
In this study,we investigated which members of the duodenal microbiota could aid in diagnosing PB Ca and foundClostridiumcluster XVIII to be more useful than CA19-9 Levels and other bacteria for diagnosing PB Ca.Notably,the combination ofClostridiumcluster XVIII and CA19-9 Levels showed high sensitivity,indicating that this combination is valuable for screening patients for PB Ca.
As mentioned above,the oral microbiota has been considered to be a biomarker in pancreatic cancer.First,Michaudet al[30] reported that a history of periodontal disease was a risk factor for pancreatic cancer.After that,several oral microbes were reported to be more abundant and possible predictors and risk factors for survival in pancreatic cancer (Table 6)[27-29,39,40].In addition,antibodies againstPorphyromonas gingivaliscan serve as a risk factor for pancreatic cancer onset[27].
Although the salivary microbiome may be useful for the medical care of patients with pancreatic cancer,it is influenced by differences in oral hygiene,mastication,and swallowing among individuals[27,41,42].In contrast,the duodenal microbiota is more relevant to the pancreas and bile duct than is the salivary microbiota.Therefore,the duodenal microbiota was hypothesized to directly reflect the dysbiosis associated with PB Ca,and in fact,the results of this study reveal that the duodenal microbiota might be beneficial for screening PB Ca.The microbiota around the pancreas and biliary duct have been reported.Microbes of the duodenal mucosa,bile juice,cancer tissue,and cyst fluid of IPMN in pancreaticobiliary tumor patients have been investigated(Table 6)[30,43-47].However,the methods used to analyze the microbiota around the pancreas and bile duct in these reports were more invasive than duodenal juice sampling.
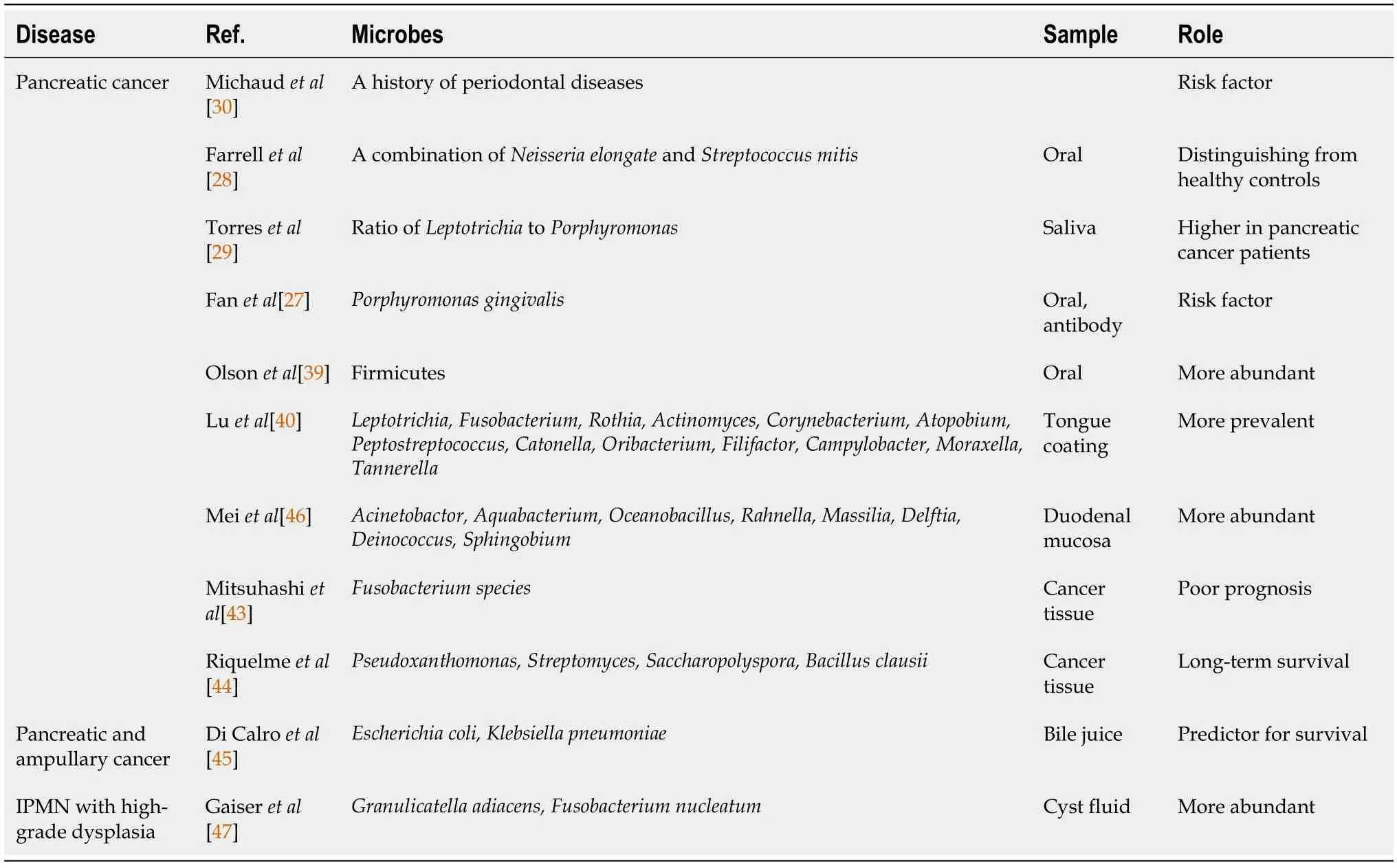
Table 6 Past reports on microbes and pancreaticobiliary cancer
The mechanism by which microbes lead to PB Ca remains unknown.The etiology with respect to the salivary microbiota has been considered in past reports.P.gingivaliscan interrupt signaling pathways by modulating receptor expression and cytokine secretion to evade the host’s immune system[48-52].Moreover,P.gingivalisactivates the Toll-like receptor signaling pathway[53,54],which has been reported to be related to pancreatic carcinogenesis[55,56].In other reports,oral bacteria were found outside of the oral cavity in the gastrointestinal tract.Immune responses against these bacteria can cause inflammation and carcinogenesis in the pancreas.Lipopolysaccharide has also been reported to drive pancreatic carcinogenesis by blocking the MyD88-dependent,Toll-like receptor 4 and MyD88-independent pathways[57].In another report,the mechanism was described as follows.Bacterial ligands detected by Toll-like receptors cause a Th1/Th2/Th17 imbalance in the tumor microenvironment,promoting tumorigenesis in combination withKrasmutation.In the duodenum,microbes may reach the pancreatic duct or biliary duct through the Vater papilla.In the pancreas or bile duct,pattern recognition receptors (such as Toll-like receptors) are stimulated by the pathogenic molecular patterns of bacterial ligands and induce lower levels of immune suppression,leading to the development of PB Ca[58].These results from past reports suggest that some type of immune system response is the link between the duodenal microbiota and PB Ca.
On the one hand,the relationship betweenClostridiumcluster XVIII and carcinogenesis has not been reported,even thoughClostridiumcluster XVIII is reported to have the potential to enhance regulatory T (Treg) cells[59].Many Treg cells exist in tumor tissue and prevent the immune response to tumors.Therefore,Tregs contribute to tumor progression and poor prognosis[60-64].Thus,Clostridiumcluster XVIII may increase in response to cancer and activate Tregs.Alternatively,Clostridiumcluster XVIII may activate Tregs,with oncogenesis advancing.
This report has some limitations.First,this study was small and performed at a single institution.However,based on the data fromClostridiumcluster XVIII,the average value of the malignant group was 4.5%,and that of the benign group was 1.5%.Total thirty patients were needed to achieve an α error of 5% and a β value of 0.2.WhenClostridiumcluster XVIII was the main outcome,the minimum necessary sample size was secured.Although this is the first report to describe the relationship between the duodenal juice microbiota and PB Ca,the diseases in the malignant group were not uniform.If subgroup analyses of pancreatic diseases were performed,the abundance of some duodenal microbes would be significantly different between the benign and malignant groups (Table 7).We hope that a future study with a larger number of patients will confirm our results for both pancreatic cancer and biliary cancer.Second,healthy control subjects were not enrolled in this study.However,as esophagogastroduodenoscopy under sedation is rarely performed in healthy patients,this limitation was unavoidable in the study design.Third,T-RFLP was applied.Investigations into the duodenal microbiota have been limited because duodenal juice cannot be collected in large volumes (less than 0.5 mL is typically collected).However,the measurement of the duodenal microbiota was demonstrated to be possible.Follow-up studies using next-generation sequencing are warranted[65].Fourth,examining the duodenal microbiota requires a somewhat invasive technique.In the future,the development of serum antibody testing for the duodenal microbiota should be pursued.
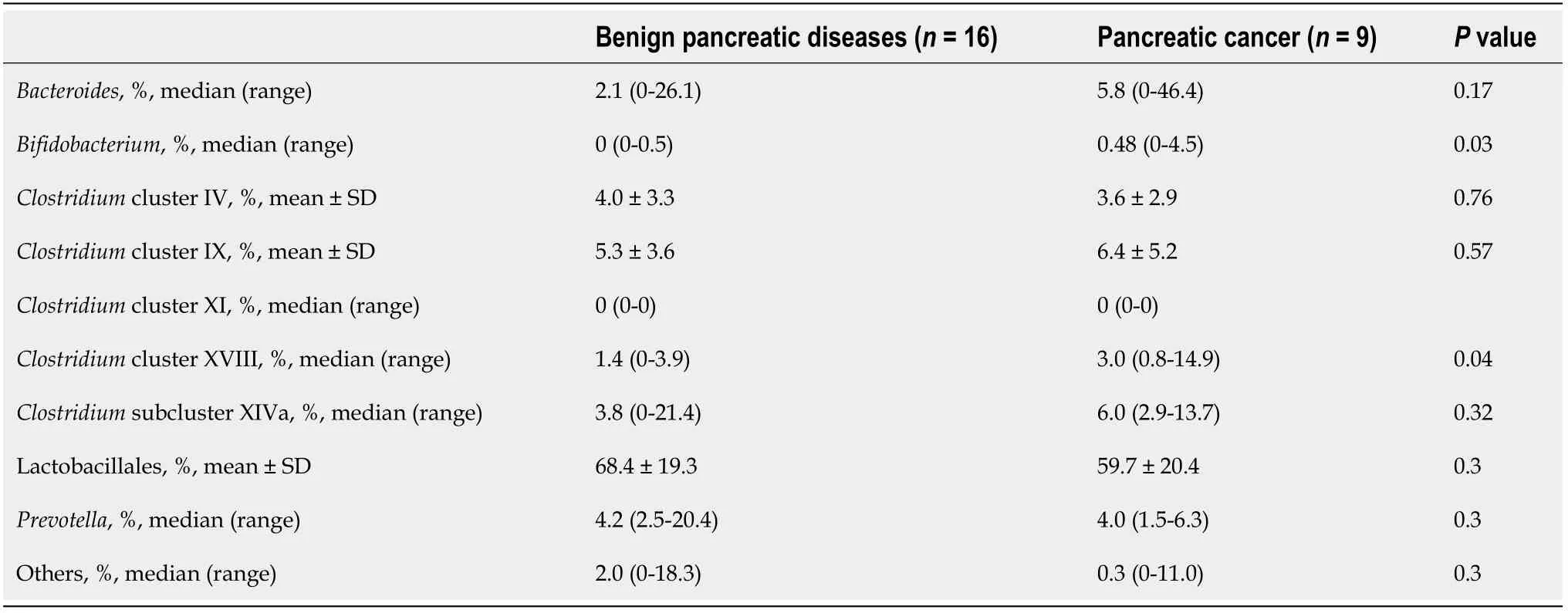
Table 7 Microbiome comparison in patients with pancreatic disease
CONCLUSION
In conclusion,the duodenal microbiota may contribute to PB Ca screening.
ARTICLE HIGHLIGHTS
Research background
Pancreaticobiliary cancer (PB Ca) is a lethal disease;however,there are currently no appropriate diagnostic and prognostic markers.Recently,the human microbiota was reported to be a causative factor,diagnostic marker,and prognostic marker for gastrointestinal malignant diseases.
Research motivation
The oral and fecal microbiota have been reported to be useful diagnostic markers for gastrointestinal cancer.The duodenum is located closer to the pancreas and bile duct than the oral cavity and colon.Therefore,we hypothesized that assessment of the duodenal microbiota might improve the diagnostic accuracy for PB Ca.
Research objectives
To investigate the diagnostic accuracy of duodenal microbiota evaluation for PB Ca.
Research methods
Thirty-four PB Ca and benign pancreaticobiliary disease patients were recruited for this study,and their duodenal juice was aseptically collected by endoscopy.The duodenal microbiota was analyzed,and the relative abundances of species in the duodenal microbiota were compared between PB Ca patients and benign pancreaticobiliary disease patients.The PB Ca diagnosability was compared between a conventional tumor marker and species in the duodenal microbiota with significantly different abundances in PB Ca patients vs benign pancreaticobiliary disease patients.
Research results
The abundances of cancer antigen 19-9 (CA19-9),Bifidobacterium,Clostridium cluster XVIII,and Prevotella were significantly different between PB Ca patients and benign pancreaticobiliary disease patients.The diagnostic capacity of Clostridium cluster XVIII was the highest among the four markers (CA19-9,Bifidobacterium,Clostridium cluster XVIII,and Prevotella).The combined assessment of Clostridium cluster XVIII and CA19-9 Levels was useful for PB Ca diagnosis.
Research conclusions
It was possible to investigate the microbiota of duodenal juice.Duodenal microbiota evaluation may contribute to the diagnosis of PB Ca.
Research perspectives
In the future,novel diagnostic and prognostic markers and treatments could be developed by investigating the relationship between the duodenal microbiota and PB Ca.
ACKNOWLEDGEMENTS
We thank all the staff at the Department of Gastroenterology of Fukushima Medical University,the Department of Endoscopy of Fukushima Medical University Hospital,and the Gastroenterology Ward of Fukushima Medical University Hospital.We also thank TechnoSuruga Laboratory for T-RFLP.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Management of obstructive colon cancer:Current status,obstacles,and future directions
- Role of endoscopic ultrasound in anticancer therapy:Current evidence and future perspectives
- Mesenchymal stem cell-derived exosomes for gastrointestinal cancer
- Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated
- Pancreatic intraductal papillary mucinous neoplasms:Current diagnosis and management
- Combined treatments in hepatocellular carcinoma:Time to put them in the guidelines?
