Combined treatments in hepatocellular carcinoma:Time to put them in the guidelines?
Zeno Sparchez,Pompilia Radu,Adrian Bartos,Iuliana Nenu,Rares Craciun,Tudor Mocan,Adelina Horhat,Mihaela Spârchez,Jean-François Dufour
Zeno Sparchez,Iuliana Nenu,Rares Craciun,Tudor Mocan,Adelina Horhat,3rd Medical Department,“Ïuliu Hatieganu” University of Medicine and Pharmacy,Institute for Gastroenterology and Hepatology,Cluj-Napoca 400162,Romania
Pompilia Radu,Department of Visceral Surgery and Medicine,Hepatology,Inselspital,Bern University Hospital,University of Bern,Bern 3010,Switzerland
Adrian Bartos,Department of Surgery,“Ïuliu Hatieganu” University of Medicine and Pharmacy,Institute for Gastroenterology and Hepatology,Cluj-Napoca 400162,Romania
Mihaela Spârchez,Department of Mother and Child,2nd Paediatric Clinic,“Ïuliu Hatieganu”University of Medicine and Pharmacy,Cluj-Napoca 400177,Romania
Jean-François Dufour,Department for BioMedical Research,Hepatology,University of Bern,Bern 3008,Switzerland
Abstract The time for battling cancer has never been more suitable than nowadays and fortunately against hepatocellular carcinoma (HCC) we do have a far-reaching arsenal.Moreover,because liver cancer comprises a plethora of stages-from very early to advanced disease and with many treatment options-from surgery to immunotherapy trials-it leaves the clinician a wide range of options.The scope of our review is to throw light on combination treatments that seem to be beyond guidelines and to highlight these using evidence-based analysis of the most frequently used combination therapies,discussing their advantages and flaws in comparison to the current standard of care.One particular combination therapy seems to be in the forefront:Transarterial chemoembolization plus ablation for medium-size non-resectable HCC (3-5 cm),which is currently at the frontier between Barcelona Clinic Liver Cancer classification A and B.Not only does it improve the outcome in contrast to each individual therapy,but it also seems to have similar results to surgery.Also,the abundance of immune checkpoint inhibitors that have appeared lately in clinical trials are bringing promising results against HCC.Although the path of combination therapies in HCC is still filled with uncertainty and caveats,in the following years the hepatology and oncology fields could witness an HCC guideline revolution.
Key Words:Hepatocellular carcinoma;Transarterial chemoembolization;Radiofrequency ablation;Microwave ablation;Systemic therapy;Immunotherapy combined treatments
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver cancer,ranking sixth overall among malignancies in incidence and,disproportionately,fourth in mortality[1].It is a multifaceted disease,atypical among cancers due to its intricate and non-linear prognostic indicators,as survival is closely intertwined with the tumor extension,the severity of the underlying liver disease,and overall fitness.To this point,there is a wide array of available techniques in the therapeutic arsenal against HCC.The options range from curative-intent solutions such as surgery or transplantation to local ablation,interventional radiology,and systemic therapies[2,3].However,despite recent advances and potentially game-changing developments,HCC is still associated with a poor prognosis,with an incidence to mortality ratio dismally approaching number one[4].
Currently,the most frequently employed algorithm for standardizing care is the Barcelona Clinic Liver Cancer Classification (BCLC) and its subsequent updates[5].Arguably the best algorithm to this point and backed by extensive validation,it still poses significant clinical dilemmas,especially for cases that do not fit perfectly in its boxes.In such scenarios,the comfort of evidence-based guideline recommendations tends to fade,leaving both the clinicians and the patients in uncharted waters,seeking the best path forward.
Consequently,multiple approaches have been attempted,combining available techniques in various shapes and forms with the ultimate goal of improving the overall outcome.These combinations try to negate the individual deficiencies of each method while augmenting their strengths,hoping to provide a perfect match for any specific clinical scenario.Ranging from an early tumor in an advanced,decompensated liver disease,to an advanced tumor in an otherwise relatively normal liver and anything in-between,the severity spectrum of HCC leaves room for epistemic,databased improvisation.However,as the range of therapies is ever-increasing,there is a thin line between being too conservative and overtreating,as both extremes could lead to additional harm and cost.The current review aims to provide an evidence-based analysis of the most frequently used combination therapies,discussing their advantages and caveats in comparison to the current standard of care.
COMBINED TREATMENT TO FACILITATE CURE
Hepatic resection plus intraoperative ablation
Although surgical resection (SR) still represents the ideal and best option as treatment,having curative potential,unfortunately for patients diagnosed with liver malignancies,this treatment is feasible in only 10%-20% of cases[6].The remaining 80%-90%of patients,which are not suitable for radical intervention,include patients with multiple tumors,located in both hepatic lobes with insufficient hepatic reserve[7].In the case of HCC,according to the guidelines used in clinical practice (BCLC staging system),the patients with unresectable multicentric neoplasia and preserved liver function fall into stage B,being candidates only for transarterial chemoembolization(TACE)[8].However,the results of various studies from the literature are beginning to support the use of combined techniques for this category of patients[9].According to the literature,there are several radical options for bilobar localization,including twostage resections,ALPPS technique (associating liver partition and portal vein ligation for staged hepatectomy),and combined techniques:Hepatic resections and ablative techniques[9].
Thereby,this complex treatment,which involves the combination of hepatectomies with simultaneous tumor ablations,has the role of increasing the proportion of patients who can become candidates for a radical,potentially curative treatment.However,although there are numerous reports in the literature underlining the clinical outcomes,a standard conduit and a therapeutic consensus in this field have not been yet established[6,10].
General indications for combined therapy are patients with multicentric,bilobar,unresectable neoplasms who are not candidates for curative resection but present with a compensated liver disease[11,12].Other indications include inoperable tumors due to proximity to major vascular structures and/or the presence of liver cirrhosis with functional liver parenchyma,but not being able to tolerate a major resection[6,10].Ablative techniques are indicated even if the tumors are in the proximity of a main portal branch,hepatic vein,or inferior vena cava[12].
The extrahepatic presence of neoplasia represents a contraindication for combined treatment,although there are authors who advocate for this treatment in the case of the associated resectable lung tumors or local invasion from liver tumors (in the diaphragm or adrenal gland)[9].The association of ablation is not indicated when the tumor involves the right or left liver duct[12].Incontestably,patients with decompensated liver disease,refractory ascites,coagulation disorders,and/or low-performance status (PS) cannot benefit from the combined resection-ablation treatment.
The intraoperative technique involves performing simultaneously,under general anesthesia and by laparotomy,both liver resections,and HCC ablation sessions.Most authors recommend performing resection first,followed by ultrasound-guided ablation,most commonly by tissue destruction by radiofrequency ablation (RFA)[6].Microwave ablation (MWA) comes with some advantages,these being cited by some authors,but with a lower usage than RFA[9].Although feasible,with comparable outcomes with “conventional” open surgery,the combined treatment performed by a laparoscopic approach is not a standardized technique,with only a few reports being found in the literature[13,14].
To exclude extrahepatic neoplasia it is mandatory to do a complete exploration of the entire abdominal cavity and intraoperative hepatic ultrasound (IOUS) to assess the topography and tumor relationships[15,16].Anatomical resections are preferred whenever possible[17].The indication for atypical,minor (1-2 segments),or major (> 3 segments) hepatectomy is determined by the preoperative assessment of the liver function,of the patient status,and by tumor extent.Also,to prevent blood loss,intermittent clamping of the afferent hepatic pedicle (Pringle maneuver) may be necessary.According to Qiuet al[6],it is required in less than 50% of cases[6].Another advantage of this maneuver is the reduction of the cooling effect induced by the proximity of tumors to main vascular branches (“heat sink”),the rate of achieving a complete ablation being higher[18].Evaluation of the necrotic area and the efficacy of RFA can be performed immediately by contrast-enhanced-IOUS (CE-IOUS),making possible repeated ablation sessions immediately[19].
Doing a literature survey guided by the words “hepatocellular carcinoma”,“combined”,“liver resection” and “ablative treatment”,using the PubMed database for titles in English published from 2010 to 2020,we found that the mortality reported for the surgery-ablative combined technique is around 1%-2% with a general incidence of complications in the range 22%-70%[6,20-25] (Table 1).
In general,the literature supports the combined technique as feasible and safe,although the cited recurrence rate is high[6,20,23] (Table 1).The rate of major complications is reported to be around 15%,with a rate of acute liver failure of 1.8% and postoperative bleeding of 0.9%[6,21].Other specific complications are biliary leaks(8.9%),postoperative ascites (11.6%),perihepatic abscesses (1.8%).0.9% of patients with post-operative complications may require reinterventions[6,21].No significant differences were reported between the rates of complications after combined techniques and conventional liver resections.Doing a literature survey guided by the words “hepatocellular carcinoma”,“combined”,“liver resection” and “ablative treatment”,using the PubMed database for titles in English published from 2010 to 2020,we found that the mortality reported for the surgery-ablative combined technique is around 1%-2% with a general incidence of complications in the range 22%-70%[6,20,21].
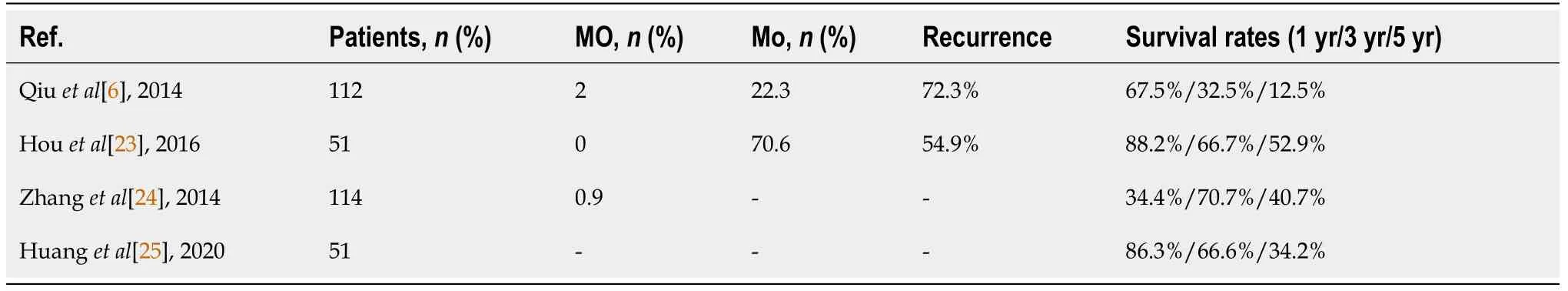
Table 1 Morbidity,mortality,recurrence and survival after hepatic resection plus intraoperative ablation
In regards to long-term survival,we found a range between 12% and 88%,depending on 1-,3- and 5-year survival reports (Table 1).As can be seen,there is a wide range of results,most likely explained by the lack of standardization of the combined procedures and by the contribution of the case selection bias.
Most of the authors concluded that whenever resection can be performed,it must be chosen instead of ablation.However,RFA remains a feasible alternative in cases that are not suitable for resection,except for large tumors,over 5 cm[22].Moreover,whenever possible,combined therapy should be indicated to the detriment of TACE,the latter being followed by a shorter 5-years survival:52.9% (combined treatment)vs9.8% (TACE)[23].
The results are more optimistic when comparing combined treatment with resections only,with overall survival (OS) at 1-,3- and 5-years being comparable between the two groups:86.3%,66.6% and 34.2%vs92.8%,67% and 37%,respectively (P=0.4)[6,21,24,26].
Supported by the data mentioned in the literature above,the combined treatment(resection plus ablation) is a feasible alternative to the therapeutic options already existing in current guidelines.The fact that more and more studies highlight the increase in the number of curative resections by using these techniques will certainly support their integration into the standard conducting algorithm for HCC.
Combination of locoregional therapies for early and intermediate stage including comparison with other therapies
Locoregional therapies (LRT) include TACE and local ablation techniques.Local ablation including percutaneous ethanol injections,RFA,MWA,laser- and cryoablation (CA) are considered alternative curative methods for early-stage HCC[27,28].As 75% of HCCs nodules are inoperable at the time of the diagnosis,TACE plays an important role in the management of unresectable HCC and is considered the first-line therapy for BCLC stage B HCC based on Barcelona Clinic Liver Cancer guidelines[27,28].
Limits of LRT and rationale for combination therapy:For some patients undergoing TACE procedure the tumor necrosis rate is low with consequent frequent tumoral residue and high intrahepatic recurrence[27,29].Along these lines there are several reasons for this limited efficacy:(1) The difficulty to embolize all the feeding arteries of the tumor;(2) Recanalization and angiogenesis which may occur after TACE with consequent tumor recurrence and metastasis;and (3) The re-establish of collateral circulation[27].Ablations techniques like RFA present a high performance in tumors below 2-3 cm where complete necrosis may be achieved in up to 90% of cases[21].However,RFA is less effective in medium-sized (3-5 cm) or large tumors (5-9 cm)where the efficacy dismally drops to 61% and 24% respectively[21].When RFA was performed for lesions located near major vessels a heat sink effect was reported leading to an increased recurrence rate as well.
In this regard,the location of the lesion is a crucial factor when considering ablation on the grounds that some lesions cannot be successfully treated with thermal ablation without damaging adjacent structures (bile ducts,colon,diaphragm)[30].Thereby,one intention-to-treat analysis found that 9% of small HCCs were not amenable to percutaneous ablation because of their location[30].
The rationale for combining TACE with ablation is to maximize the percentage of complete tumor response rate and thereby to reduce local recurrence rate due to incomplete or inadequate treatment of the adjacent hepatic parenchyma[9].As follows,the synergy of the therapies leads to larger volumes of destructed tumoral tissue with consequent efficient treatment of presumed microsatellite nodules and microvascular invasion[9,27].The sequencing of combined therapies is controversial,with most authors preferring TACE followed by RFA,although some prefer RFA followed by TACE or both techniques in the same session[9,27].The theoretical advantages of performing TACE before ablation include (1) TACE reduces hepatic artery blood flow,thus diminishing heat sink effects and maximizing the size of the ablation zone;and(2) TACE can detect satellite lesions not seen on cross-sectional imaging[9,27].
TACE combined with RFA vs TACE:There are several papers published on this combination with different clinical scenarios.The most important ones are presented in Table 2.
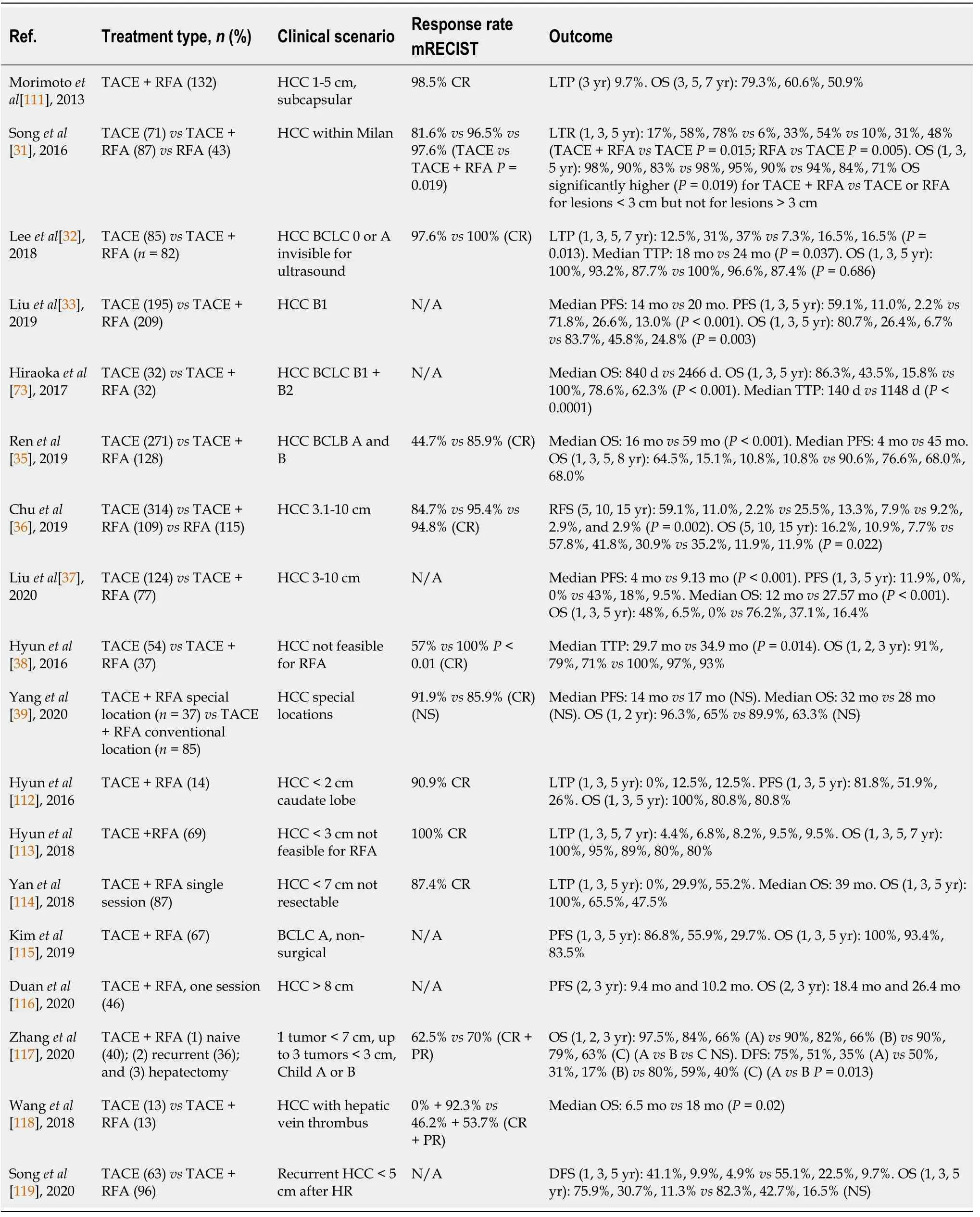
Table 2 Comparison of transarterial chemoembolization plus radiofrequency ablation to transarterial chemoembolization
By analyzing these studies,it was discovered that combination therapy might be beneficial compared to TACE alone,or RFA alone in several clinical scenarios.
BCLC-A patients:Songet al[31] have compared the results of 71 patients with HCC within Milan criteria treated by TACE to 87 and 43 patients treated by TACE + RFA and respectively RFA.The combination therapy yielded a significantly higher complete response rate according to mRECIST criteria in comparison to TACE (96.5%vs81.6%,P=0.019) and a lower rate of local tumor progression at 1,3 and 5 years (6%,33%,and 45%vs17%,58% and 78%).Nonetheless,what is surprising is the fact that OS was significantly higher for TACE + RFAvsTACE or RFA alone for lesions below 3 cm but not for lesions larger than 3 cm[31].Similar results were reported as well by Leeet al[32] with lower recurrence rate at 1 and 3 years 7.3% and 16.5%vs12.5% and 31% but no difference in OS[32].
BCLC-B patients:Liuet al[33] have compared a B1 HCC population treated by TACE(195 pts)vsTACE + RFA (209 pts) and found a significant difference in favor of combination therapy regarding progression-free survival (PFS) and OS[33].Also,the same results [significantly higher median OS-840vs2466 d and median time-to-tumor progression (TTP)-140vs1148 d] were communicated by Hirookaet al[34] although on a small sample of patients[34].Likewise,Renet al[35] have investigated a large BCLCA and B population treated either by TACE (271 pts) or TACE + RFA (128 pts) and concluded by all means that the combination therapy is significantly superior to TACE in terms of PFS and both median and cumulative OS[35].The superiority of TACE +RFA over TACE in terms of longer OS and PFS was demonstrated also for medium and large nonresectable HCCs in 2 large recent trials[36,37].
The combination therapy was assessed also in patients with small lesions not feasible for RFA and lesions in unreachable locations (e.g.,near the hepatic hilum,subdiaphragmatic,subcapsular).TACE procedures resolve this dilemma due to intratumoral accumulation of radio-opaque iodized oil used and give radiographic contrast to a small tumor either of poor conspicuity or even ultrasound (US) blind and also difficult to target spots such as hepatic dome[27].An undoubtedly higher complete response rate (100%vs54%,P<0.01) and lower TTP rate were revealed by Hyunet al[38] in a series of patients with HCC not feasible for US-guided RFA treated by TACE(54 pts) or TACE + RFA (37 pts)[38].However,Yanget al[39] compared the efficacy of combination therapy in 37 patients with HCC in special locations to 85 patients with HCC in convenient locations and found no differences in PFS and OS[39].Three metaanalyses concerning the comparison of TACE + RFAvsTACE have been already published[40-42].The last one issued in 2017 found that for intermediate-stage HCC,TACE plus RFA attained higher tumor response rates (OR=6.08,95%CI:4.00-9.26,P<0.00001),achieved longer recurrence-free survival (RFS) rates (ORRFS=3.78,95%CI:2.38-6.02,P<0.00001) and OS rates (OR1-year=3.92,95%CI:2.41-6.39,P<0.00001;OR3-year=2.56;95%CI:1.81-3.60;P<0.00001;OR5-year=2.78;95%CI:1.77-4.38;P<0.0001) when comparing to TACE alone.Unfortunately,as expected the number of complications were higher in the TACE plus RFA group than in the TACE alone group(OR=2.74,95%CI:1.07-7.07,P=0.04)[40].
The combination therapy TACE + RFA was also compared to RFA in several other meta-analyses[43-46].The last one including 8 trials and 648 patients showed that RFA plus TACE is associated with a significant advantage in RFS [hazard ratio (HR)=0.58;95%CI:0.42-0.80,P=0.001],and OS (HR=0.60;95%CI:0.47-0.76,P<0.001).The authors concluded that TACE combined with RFA is more competent and appealing than RFA alone,especially for intermediate and large-size hepatic tumors or younger patients with HCC[47].
TACE combined with RFA vs hepatic resection:According to the BCLC guidelines published in 2018,the mainstay of curative therapy for early HCC is RFA or surgeryeither HR or liver transplantation.As mentioned earlier,even though HR is a cornerstone treatment in non-metastatic disease,some patients are considered inappropriate candidates due to underlying liver disease[48].We have emphasized in the previous lines that the combination therapy of TACE and RFA is superior and beneficial for the treatment of early and intermediate HCCvsthe therapies used individually.Along these lines,the comparison of the outcome of TACE + RFAvsHR is of paramount importance as some of the patients are not ideal surgical candidates.Several comparative studies are presented in Table 3.
For small single HCC (2-3 cm) TACE + RFA have similar outcomes to HR in terms of disease free survival (DFS) and OS but with significantly lower complications rate and hospital stay[49].However,as expected for medium size and larger lesions tumor recurrence (75%vs35.4%,P=0.005) and local tumor progression (LTP) (55.7%vs16%,P=0.013) are significantly lower for HR[49].Moreover,it seems that for patients fitting the up-to seven criteria both treatment options provide similar DFS and OS.However for patients outside the Milan criteria but within up to seven criteria there was an increased median OS when HR was performed[50].For patients within the Milan criteria,Takuma and colleague reported similar DFS and OS,whereas Liuet al[51] found a significantly increased DFS and OS for patients who had undergone HR[51].In a recent study,Linet al[52] demonstrated a significantly higher 5 years OS(61.2%vs38.2%,P=0.009) for HR in patients with HCC BCLC-B[52].
Beyond a shadow of a doubt,the association of TACE with RFA has proved its benefit in terms of controlling much more suitably early and intermediate primary liver cancer compared to the therapies used alone and we consider that it has gained its place in the treatment of HCC.Moreover,combination therapy is also an important alternative when surgery is not feasible.
TACE combined with MWA:MWA is a dielectric heating technique that generates an electromagnetic field surrounding the needle tip,consequently producing the coagulation necrosis of the target area.Along with RFA,MWA is an established thermal ablation method,best-suited for treating early-stage HCC with curative intent.Being non-inferior to RFA in the standard guideline setting,MWA boosts some theoretical advantages:Higher efficacy in larger nodules,quicker heating,lower procedural time,and higher heating temperatures[53].Therefore,like RFA,MWA has often been combined with TACE in a multitude of clinical scenarios.These situations either fit outside the standardized BCLC boxes or aim to improve the relatively dim prognosis of the well-established path in intermediate or advanced HCC.
As addressed by Renzulliet al[54],intermediate-and advanced-stage HCC often leads the clinicians in the uncertain waters of an imperfect solution[54].The typical case resembles the following:Curative intent solution off the board,unsatisfactory gold-standard (TACE),and technically treatable nodules within ablative reach.This has led to a recent surge of interest with regards to combining TACE and MWA,with numerous papers published on this topic in the past decade.The most important articles are comprised in Table 4,which will provide the cornerstone for the upcoming discussion.
As shown in Table 4,there is a wide array of clinical scenarios in which the TACE +MWA approach was tested.However,the large majority of the data comes from the most heterogeneous class:Intermediate-stage,BCLC-B.However,most of the HCC disease spectrum stood for trial,ranging from small,solitary unresectable nodules,up to 10 nodules and more advanced BCLC-C tumors[55-58].The overall results were promising,if not always definitely positive.Therefore,both the empirical and the epistemological conclusions suggest that TACE + MWA can be safely employed whenever it is technically feasible with regards to tumor characteristics,vascularization,and percutaneous approach.
Three key aspects define therapeutic efficacy in HCC:Treatment response according to the mRECIST criteria,PFS,and OS.In this regard,the TACE + MWA combination appeared to exceed the standard of care in most of the clinical scenarios,in all three aspects (Table 4).Of course,the precise survival data varies widely,as the study designs were extremely heterogeneous,even within the same BCLC class.In intermediate-stage HCC,OS ranged from 17.1 mo[57] to 35 mo[59] as it reliably extended OS compared to TACE alone by 4 to 12 mo[56,57,60,61].To objectivize the apparent benefit,a meta-analysis was recently performed by Liuet al[62],comparing TACE with TACE + MWA in single and up to three HCC nodules exceeding 5 cm[62].The analysis included over 1700 patients,all from Chinese-conducted studies,and showed a significantly higher OS for the latter (1-year OS rate:RR=1.36,95%CI:1.28-1.44;2-year OS rate:RR=1.56,95%CI:1.40-1.74,and 3-year OS rate:RR=2.07,95%CI:1.67-2.57,P<0.001).However,when compared to other non-standard therapies,such as radiation segmentectomy[55] or CA[63] the differences in outcome were not statistically significant,which might further suggest the rather suboptimal standard of care for intermediate HCC.Other clinical scenarios have shown a benefit of combination therapy,as TACE + MWA + Sorafenib outperformed TACE +Sorafenib in off-guideline advanced HCC[58].However,it is unclear whether the available reports provide sufficient grounding for altering the current time-tested recommendations since no major randomized controlled trials are available.Furthermore,the overall quality of the data can be improved,as most studies are retrospective.

Table 3 Comparison transarterial chemoembolization + radiofrequency ablation to other curative therapies
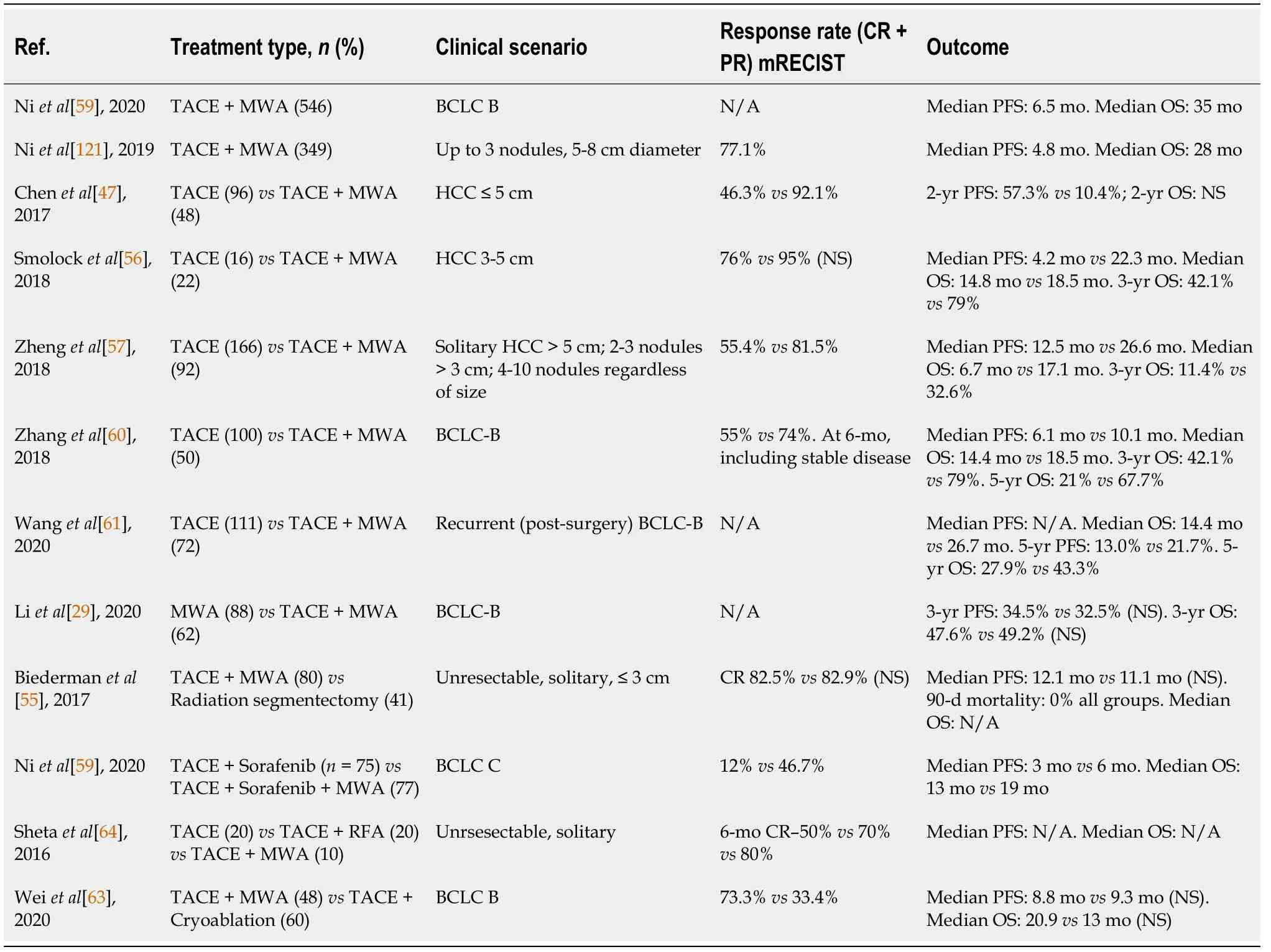
Table 4 Available studies on the transarterial chemoembolization plus microwave ablation
There are only a few small sample studies directly comparing MWA to RFA in combination with TACE,with no significant differences in major outcomes such as PFS and OS[64-66].However,subgroup analysis suggests a higher response rate in larger tumors for MWA.
Peravailable data,TACE + MWA appears to laterally exceed the BCLC-B stage.On one hand,it might secure substantial survival benefits for nodules not amenable to curative intent solutions.One such scenario might be large,borderline BCLC-A nodules,unfit for resection due to portal hypertension,yet fit for transplantation,but living in a low-transplantation rate medical system.On the other hand,for BCLC-C patients,TACE + MWA appears to bring survival benefits,especially when adding sorafenib into the mix.The further one navigates beyond BCLC-B,the lesser the strength of the data.Within BCLC-B though,TACE + MWA shines the brightest,appearing to add a substantial survival benefit[67].
TACE combined with other local therapies:In a RCT comparing CA + drug-eluting bead (DEB)-TACE to CA monotherapy in large tumors (mean:7.2 ± 4.5 cm and 6.5 ±3.8 cm,respectively),the combination group demonstrated superior OS [16.8 movs13.4 mo,respectively (P=0.0493)] and significantly increased PFS [8.1 movs6.0 mo,respectively (P=0.0089)].Interstitial laser therapy (ILT) uses optical fibers to create cytotoxic temperaturesviathe conversion of absorbed infrared light to heat with consequent coagulative necrosis[68].TACE followed by ILT was used in patients with tumors up to 8 cm that has obtained a median OS of 36 mo (95%CI:29.3-42.6)[69].
Stereotactic body radiation therapy (SBRT) can provide satisfactory local control with a low incidence of radiation-induced liver disease in patients with unresectable HCC that are not amenable to thermal ablation[68].Viaradiosensitization of tissue TACE/TAE may serve as targeting fiducials for SBRT.TACE + SBRT was compared to SBRT in a retrospective study including patients with tumors with a median size of 8.5 cm.An increased 5-year OS was reported in the combination armvsSBRT arm (46.9vs32.9%,P=0.047)[70].In addition to cytotoxic properties,SBRT seems to be a potent activator of the adaptive immune system,and thus might stand as an interesting player in the field of immunotherapy[68].
COMBINED TREATMENT TO ENHANCE PALLIATION AND INCREASE SURVIVAL
TACE + systemic therapies-the downfall of an era
As mentioned previously,current guidelines recommend local ablative therapies(RFA/MWA) for patients with early-stage HCC (BCLC-0) and TACE for patients with intermediate-stage BCLC-B or BCLC-A patients not suitable for resection or ablation[2].Unfortunately,many of these patients experience a progression of the disease and a worsening of the liver function after repeated sessions of percutaneous ablation or TACE[71].The OPTIMIS study,which assessed the outcomes of HCC patients treated with TACE alone,or with TACE followed by sorafenib found that the proportion of patients with a progressive disease increases with each subsequent TACE,while the objective response rates decline as the number of TACE sessions increased (first TACE:40%;second TACE:26%;third TACE:24%;and fourth TACE:25%)[72].Moreover,up to 30% of these patients experienced a deterioration of liver function.Similarly,Hiraokaet al[73] reported that the liver function deteriorated with repeated TACE[73].These findings emphasize the importance of the appropriate timing of switching from local therapy to systemic therapies to obtain the maximal benefits of other therapies and consequently improve the OS.
As a result of an exclusively arterial vascularization of HCC tumors and comprising the fact that the normal surrounding liver parenchyma is vascularized from branches of the portal vein,TACE and other image-guided transcatheter treatments were born to destruct arterial tumoral vessels and hence inducing tumor necrosis[74].TACE procedure is based on an intra-arterial infusion of a chemotherapy agent such as doxorubicin or cisplatin,frequently embedded in lipiodol as a vehicle to increase the availability of the drug.Furthermore,the tumoral blood vessels could be embolized with different agents such as gelatine sponge particles,metallic coils,polyvinyl alcohol,starch microspheres and autologous blood clots leading to an increased tumoricidal and ischemic effect[27].According to the European Association for the Study of the Liver (EASL),the median survival for untreated patients at an intermediate-stage [BCLC-B-multinodular disease,good PS,without vascular invasion or extrahepatic spread] is around 16 mo and in rigorously selected candidates TACE can increase the survival up to 3 years[2].
TACE causes local hypoxia in the tumor,building up an expression of hypoxia response genes in tumor cells regulated by hypoxia-inducible factor-1 alpha (HIF-1α).The response triggers vascular endothelial growth factor (VEGF) expression and thus leading to the formation of neovascularization,and thereby forming a vicious cycle leading to tumor recurrence and metastasis[75].In this regard,studies have conceded that dynamic changes in serum HIF-1α and VEGF levels occur after TACE in HCC patients[76-78].Along these lines,Jiaet al[76] investigated the expression levels of serum HIF-1α and VEGF before and after TACE and analyzed the correlations between prognosis factors and serum HIF-1α as well as VEGF levels[76].The serum HIF-1α and VEGF levels of HCC patients pre-TACE,1 d,1-wk,1-mo post-TACE were analyzed using ELISA and compared with that of 20 healthy volunteers[76].The study revealed that the expression levels of serum HIF-1α and VEGF in HCC patients were significantly higher than those in the control group.One day after TACE,both serum HIF-1α and VEGF levels reached the peak values.One-week post-TACE,expression levels of them were decreased,but still significantly higher than those before TACE.The levels of both HIF-1α and VEGF incomplete response group 1-mo post-TACE were significantly lower than those in partial response,stable disease,or progressive disease groups.Thus,HIF-1α and VEGF might be important predictors of TACE efficacy.
Along these lines,hepatologists and oncologists hypothesized whether the combination of systemic therapy and TACE might be beneficial in terms of survival in HCC patients.In an effort to address this problem,several trials with TACE and antiangiogenic therapies have emerged.Nevertheless,some challenges have arisen.Firstly,TACE is addressed to BCLC-B class patients which is a heterogeneous group due to the wide range of liver function (Child-Pugh A or B cirrhosis) and variable lesion number and dimension.Secondly,the use of chemotherapy,degree of selectivity and management of adverse effects have to be considered.The GIDEON trial,the first observational trial of more than 3000 patients with HCC BCLC A to C treated with sorafenib or in combination with TACE reported that sorafenib could be safely associated or used sequentially with TACE[79].Thereby,taking this assumption into account,several randomized controlled trials have been reported,as seen in Table 5.
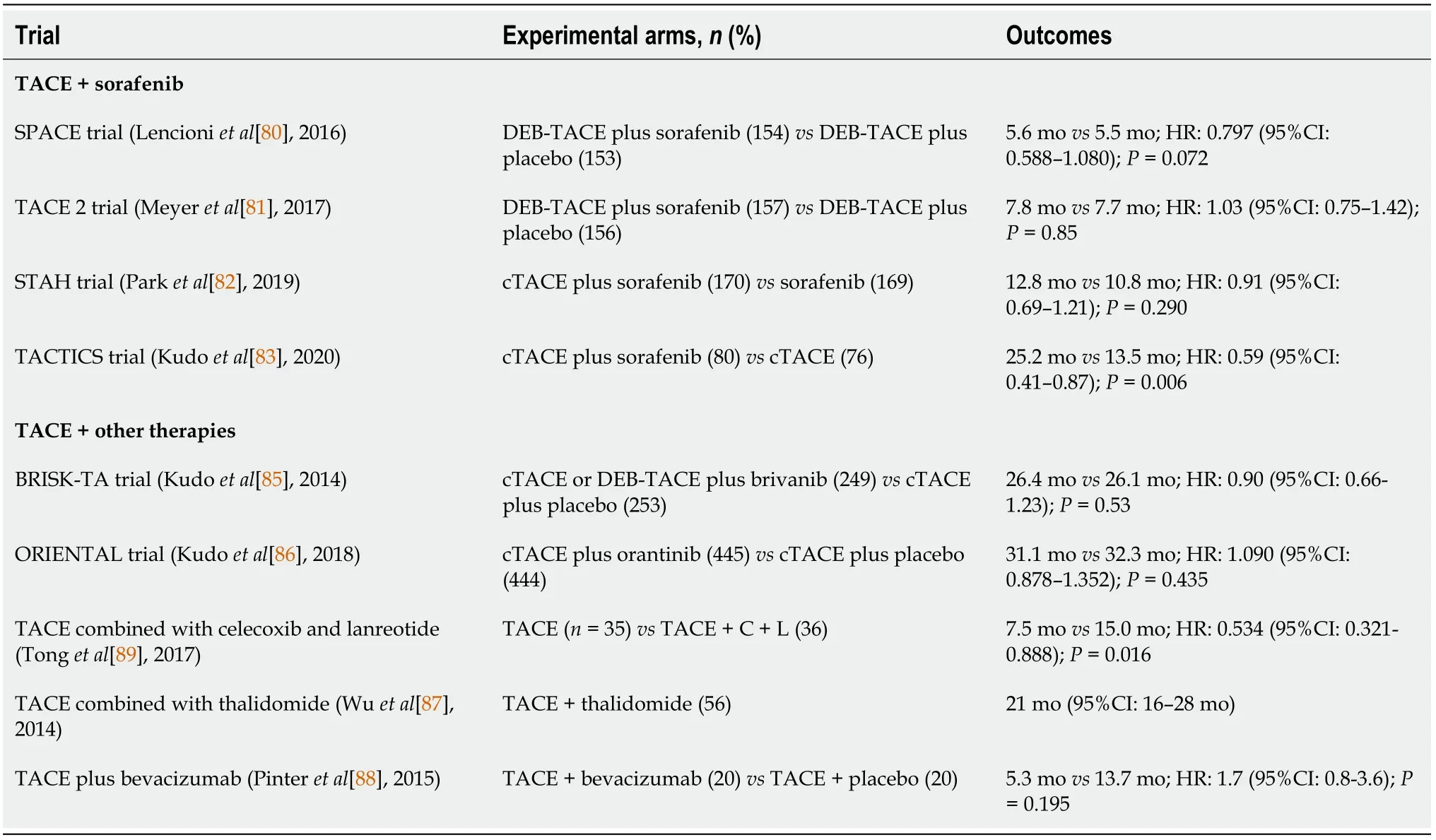
Table 5 Chemoembolization plus systemic therapies
SPACE prospective randomized phase II trial included 307 patients with BCLC-B HCC randomly allocated to DEB-TACE with sorafenib 400 mg twice daily and DEBTACE with placebo.Unfortunately,there was no difference in TTP between the 2 arms(169 dvs166 d in the sorafenib and placebo arms,respectively,P=0.072) and no impact on OS (P=0.29) was observed[80].
A year later a phase III trial of TACE with sorafenib (TACE-2) from the United Kingdom emerged and included 313 patients randomized to sorafenib or placebo with DEB-TACE 2-5 wk later and additional TACE on demand[81].The study aimed to reduce the adverse effects induced by combination treatment and to increase the prospect of continuing the drug at the time of the TACE procedure.Sadly,as in the SPACE trial,this RCT was also negative with a median PFS of 7.9vsonly 7.8 mo in the sorafenib and placebo arms,respectively (P=0.94),and median OS of 21.1 and 19.7 mo in the sorafenib and placebo groups,respectively (P=0.57).Moreover,discouraging results were also reported by the STAH trial in 2019 when comparing the combined treatment with sorafenib alone.The authors justify their results due to delays in starting sorafenib after TACE and/or low daily sorafenib doses[82].
TACTICS trial was the only phase II RCT that attested to the benefits of TACEsorafenib synergy and met its primary endpoint for the treatment of intermediate stage HCC[83].The authors reported a median PFS significantly longer in the TACE plus sorafenib groupvsTACE alone group (25.2vs13.5 mo;P=0.006).Moreover,the innovation of the trial stands in the modification of PFS,defined as time-tounTACEable progression (TTUP),characterized as untreatable tumor progression,transient deterioration to Child-Pugh C,or appearance of vascular invasion/extrahepatic spread.Patients in the combination group received sorafenib 400 mg once daily for 2-3 wk before TACE,followed by 800 mg once daily during on-demand conventional TACE sessions until time to untreatable TTUP.Howbeit,a further analysis conceded that the TACTICS trial did not show an improved OS in the combination group as compared with TACE alone although significantly better PFS was consistently observed.However,the OS in TACE plus sorafenib arm showed the longest OS (36.2 mo) with the longest ΔOS (5.4 mo) as compared with the previous TACE combination trials[84].The authors explain that the major reason for the negative OS result was due to many post-trial active treatments (other systemic treatments and immunotherapy agents) performed in the sorafenib group (76.3%),which implies that the OS endpoint in the TACE combination trial may not be feasible anymore in the current era of personalized medicine and immunotherapy.
It seems likely that other TKIs (brivanib,orantinib) and thalidomide derivatives as well combined with conventional TACE failed to meet the primary endpoint of OS[85-87].Moreover,the addition of bevacizumab to TACE raised some safety concerns related to sepsis and vascular complications of the combination treatment[88].Nevertheless,encouraging results were published by Tonget al[89] which have compared TACE alone with TACE combined with the selective COX-2 inhibitor,celecoxib and the somatostatin analog,lanreotide in advanced HCC[89].The patients receiving the combination therapy had a median OS of 15 mo compared to 7.5 mo for those receiving TACE alone,and a subgroup analysis of advanced patients demonstrated an OS of 13 mo for the combination and 4.5 mo for TACE alone (P=0.013).Likewise,encouraging results published in 2020 confirmed that the use of lenvatinib-TACE sequential treatment after progression during lenvatinib therapy was associated with better post-progression survival (HR=0.08;95%CI:0.01-0.71;P=0.023)[90].
Overall,up to this point,the literature has failed to support the use of multi-kinase inhibitors in combination with TACE.However,with the emergence of immunotherapy,combined strategies are encouraged.Moreover,because intermediate stage liver cancer is very heterogeneous a personalized approach is the key to a better outcome for patients.
TACE + immunotherapy-a new beginning?
It has been noted for several years that LRTs result in the release of tumor antigens,which are taken up by antigen-presenting cells (mainly dendritic cells) and which have been shown to activate a tumor-specific immune response a[91,92].This evidence suggests that l LRTs may boost the response to immune-oncology drugs.Preliminary results of the phase I/II PETAL clinical trial (NCT03397654) showed good tolerability of pembrolizumab after TACE without cumulative side effects.Additionally,several clinical trials testing immune checkpoint inhibitors (ICI) as neoadjuvant or adjuvant therapy in patients treated with LRTs are currently running (Table 6).
However,despite the strong antitumor response induced by ICI,not all patients experienced an objective response[93].Currently there is no biomarker to predict response or resistance to immunotherapy in HCC.Emerging evidence revealed that VEGF is not only a proangiogenic factor but that VEGF also plays an important role in the development of the immunosuppressive tumor microenvironment (e.g.inhibition of dendritic cell maturation,accumulation of dendritic cell maturation,accumulation of myeloid-derived suppressor cells and induction of T reg cells).Voronet al[94]showed that targeting VEGF-A can decrease the VEGF-induced expression of inhibitory receptors mediating CD8+T cell exhaustion[94].Given these results,the association of anti-angiogenic therapy [e.g.inhibitors of VEGF (bevacizumab) or TKIs(lenvatinib)] with ICI seems to overcome tumor-intrinsic resistance to immune checkpoint blockade.
LRTs are minimally invasive therapies;however,it has become clear that the treatment regimens adopted 15 years ago will change in the next few years.In the light of new evidence,three groups might benefit from the combined therapy (locoregional therapy and immunotherapy),including (1) Patients with a high risk of recurrence after a complete response by local ablation;(2) Patients who progressed under TACE;and (3) Patients with poor predictors of response to immunotherapy,when such predictors will be validated,possibly including NAFLD as underlying liver disease.
Currently,there are several trials underway evaluating different combinations (ICI± anti-angiogenic therapy) as options for patients treated with LRTs (Table 6).It is important to remember that immunotherapies represent a two-edged sword,thus we must find the right timing,dose and combination of immunotherapy for a robust response and minimal side effects.
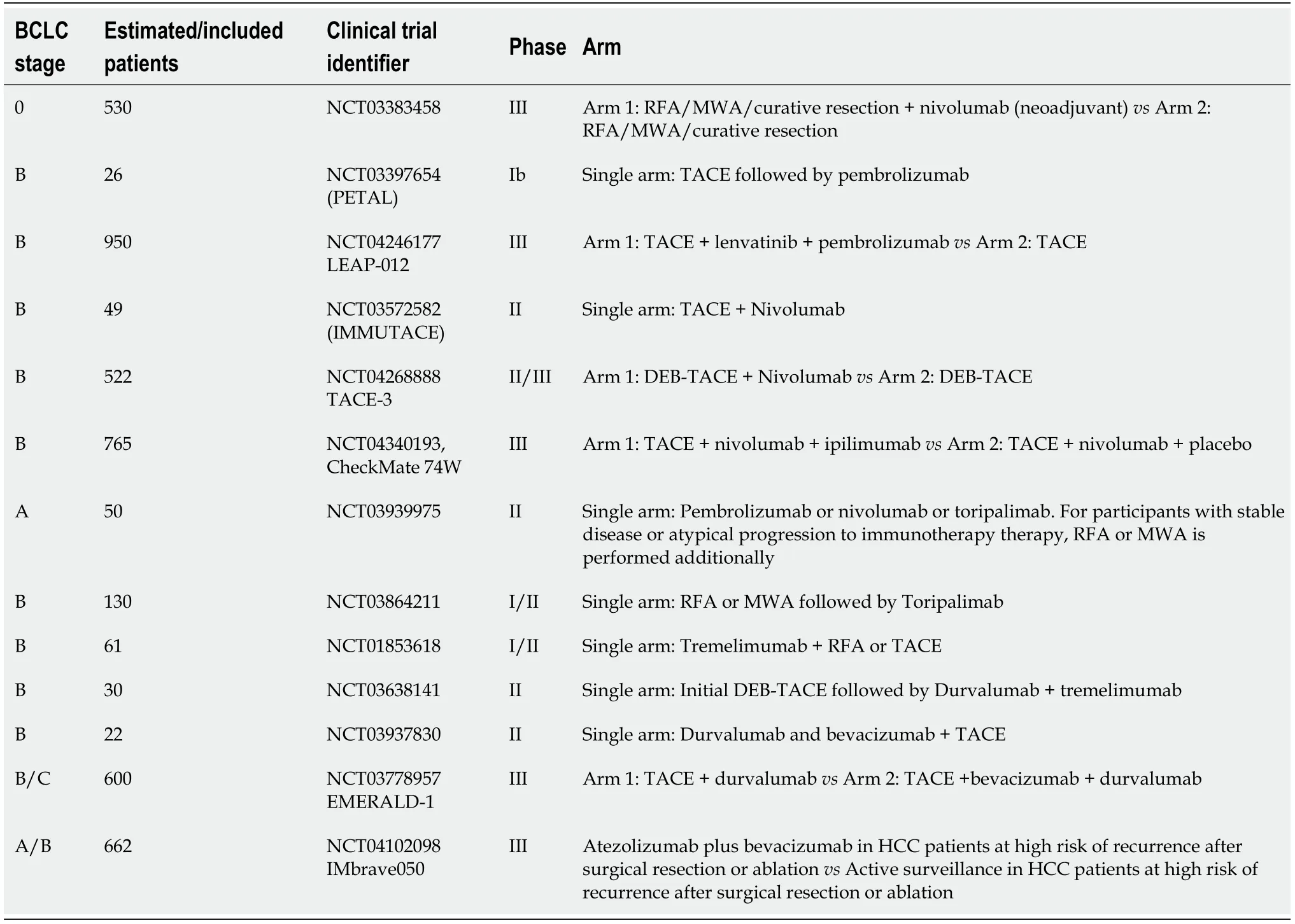
Table 6 Summary of ongoing clinical trials evaluating combination therapy of immune checkpoint inhibitors with locoregional therapies
Ablation + other treatments
HCC is an attractive target for immunotherapy due to several reasons:(1) Usually,HCC develops on a background of chronic inflammation (cirrhosis or chronic hepatitis);(2) In the context of cirrhosis there is an immunosuppressive environment;and (3) Immune evasion was described in patients with liver cancer[95].
Combining thermal ablation with immunotherapy is a very appealing approach.Thermally-induced necrosis can act as a permanent source of tumor antigens,the sublethal zone around the necrotic zone can generate inflammatory cytokines,and the thermal stress is capable of making HCC cells more sensitive to immune therapies[48,96,97].The field of immunotherapy in HCC (different from other cancer entities) was only recently unraveled.However,some preliminary studies with RFA and immunotherapy combinations (immune-ablation) have been already published.When used in a palliative setting,tremelimumab (anti-CTLA4) in combination with RFA or TACE in 32 HCC patients showed indeed interesting results.The patients received a total of six doses of tremelimumab at a 4-wk interval followed by an intentionally incomplete RFA or TACE to induce anti-tumor response at the ablation tumor junction.Patients with clinical response had an increase in CD8+ T cells in tumor biopsies obtained 6 wk after treatment.More interestingly,some patients experienced tumor responses in untreated lesions[98].In another study,Maet al[99] injected RetroNectin activated killer cells 14 d after RFA in patients with an HCC less than 4 cm[99].They reported no severe adverse events,recurrences,or deaths during a seven-month follow-up.Using a similar approach,Cuiet al[100] studied the combination of RFA and cellular therapy.Mononuclear cells from 30-HCC patients (early,intermediate and advanced stage)were harvested and induced into natural killer cells,γδT cells and cytokine-induced killer (CIK) cells which were subsequently infused back into RFA-treated patients for three or six courses.The combination improved PFS and reduced HCC recurrence compared to RFA alone[100].
Adjuvant treatment for HCC patients is an unsolved medical need.Using cellularbased immunotherapy Leeet al[101] studied the use of CIK cells injected after RFA (n=69),ethanol injection (n=13),or surgery (32) in patients with early-stage HCC[101,102].They reported a better OS and cancer-specific survival in patients treated with a combined approachvsthose treated with RFA,percutaneous ethanol injection (PEI),or surgery alone (P=0.006 andP=0.02).Similar findings were also reported in one multicentre randomized open-label phase 3 trial of adjuvant immunotherapy with CIK cells.The study included 230 patients with HCC treated by SR,RFA,or PEI.Patients were assigned randomly to receive immunotherapy or no adjuvant therapy.Adjuvant CIK cell therapy increased both recurrence-free survival and OS[103].
The use of monoclonal antibodies in combination with RFA was also studied in HCC.Either injected during RFA (131I-chTNT) or after RFA (131I metuximab) in an adjuvant setting both combinations showed improved PFS or OS[103,104].More data about studies investigating the combination of RFA with different immunotherapy strategies are depicted in Table 7.
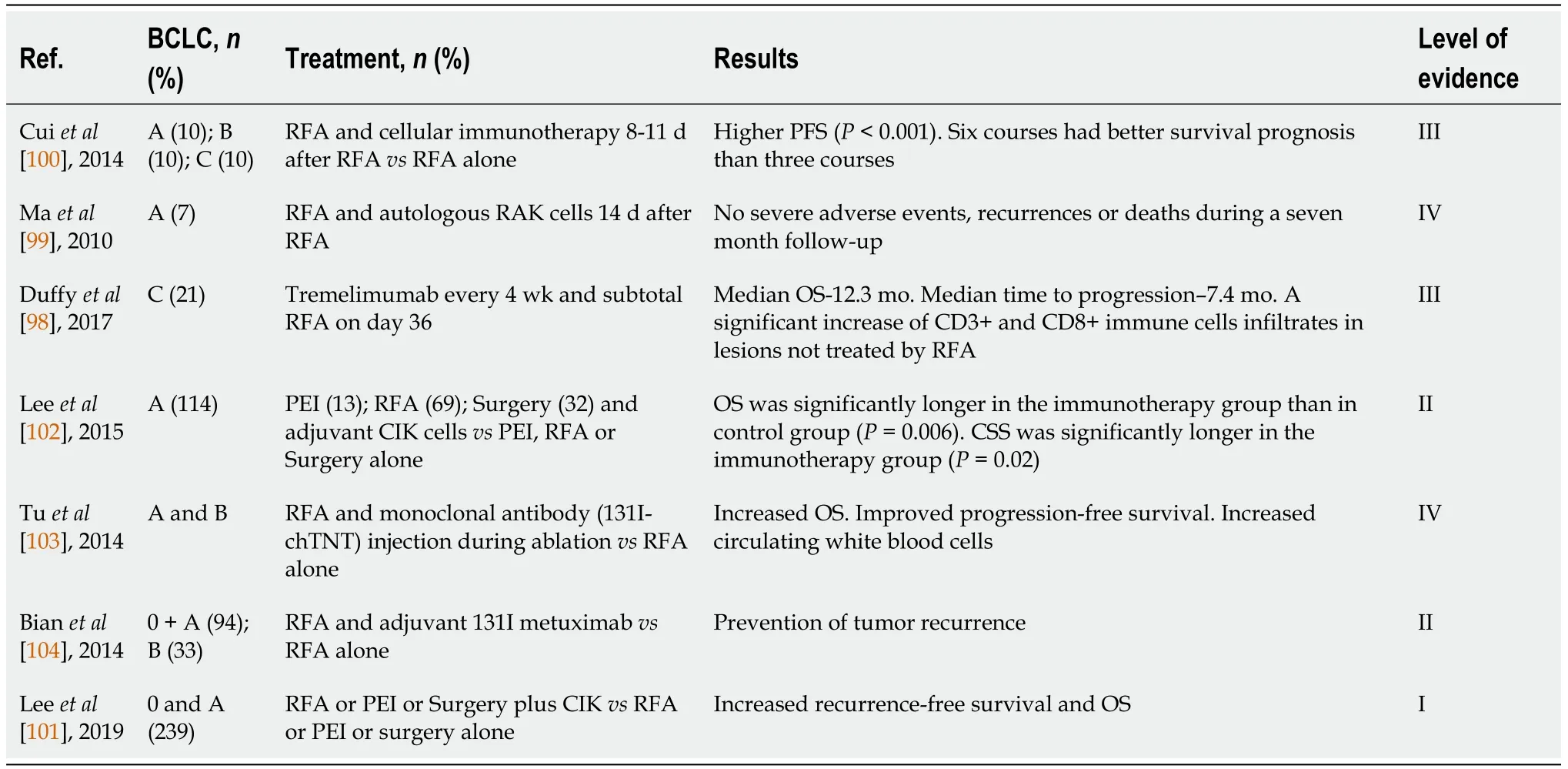
Table 7 Radiofrequency ablation combined with immunotherapy
Nevertheless,the treatment of HCC will change over the following years.Not much has changed in the last 20 years but the era of immunotherapy has started and we will probably witness groundbreaking changes in the years that will come.New treatments,new guidelines,from single option to multiple options and from RFA to“immune-ablation” the burden has moved from scientist to clinicians:It is an interesting world out there.
Assuming similar suppositions with the TACE procedure,RFA was also combined with VEGF inhibitors.One study showed that Bevacizumab is useful in preventing the rapid progression of residual HCC following RFA in a rat model[105,106].EMERALD-2 is an ongoing A Phase III,multicenter study of Durvalumab monotherapy or in combination with Bevacizumab as adjuvant therapy in patients with HCC who are at high risk of recurrence after resection or RFA (NCT03847428).Last but not least,nanoparticle-mediated drug delivery systems have also gained ground in oncology.The lyso-thermosensitive liposomal doxorubicin (LTLD) treatment aims to deliver doxorubicin at the peripheral thermal ablation zone,where the thermal elevation is suboptimal.When heated to 40 °C,LTLD releases a 25-fold greater concentration of Doxorubicin.The HEAT study is a global randomized,double-blind,dummycontrolled trial comparing RFA plus LTLDvsRFA alone that enrolled 701 patients with ≤ 4 unresectable up to seven HCC lesions.No differences in PFS and OS were found.The subgroup post hoc analysis showed improved efficacy when the thermal ablation indwell time for a solitary lesion was ≥ 45 min and increased treatment timepertumor volume was associated with better OS in the RFA + LTLD group[107,108].The subsequent phase III OPTIMA study (NCT02112656) was halted in the interim analysis for futility reasons.
An exhaustive report about the combination of different tyrosine kinase inhibitors,distinctive ICI,or even their combo in the advanced liver cancer setting is beyond the scope of our review.However,we consider it far-reaching to mention one of 2020’s revolutions-the association of bevacizumab (VEGF inhibitor) with atezolizumab (PDL1 inhibitor) that brings encouraging data for unresectable HCC patients[109].
Although the combination of TACE and TKI’s seemed promising in terms of inhibiting hypoxia-activated tumoral growth factors,studies do not appear to benefit any amalgam therapy compared to TACE monotherapy.To such a degree,one might say that the association of TACE and TKI’s might have seen its downfall.Hence,the attention of the hepatology and oncology community was diverted to a new starimmunotherapy.In both the association with TACE and ablation,ICI have quietly demonstrated their benefit in trials.Although this path is still filled with uncertainty and caveats,in the following years we will witness an HCC guideline revolution.
THE PLACE OF COMBINED THERAPY IN THE BCLC/EASL-HCC GUIDELINES
In tumor boards across the world,the most debatable section of the BCLC classification appears to be ranging from non-resectable BCLC-A to the less severe spectrum of the BCLC-C class,which is,by excellence,the appanage of interventional therapies[110].Conventionally,the available treatments are dichotomized in curative intent and,possibly mislabelled,palliative therapies.The first group comprises RFA,MWA,PEI,CA,irreversible electroporation,and the latter includes bland trans-arterial embolization,conventional TACE,DEB-TACE,and endovascular radiotherapyselective internal radiation therapy[2,3].
However,the basis for the aforementioned dichotomy might be fading,as research published in the past decade has shown that combination therapy is at least technically feasible,with the most relevant results being discussed in the previous sections.This has prompted a discussion with regards to the place of combination therapy in the therapeutic algorithm,as some of the approaches might be suited for second-,or even first-line choices for a select group of patients.On the other hand,it might be important to recognize that over-complicating a relatively straightforward algorithm could lead to disputable therapeutic choices and widespread heterogeneous interpretation,rendering data collection difficult.
As discussed earlier,one particular combination therapy appears to stand-out among other approaches:TACE-ablation for small,non-resectable HCC (3-5 cm),which is currently at the threshold between BCLC-A and -B.Not only does it improve outcomes in comparison to each individual therapy alone,but,according to limited data,it also appears to generate outcomes similar to surgery,which otherwise would have not been available[30].A proposed alteration of the BCLC classification,which speculates on the potential role of combination therapies based on the available data previously discussed,is shown in Figure 1.
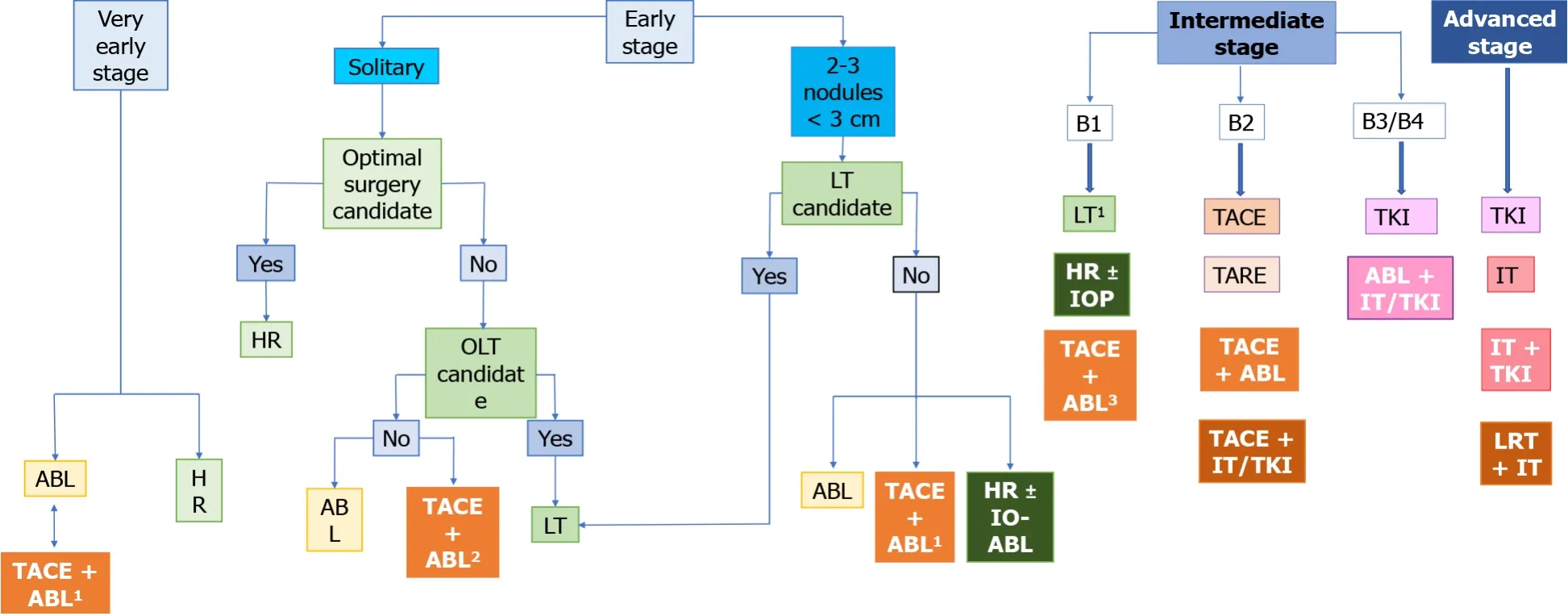
Figure 1 The place of combined therapy in the Barcelona clinic liver cancer classification algorithm.
CONCLUSION
Of course,our proposal is based on the best available data and still needs further consensus validation,but might provide a foundation for future recommendations,as well as hinting towards potential areas of future development.There is a great need for well-designed,large-scale randomized controlled trials to adequately assess the benefits of combination therapy.Moreover,there are multiple nuances open for debate.Which is the best radiological method for combination therapy:Bland TAE,TACE,or DEB-TACE? Which ablative technique has the most benefits? Should treatments be applied in the same session or sequential? Which is the best sequence?The authors strongly believe that methodically addressing these questions could ultimately lead to a truly personalized approach,hoping to improve the quality of life and OS of HCC patients.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Management of obstructive colon cancer:Current status,obstacles,and future directions
- Role of endoscopic ultrasound in anticancer therapy:Current evidence and future perspectives
- Mesenchymal stem cell-derived exosomes for gastrointestinal cancer
- Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated
- Pancreatic intraductal papillary mucinous neoplasms:Current diagnosis and management
- Unique situation of hepatocellular carcinoma in Egypt:A review of epidemiology and control measures
