Macrophages play a role in inflammatory transformation of colorectal cancer
Lu Lu,Yu-Jing Liu,Pei-Qiu Cheng,Dan Hu,Han-Chen Xu,Guang Ji
Lu Lu,Yu-Jing Liu,Pei-Qiu Cheng,Han-Chen Xu,Guang Ji,Institute of Digestive Diseases,Longhua Hospital,Shanghai University of Traditional Chinese Medicine,Shanghai 200032,China
Dan Hu,Han-Chen Xu,Shanghai Pudong New Area Hospital of Traditional Chinese Medicine,Shanghai 200120,China
Abstract Colorectal cancer (CRC) is one of the most common and fatal cancers worldwide,and it is also a typical inflammatory cancer.The function of macrophages is very important in the tissue immune microenvironment during inflammatory and carcinogenic transformation.Here,we evaluated the function and mechanism of macrophages in intestinal physiology and in different pathological stages.Furthermore,the role of macrophages in the immune microenvironment of CRC and the influence of the intestinal population and hypoxic environment on macrophage function are summarized.In addition,in the era of tumor immunotherapy,CRC currently has a limited response rate to immune checkpoint inhibitors,and we summarize potential therapeutic strategies for targeting tumorassociated macrophages.
Key Words:Colorectal cancer;Macrophages;Inflammatory transformation;Tumor microenvironment
INTRODUCTION
Colorectal cancer (CRC) is a common malignant tumor.Changes in bowel habits and stool characteristics,abdominal discomfort,thigh lumps,intestinal obstruction,anemia,and other systemic symptoms can be related to disease progression,but no obvious clinical manifestations are present in the early stage[1].According to the latest statistics by the American Cancer Society,the incidence and mortality of CRC rank third among all malignant tumors[2].The latest statistics on cancer from China show that CRC has become the third most common cancer in terms of incidence,with the fifth highest mortality rate[1].Most patients are already in a moderate or advanced stage when they are diagnosed,which imposes a great burden on their family and society.Therefore,early detection and screening,correct diagnosis of CRC,and early intervention and treatment to slow down the progression of the disease are particularly important.In recent years,an increasing number of studies have shown that macrophages play an important role in the occurrence and development of CRC.This article will review the research status of intestinal macrophages,the role and regulatory factors of tumor-associated macrophages (TAMs),and the research progress related to targeted TAM therapy to provide new ideas for the clinical diagnosis and treatment of inflammatory bowel disease and CRC.
INTESTINAL MACROPHAGES
Macrophages play an important role in intestinal inflammatory immunity,injury repair,epithelial-mesenchymal transition,and tumor development.Traditionally,macrophages differentiate from monocytes and play an immunomodulatory role[3].Further study found that there are two main sources of intestinal macrophages:Gutresident macrophages (gMacs) and monocytes (monocyte-derived macrophages).Resident tissue macrophages (RTMs) are derived from embryonic precursors,which accumulate in tissues before birth and are maintained by renewal in adulthood[4].In contrast to the self-renewal and self-maintenance of Kupffer cells and microglia,whether the gMac population is maintained by contributions from mononuclear macrophages is not clear.Although traditional studies have concluded that embryonic macrophages in the intestinal tract are replaced by bone marrow-derived Ly6Chi monocytes in a microorganism-dependent manner,an experiment evaluating intestinal macrophage heterogeneity determined that the self-sustaining population of macrophages is produced by embryonic precursors and adult bone marrow-derived monocytes,which persist throughout adulthood,and that these cells settle in specific niches,including the vascular system,submucosa,muscular plexus,sites of Pan’s cells,and Peyer’s patches.Single-cell analysis has shown that gMacs have a unique transcriptional profile,which supports the vascular structure and permeability in the lamina propria (LP) and also regulates neuronal function and intestinal peristalsis in the LP and muscularis externa[5].
Origin and differentiation of intestinal macrophages
The gene expression profiles of macrophages in tissues and sites vary[6].Although no study has shown that the origin changes the macrophage life span or biological functions[7],recent studies have shown that macrophage origin influences the gene expression profile[8,9].After treatment with chlorophosphate liposomes,mice with a monocyte-derived Kupffer cell population reacted more acutely to excessive paracetamol than mice with an intact embryonic Kupffer cell population.However,this functional difference might also be attributed to tissues because the difference disappeared after monocyte-derived Kupffer cells were placed in the liver for 60 d without an overdose of acetaminophen[10].Epigenetic analyses show that macrophages of different cell origins are relatively similar and are mainly influenced by living tissues.There are some epigenetic differences among macrophages derived from different precursors,which may be related to the changes in the local tissue environment caused by whole body irradiation[8].To date,it is necessary to explore the differences in epigenetics and function,not only origin,among different macrophages in detail.
Surface markers of intestinal macrophages
The unique transcriptome of tissue macrophages endows different functions to these cells and allows them to play specific biological functions in the microenvironment[11-13].
Some of the main challenges in this field are to identify intestinal macrophages and their subgroup markers and determine how to regulate these cells to meet the biological functional requirements of their living environment.In mice,F4/80 is the best and most commonly used marker to identify macrophages[14].However,conventional dendritic cells (cDCs) and eosinophils can also express F4/80[15,16].Intestinal macrophages highly express CD11C and MHCII,which can identify cDCs and are related to the polarization of M1 macrophages[17].However,intestinal macrophages also express CD206 and CD163 but do not express arginine[18].Therefore,intestinal macrophages are not suitable for M1 and M2 typing.The identification of intestinal macrophages requires a multiparameter method.
gMacs and cDCs can be distinguished by CX3CR1 and CD64 in combination with CD11C and MHCII.Compared with cDCs,gMacs highly express the chemokine receptor CX3CR1[18,19],which is mainly located in the LP of the intestine,connective tissue under the skin,intestinal wall,submucosa,and muscle[19-22].CX3CR1 is a key regulator of macrophage function in the inflammatory state[23,24],while the CX3CR1+myeloid cell-Treg axis plays a central role in maintaining intestinal homeostasis[25].CX3CR1+macrophages resident in the mucosa can recruit and activate antigenpresenting cells displaying epitopes to CD4+T cells and B cells at an invasion site[26],effectively inhibiting the production of IL-17 by CD4+T cells by promoting Treg activity dependent on IFN-β[27].Although there are reports that IFN-β can inhibit the production of IL-17 in mouse and human CD4+T cells,the mechanism is not clear[28,29].The expression of CD11c differs among gMacs at different sites;CD11c+gMacs are enriched in the LP,while CD11c-/loCX3CR1higMacs are enriched in the muscle[29,30].LP gMacs actively participate in host defense,maintain the integrity of the barrier,have high phagocytic activity,promote the constitutive secretion of interleukin-10 (IL-10),maintain FoxP3+T cells,and protect mucous membranes[31].The development and survival of CD64+mononuclear phagocytes are highly dependent on colonystimulating factor 1 (CSF1),while CD64-CD11c+MHC II+mononuclear phagocytes,which are highly dependent on the CDC-specific growth factor FLT3[32],migrate to the mesenteric lymph nodes (MLNs) and participate in the initiation of T cell responses in a CCR7-dependent manner[33,34].An experiment evaluating Tim-4- and CD4-labeled gMacs also provided evidence for the development and heterogeneity of intestinal macrophages[35].However,the function of these cells is not clear.Tracking CD64+gMacs with YFP in hybrid offspring from Cx3cr1CreERT2mice and Rosa26-LSLYFP mice successfully identified self-sustaining gMac subsets[5].
Intestinal macrophages under steady-state and inflammatory conditions
Intestinal macrophages are the main participants in establishing and maintaining intestinal homeostasis.gMacs produce a variety of cytokines and mediators (PGE2,BMP2,WNT ligand,etc.) to maintain the proliferation of intestinal epithelial cells and the physiology of intestinal neurons and endothelial cells[36].gMacs also promote the expansion of antigen-specific CD4+CD25+regulatory T cells by producing IL-10,prevent inflammatory reactions in the microbial environment,and support intestinal tolerance[37].Intrinsic receptors (including LPS (CD14),fcα (CD89),fcγ (CD64,CD32,and CD16),Cr3 (CD11b/CD18),and Cr4 (CD11c/CD18)) are not expressed in gMacs[38].gMacs also lack trigger receptors expressed on myeloid cells 1 (TREM-1)[39],which is a cell-surface molecule expressed on neutrophils and monocytes/macrophages in the peripheral blood.The activation reaction mediated by TREM-1 can increase the expression of proinflammatory mediators (such as TNF,IL-1β,and IL-6)and upregulate the levels of cell-surface molecules (CD40,CD86,and CD32)[40],leading to oxidative stress.Therefore,when intestinal macrophages play an effective scavenging role,they usually do not induce inflammation or damage intestinal homeostasis.
Monocytes and macrophages can induce cytotoxicity and proinflammatory mediators,eliminate apoptotic and damaged cells,and promote tumor progression when tissue is damaged[41,42].The CCL2-CCR2 axis plays an important role in the migration of monocytes from the bone marrow to the peripheral blood.CCR2-deficient and CCR2-positive mice have been widely used in the study of monocytes and monocyte-derived cells in the development of tissue damage and elimination of pathogens[43,44].During inflammation,the transportation of CCR2-/-monocytes to the small intestine is obviously decreased,but interestingly,the recruitment of circulating monocytes to other tissues,such as the liver and spleen,is not affected by CCR2 deficiency[45].Silencing CCR2 also significantly reduces repaglinide tolerance,which may be related to the stability of β-catenin regulated by AKT/GSK3[46].Recent studies have shown that the exogenous antiaging factor Klotho can inhibit the progression of CRC by inhibiting the expression of CCL2[47].The chemokines CCL2 and CXCL12 synergistically induce M2 macrophage polarization[19].Targeting CCL2/CCR2 without affecting transport to other tissues provides new hope for the treatment of CRC.
Under steady-state conditions,monocytes gradually differentiate into CX3CR1himacrophages that express genes related to the function of tolerant macrophages.According to the expression of Ly6C and MHCII,monocytes and macrophages in the small intestine can be divided into three subgroups:Ly6C+MHCII-,Ly6C+MHCII+,and Ly6C-MHCII+.Based on the expression of CX3CR1,Ly6C-MHCII+cells can be divided into CX3CR1intand CX3CR1hicells,which can reflect the different stages of monocyte differentiation in the small intestine and colon[18,48,49].Transcriptomic analysis also shows significant differences in gene expression among different stages.In addition to CX3CR1,the expression of CD64,CD11c,and CD206 increases with the development of Ly6C+MHCII monocytes into small intestinal Ly6C-MHCII+CX3CR1himacrophages.In contrast,monocytes immediately adapt to different expression patterns in a TREM-1-dependent manner after they enter the intestine in an inflammatory state.Inflammation fundamentally changes the kinetics and mode of monocyte differentiation in tissues[45].In contrast to intestinal homeostasis,inflammatory injury results in the accumulation of Ly6C+monocytes in large numbers.In a study,the expression of CD64 was high,while that of CX3CR1 was always low.On the third day of inflammation,CD64+ Ly6C-MHCIIintmonocytes were divided into two subsets:MHCIIhiCX3CR1int(seen in the inflamed colon)[50]) and MHCII.In the Ly6C-MHCIIintpopulation,the CX3CR1 expression level was slightly higher than that in the Ly6C+MHCII-and Ly6C+MHCIIintpopulations but lower than that in Ly6C-MHCIIhimacrophages.These cells may represent the intermediate stage of monocyte differentiation in intestinal inflammation.However,there was no differential expression of genes with enhanced expression during homeostasis in the inflammatory intestinal environment.The levels of some inflammation-related genes gradually decreased,while that of CD169 increased significantly.
Studies have shown that macrophages play a key role in the pathogenesis of IBD and that these cells are present throughout the occurrence,progression,and recovery of intestinal inflammation in both humans[18,51] and mice[52,53].Macrophages regulate the progression of colitis by producing proinflammatory factors,such as TNF,IL-1β,IL-23,IL-6,reactive oxygen species (ROS),and NO[50].Intestinal macrophages release IL-1β,IL-6,IL-23,and TGF-β and mediate the Th17 immune response,which plays an important role in the pathogenesis of IBD[54].
Intestinal flora and intestinal macrophages
The intestinal flora maintains the integrity of the epithelial barrier,shapes the mucosal system,and balances host defense through metabolites,its own components,and adhesion to host cells.The metabolites and bacterial components of intestinal microorganisms can send signals to immune cells and regulate intestinal immunity.
Dietary fiber can directly enter the cecum and colon,where it can be fermented and metabolized by microorganisms to produce short-chain fatty acids (SCFAs)[55].SCFAs are the energy source of colon cells and regulate the physiological functions of intestinal epithelial cells and intestinal immune cells.SCFA-mediated histone deacetylase (HDAC) inhibition has anti-inflammatory effects.Butyrate inhibits the differentiation of dendritic cells and proinflammatory macrophage effectors from bone marrow stem cells in the LP through HDACs and reduces the immune system response to beneficial symbionts[56].In addition,macrophages and dendritic cells develop anti-inflammatory properties under the stimulation of butyrate-mediated GPR109A signaling.Foxp3+Tregs and CD4+T cells accumulate in the colon,activating immunosuppressive mechanisms and maintaining intestinal homeostasis[57].
CX3CR1himononuclear phagocytes do not migrate during intestinal homeostasis[58].Symbiotic bacteria and pathogenic bacteria can regulate the host immune response by activating TLR pathways in the intestine.TLR/MyD88 signal transduction limits the transport of CX3CR1himonocytic phagocytes from the LP to the MLNs[59].MyD88 deficiency and malnutrition lead to the migration of CX3CR1himononuclear phagocytes to the MLNs,enhance the Th1 response to noninvasive pathogens in the MLNs,and increase IgA.TLR signaling mediated by the intestinal microbiota can regulate IL-10 production by intestinal macrophages[60].The probioticClostridium butyricumpromotes the accumulation of F4/80+CD11b+CD11c macrophages in the inflamed intestinal mucosa through the TLR2/MyD88 signaling pathway and the production of IL-10 and prevents colitis in mice[52].It has also been shown that the LPS/TLR4 pathway can trigger CCL2 and promote the accumulation of monocyte-like macrophages (MLMs)[61],which can produce IL-1β,promote Th17 cell expansion,aggravate malnutrition and inflammation,and lead to tumor progression tumor formation[62].
TUMOR-ASSOCIATED MACROPHAGES
Peripheral mononuclear cells or RTMs infiltrate near tumor masses or into tumor tissue to form TAMs,which are the main inflammatory cells in the tumor matrix[63].
Recent studies have shown that TAMs originate from RTMs and newly recruited monocytes[64].The evolution of cells was inferred by the RNA velocity of single cells,and it was confirmed that FCN1+monocyte-like cells with tumor enrichment may be the precursors of TAMs and have a tumor-promoting transcriptional program.Transcriptional tracking of macrophages[65,66] indicated that FCN1+monocyte-like cells produce C1QC+TAMs and SPP1+TAMs from different RTMs.C1QC+TAMs may develop through IL1B+RTMs and express genes involved in phagocytosis and antigen presentation.SPP1+TAMs are linked to NLRP3+RTMs,which are rich in angiogenesisregulating factors and have specific enrichment of rectal adenocarcinoma and metastatic liver cancer pathways,suggesting that SPP1+TAMs can promote tumor development and metastasis[67].However,these subsets do not conform to the M1 and M2 classification of TAMs[68].
Dual role of tumor-associated macrophages
The plasticity of macrophages determines the polarization state,and the function of macrophages varies with the macrophage phenotype and tumor type[69,70].The phenotype of polarized TAMs depends on the stage of tumor progression:In the early stage of cancer,that is,the stage of tumor elimination with local chronic inflammation in the tumor,cytokines and chemokines induce TAM polarization to the M1 type[71],which can induce an inflammatory response and phagocytosis[72].Subsequently,M2 polarization occurs,and these cells secrete cytokines or chemokines and inhibit the antitumor immune response with changes in the tumor microenvironment (TME) and external stimuli as the tumor progresses[73].
In most human cancers,a large number of TAMs are significantly related to a poor disease prognosis,and basic research also shows that macrophages have a tumorpromoting function[74,75].A study of 120 CRC patients with liver metastasis showed that M1 macrophages were negatively correlated with tumor metastasis,while M2 macrophages were positively correlated with lymph node and liver metastasis and the degree of tumor differentiation.M2 macrophages and the M2/M1 ratio can be used as accurate predictors of liver metastasis in CRC patients[76].Based on an analysis of peripheral blood mononuclear cell samples from 360 CRC patients at the European Oncology Center,polarized circulating mononuclear cells can be used as biomarkers for CRC diagnosis and may be useful for follow-up and treatment evaluation[77].
M1 macrophages have high expression of major histocompatibility complex-II(MHC-II),exhibiting an effective antigen-presenting ability,and secrete proinflammatory factors and immunostimulatory cytokines,such as IL-12,IL-23,CXCL9,and CXCL10;thus,these cells function to kill bacteria and viruses,promote TH1 cell polarization and recruitment,and enhance the type 1 immune response[78].M2 macrophages express a large number of anti-inflammatory cytokines (IL-10),immune mediators (TGF-β),prostaglandins,indoleamines,growth factors (VEGF),chemokines(CCL2,CCL17,and CCL22),and matrix metallopeptidases;thus,M2 macrophages participate in anti-inflammatory activity,tissue remodeling,wound healing,angiogenesis,and tumor development[79].Prior research and a meta-analysis showed that M1 macrophages prevent the occurrence and development of tumors,while M2 macrophages promote tumor cell proliferation and invasion,enhance angiogenesis,and accelerate tumor growth and metastasis[80,81].However,CRC exhibits a paradox in the function of specific groups of immune cells.A study of 205 CRC patients showed that there were a large number of infiltrating CD163+macrophages in the CRC patients with less lymphatic metastasis and a lower tumor grade,and the patients with more CD163+macrophages exhibited a survival benefit.Unexpectedly,iNOS+macrophages did not show any advantage[82].In CRC stage III patients,high TAM levels are related to a better prognosis in patients who receive chemotherapy but not to the prognosis of patients who do not receive chemotherapy[83].
The type 1 immune response can inhibit the progression of CRC[84].However,the molecular mechanism regulating antitumor activity and promoting tumor inflammation in CRC is still unclear.NF-κB is a key regulator of inflammation,and its activation and inhibition are controlled at a variety of regulatory levels,which can regulate the function of macrophages[85].NF-κB p50 promotes the transcriptional program of M2 macrophages[86].In a model of colitis-associated cancer (CAC)induced by AOM combined with DSS[87],the number of tumor lesions was significantly decreased in p50-/- mice,accompanied by increases in Th1/M1 inflammatory genes (Il12b,Il27,Ebi3,Cxcl9,Cxcl10,Nos2,andIfng) and gene products (TNFα,IL12,and iNOS).An analysis of CRC stage II/III patients showed that nuclear accumulation of p50 in TAMs inhibited Th1 cell/M1 macrophage-dependent antitumor reactions,which was related to the expression of M2 macrophage-related genes (IL10,TGF-β,Ccl17,andCcl22) and increases in tumor-promoting genes (TNF-αandIL23).The expression of NF-κB p50 plays important roles in the development of colitis and CAC,but negative regulators (including p50) that only block inflammatory reactions also cause adverse reactions[88].Type 1 proinflammatory factors (IL-12 and CXCL-10) can offset adverse reactions and restore antitumor immunity,which still needs to be evaluated in large-scale clinical studies.
Tumor microenvironment and tumor-related macrophages
The TME is composed of cellular components and noncellular components.The cellular components include cancer cells,mesenchymal cells,infiltrating immune cells,and tumor-related fibroblasts,while the noncellular components are composed of cytokines and chemokines[89].The TME can regulate the infiltration of macrophages and promote the development of CRC through the synergistic effects of cytokines and cells.
Chemotactic factors
The chemokine family includes important signaling molecules in the TME.CCL3,CCL4,CCL5,CCL8,and CCL22 are highly expressed in various tumors and participate in the action of TAMs[90].Recent studies have shown that CCL5 plays an important role in the development of CRC and that CD8+T cell infiltration is significantly increased in the primary colorectal tumor site of CCL5-/-mice[91].In vivoandin vitroexperiments show that CCL5 secreted by macrophages mediates the formation of the p65/STAT3 complex,induces upregulation of PD-L1,inhibits the CD8+T cell response,and promotes immune escape and CRC development in cancer cells.Macrophage infiltration decreases significantly after anti-CCL5 and C-15 treatment[92].Inhibition of the CCL5-CCR5 axis is expected to be a new cancer treatment strategy[93].
CCL2 plays an important role in regulating the TME[94].CCL22 secreted by tumor cells plays a pivotal role in immunosuppression in the tumor microenvironment by binding with Foxp3+Tregs,which highly express CCR4[95].CCL22 was recently identified to have potential as a molecular biomarker for evaluating chemotherapy and tumor progression.Moreover,M2 macrophages transfer CCL22 to cancer cells and contribute to the development of 5-FU resistance and the epithelial-mesenchymal transition (EMT) program in CRC cells[96].CCL22 and its receptor CCR4 can also promote the migration and invasion of gastric cancer cells[97],and M2 macrophagederived CCL22 can enhance the migration of tumor cells in patients with liver cancer[98].
Hypoxia
Hypoxia in the TME can lead to angiogenesis,EMT,TGF-β signal transduction,and increases in tumor cell migration and metastasis[99,100].Tissue hypoxia affects TAMs in two ways:Hypoxia can induce tumor cells and the stroma to produce monocyterecruiting factors (CCL2,CCL5,CXCL12,CSF1,and VEGF).After monocytes are recruited into hypoxic areas,the expression of cytokine receptors is downregulated,and TAMs are trapped in the hypoxic microenvironment[101].Furthermore,macrophages capture oxygen through hypoxia inducible factors (HIFs),and decreased expression of ARG1 and immunosuppressive activity occurin vitroin the absence of HIF1α[102].HIF2α deficiency weakens macrophage infiltration and cytokine production[103].TAMs secrete “vascular factors” (VEGF,Sema3A,MMP2,and MMP9)[104].TAMs in NrpL/Lmice fail to enter the hypoxic tumor area,resulting in decreased angiogenesis and a weakened immunosuppressive ability,which leads to decreased vascular branches and a Th1 cell/CTL-mediated antitumor immune response[105].Th1 cells release TAMs recruited by IFN-γ and other cytokines,initiating feed-forward circulation and enhancing antitumor immunity[106].Reduced angiogenesis and tumor perfusion also trigger feed-forward circulation,resulting in hypoxia and recruitment of more TAMs[107].However,when Nrp1 is absent,these TAMs will not enter the hypoxic area and thus maintain the antitumor phenotype,which may explain observations made with clinical tumor biopsies:A higher number of TAMs are not necessarily related to a poor prognosis,and the clinical correlations between TAMs in different locations and the prognosis and survival of tumor patients are different[108].
Cluster analysis showed that the degree of M2 macrophage infiltration increased obviously under hypoxia but that the degree of M1 macrophage infiltration did not increase.The levels of CD163+and CD206+macrophages in the hypoxic subgroup were much higher than those in the normoxic subgroup.Hypoxia activates the RAS signaling pathway independently of KRAS mutation and activates the IL-6/JAK/STAT3 signaling pathway by increasing the infiltration of M2 macrophages,thus regulating the progression of CRC[109].The effect of lactic acid on macrophages under normoxic conditions is weak,but the combination of hypoxia and lactic acid can significantly promote the M2 polarization of macrophages through HIF-1,Hedgehog,and mTOR pathways[110].
Metabolism of tumor-related macrophages
Tumor metabolism plays important roles in promoting tumor growth and metastasis[111,112].Amino acids and fatty acids provide substrates for tumor cells to produce metabolites and energy to meet the metabolic needs for proliferation and TME development.M1 macrophages mainly produce ATP through glycolysis,while M2 macrophages preferentially obtain energy through the oxidative TCA cycle coupled with oxidative phosphorylation.Compared with M1 macrophages,M2 macrophages have opposing arginine metabolism[113].Increasing evidence shows that the lipid metabolism of immune cells,especially that of TAMs,plays important roles in the occurrence and development of tumors.In recent years,research on the process of lipid metabolism in TAMs has focused on the regulatory mechanisms of lipid metabolism-related enzymes.
In vitroandin vivomouse experiments have shown that[114] the level of the lipolytic coactivator ABHD5 in CRC-associated macrophages is increased significantly,while that of monoacylcerolipase (MGLL) is decreased.ABHD5 can promote the growth of CRC by inhibiting the production of spermidine,which depends on SRM in TAMs.MGLL deficiency may lead to an increase in fatty acid glycerides[115].The upregulation of ABHD5 may lead to a decrease in triglycerides and an increase in diglyceride[116].A transplanted tumor model including mouse myeloid cells overexpressing ABHD5 showed that TAM ABHD5 could inhibit peritoneal and pulmonary metastasis of tumor cells (MC-38 and B-16 cells) and that macrophage ABHD5 regulated the migration and metastasis of tumor cells through the IL-1β/NF-κB/MMP pathway.The MMTV-PyMT mouse model of spontaneous breast cancer also verified that macrophage ABHD5 could inhibit lung metastasis of spontaneous breast cancer[114].
Phospholipid metabolism can affect the TME by regulating tumor-related immune cells[117-119].The lysophosphatidic acid acyltransferase β-AGPT4 is highly expressed in CRC patients,and the survival rate of CRC patients is reduced with high expression of AGPT4.Agpat4knockdown can increase the expression of the proinflammatory factors IL-1β,IL-6,and TNF-α by increasing the LPA content,inducing polarization of M1 macrophages and enhancing antitumor effects[124].An animal experiment performed with mice treated with ethoxymethane and sodium dextran sulfate showed that[120] Lipin-1,a phospholipid acid phosphatase,could promote the infiltration of F4/80+macrophages by participating in the production of CXCL1/2 (the infiltration of other immune cells,such as T cells,was not changed),upregulating the level of Nos2/iNOS and promoting dysplasia-cancer metastasis in colorectal tumors.
Targeting tumor-related macrophages
A large number of studies have proven the role of the CSF1-CSF1R axis in TAM recruitment,and inhibition of CSF1-CSF1R signaling leads to apoptosis and death in most TAMs[121].CSF1-CSF1R blockers can improve the efficacy of various immunotherapeutic methods,including administration of CD40 agonists or PD1 or cytotoxic T lymphocyte antigen 4 (CTLA4) antagonists and adoptive T cell therapy[122-124].Anti-CSF1R treatment can specifically deplete C1QC+TAMs but cannot deplete the entire SPP1+macrophage population,which can promote tumor growth.This finding may explain why anti-CSFR1 antibodies are not effective as monotherapies in tumor patients[67].CSF1R inhibition combined with radiotherapy or chemotherapy can improve the T cell response and enhance the therapeutic effect in a large number of animal models[125-127].
CXCR4-CXCL12 is an important signal transduction axis involved in TAM recruitment,which can promote tumor invasion and regeneration[128].Monocytes secrete the chemokine CXCL12 and express the receptors CXCR4 and CXCR7,which lead to autocrine/paracrine loops;promote the differentiation of different types of macrophages;enhance the expression of CD4,CD14,and CD163;and decrease the ability to stimulate antigen-specific T lymphocyte responses[129].The CXCR4 antagonist peptide R (PEP R) can reduce the growth of HCT116 cells and improve the therapeutic effect of conventional chemotherapy (CT) or chemoradiotherapy (RT-CT).This effect depends on the decreases in cell growth and mesenchymal stem cell transformation induced by CT/RT-CT[130].PEPR can also target CXCR4+stromal cells and further decrease EMT and chemoresistance[131].Combined administration of PEP R and the CXCL12 antagonist noxa-012 can improve the function of anti-PD1 antibodies in mice with CRC[132].
Macrophages in different functional states maintain cell activity through different metabolic pathways and metabolites[133].Mammalian target of rapamycin (mTOR)signalingviamTORC1 and mTORC2 plays a central role in tracking nutrition,oxygen,and metabolites to guide the metabolic processes of macrophages[134].Rapamycin (an mTORC1 inhibitor) can stimulate M1 macrophages and cause them to have an antitumor effect[135].mTORC1 inhibitors can reduce immunosuppressive inflammation and tumor occurrence.Rad001 (a rapamycin derivative) ameliorates CRC induced by AOM/DSS in mice by limiting inflammation[136].Signaling molecules(such as PI3Kγ,Akt,and PTEN) upstream of mTOR also participate in the polarization and remodeling of TAMs,making the mTOR pathway a potential anticancer target[137].The expression of a PI3Kγ inhibitor (PTEN) or silencing of AKT1 can also promote the polarization of antitumor M1 macrophages[138].
Iron participates in the interaction between tumor cells and their environment[139].Unlike M1 macrophages,M2 macrophages express iron transporters and downregulate ferritin and heme oxygenase,all of which promote iron release[140].In addition,conditioned medium from M2 macrophages can promote the proliferation of tumor cells,while iron chelation can inhibit the proliferation of tumor cells[141].Recent studies have shown that iron chelation can reverse the iron-processing function of M2 macrophages,switching from iron release to chelation,and block the tumorpromoting effect of M2 macrophages[142].
CONCLUSION
Macrophages play a crucial role in the occurrence and development of CRC.As the disease progresses,macrophages tend to differentiate into different subsets that play different biological functions.The dual functions of TAMs and the regulatory effects of the TME on TAMs are worthy of further study.Subsets of macrophages cannot be simply classified according to the traditional M1 and M2 phenotypes.Single-cell technology will benefit the phenotypic classification of macrophages and provide further insights into their function (Figure 1).
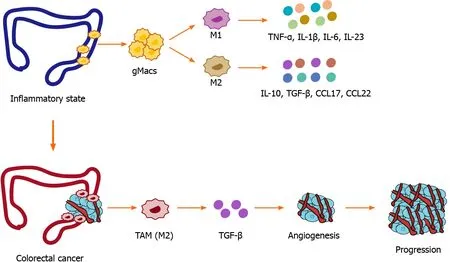
Figure 1 Etiology of macrophages in inflammatory bowel disease and colorectal cancer.
The complexity of tumors highlights the advantages of combined therapeutic approaches.The clinical application of immune checkpoint inhibitors such as PD-1 and PD-L1 monoclonal antibodies provide additional evidence for tumor immunotherapy,and studies have shown that targeting tumor-associated macrophages can significantly improve the efficacy of existing immunotherapy.Future research needs to have a clear understanding of drug mechanisms of action and drug resistance mechanisms to design effective combined therapies.In addition,more clinical data are needed to clarify the relationships between macrophage infiltration or phenotype and the prognosis of patients and to guide whether TAM antagonists can be used in patients to overcome immunotherapy resistance.Despite these challenges,the use of macrophages to improve the prognosis of cancer patients still has great potential.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Management of obstructive colon cancer:Current status,obstacles,and future directions
- Role of endoscopic ultrasound in anticancer therapy:Current evidence and future perspectives
- Mesenchymal stem cell-derived exosomes for gastrointestinal cancer
- Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated
- Pancreatic intraductal papillary mucinous neoplasms:Current diagnosis and management
- Combined treatments in hepatocellular carcinoma:Time to put them in the guidelines?
