Diabetes mellitus contribution to the remodeling of the tumor microenvironment in gastric cancer
Armando Rojas,Cristian Lindner,Iván Schneider,Ileana Gonzàlez,Hernan Araya,Erik Morales,Milibeth Gómez,Nelson Urdaneta,Paulina Araya,Miguel Angel Morales
Armando Rojas,Cristian Lindner,Iván Schneider,Ileana Gonzàlez,Erik Morales,Paulina Araya,Biomedical Research Lab.,Medicine Faculty,Catholic University of Maule,Talca 34600000,Chile
Hernan Araya,Milibeth Gómez,Nelson Urdaneta,Department of Clinical Sciences,Medicine Faculty,Catholic University of Maule,Talca 34600000,Chile
Hernan Araya,Milibeth Gómez,Nelson Urdaneta,Servicio de Oncología,Hospital Regional de Talca,Talca 34600000,Chile
Erik Morales,Servicio de Anatomía Patologica,Hospital Regional de Talca,Talca 34600000,Chile
Miguel Angel Morales,Department of Molecular and Clinical Pharmacology Program,Institute of Biomedical Sciences,University of Chile,Santiago 8320000,Chile
Abstract Compelling pieces of evidence derived from both clinical and experimental research has demonstrated the crucial contribution of diabetes mellitus (DM) as a risk factor associated with increased cancer incidence and mortality in many human neoplasms,including gastric cancer (GC).DM is considered a systemic inflammatory disease and therefore,this inflammatory status may have profound effects on the tumor microenvironment (TME),particularly by driving many molecular mechanisms to generate a more aggressive TME.DM is an active driver in the modification of the behavior of many cell components of the TME as well as altering the mechanical properties of the extracellular matrix (ECM),leading to an increased ECM stiffening.Additionally,DM can alter many cellular signaling mechanisms and thus favoring tumor growth,invasion,and metastatic potential,as well as key elements in regulating cellular functions and cross-talks,such as the microRNAs network,the production,and cargo of exosomes,the metabolism of cell stroma and resistance to hypoxia.In the present review,we intend to highlight the mechanistic contributions of DM to the remodeling of TME in GC.
Key Words:Diabetes mellitus;Gastric cancer;Tumor microenvironment;Hyperglycemia;Chronic inflammation
INTRODUCTION
At present,a compelling body of evidence suggests that diabetes mellitus (DM)patients have not only increased incidence but also worse outcomes when they develop malignant neoplasm,especially those originating from gastrointestinal (GI)organs,such as the pancreas,colon,liver,and stomach[1].
Gastric cancer (GC) is the fifth most common cancer and the fourth most common cause of cancer death globally,with a poor 5-year survival<20% for advanced stages[2].
Strikingly,data from different epidemiological data suggest that DM and chronic hyperglycemia may even increase the risk and mortality of GC patients[3,4].Although there is compelling clinical data supporting this association,the molecular mechanisms underlying this association are not fully understood.
DM is considered a systemic inflammatory disorder[5],which triggers a dysregulated metabolism,and is characterized by sustained hyperglycemia[6].However,the systemic pro-inflammatory effects induced by DM are not only mediated by chronic hyperglycemia but also enhanced by insulin resistance[7].All these features drive critical modifications in extracellular elements such as ECM and favor the dysregulation of many intracellular signaling pathways[7-9].
Furthermore,DM not only interferes in intercellular communication increasing the biogenesis of exosomes but also altering the delivery of biomolecules to recipient cells and favors a pro-angiogenic and proliferative cross-talk between stromal cells,which could act as a key element in defining the fate of tumor development in these patients[9].
A crucial role in the tumor biology of gastric carcinoma plays the complex network established between cellular and non-cellular elements that composed the tumor microenvironment (TME),which drives the cancer cell fate and plays a critical role in the initiation,progression,and immune evasion[10].
A growing body of evidence suggests that these TME remodeling mechanisms can be crucial in favoring the development of highly malignant phenotypes in DM patients who develop GC[5,11].
In the present review,we intend to highlight the different mechanisms of the contribution of DM to the remodeling of TME in GC,which leads to more aggressive tumors.
EPIDEMIOLOGICAL ASSOCIATION
DM is considered an established risk factor for either higher incidence and increased long-term all-cause mortality rates in many cancer types[12,13],especially for those originating in the digestive tract[14,15].
Although several observational studies have demonstrated a controversial association between DM and CG[4,16] or restricted only to gender differences[17] a growing body of evidence including both meta-analyses of wide-population cohorts and case-control studies demonstrate an increased risk and mortality of GC in DM patients[3,18-21].
Furthermore,a recent study with a prospective endoscopic follow-up shows that DM is an independent risk factor for GC[3].Noteworthy,this positive association remains significant even in patients who only present pre-diabetes and hyperglycemic events[22,23].
Additionally,several reports suggest that DM and hyperglycemia are not only associated with a higher incidence of GC but also increased mortality [21,24-26],and may even lead to drug resistance and tumor progression in GC patients[27-29].
At present,Helicobacter pylori(H.pylori) colonization is a crucial risk factor in the pathogenesis of GC.H.pyloriinfection leads to chronic inflammation of gastric mucosa and then leads to atrophy of the glands,intestinal metaplasia,and GC[30].In 1994,H.pyloriwas classified as a Group 1 carcinogen by the International Agency for Research on Cancer[31].
Noteworthy,recent reports have not only demonstrated an association between DM and incidence ofH.pyloriinfection[32-34] but also,as a higher risk of failure in eradication therapy[35-37].Furthermore,sustained hyperglycemia influences the expressions of severalH.pylorivirulence factors,leading to promote carcinogenesis[38].
During the last decade,a growing body of evidence has shed light on the mechanisms underlying this epidemiological association (Figure 1).
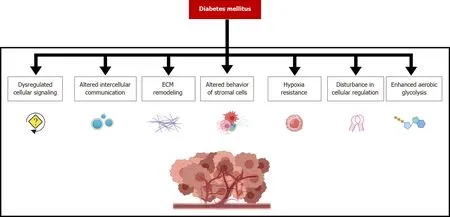
Figure 2 The molecular mechanisms involved in the contribution of diabetes mellitus to the remodeling of gastric cancer microenvironment determine crucial phenotypical changes not only on tumor cells but also in many other infiltrating cells.
THE CONTRIBUTION OF DM IN REMODELING THE TME IN GC
Changing stroma cells behavior
The TME is a complex tissue niche with a diverse repertoire of infiltrating host cells,mainly recruited by cancer cells,together with many secreted factors and components of the extracellular matrix,which profoundly influence tumor growth,and dissemination[39].
A convincing body of evidence supports that chronic inflammation caused byH.pyloriinfection is the major risk factor for the development of GC,and thus various types of cells in the gastric mucosa are exposed to an inflammatory environment for long periods.The robust inflammatory response triggered by infection,together with bacterial and host factors determines the transit from the early stages of inflammation through the development of metaplasia,dysplasia,and finally to invasive carcinoma[40].
In the GC microenvironment,the behavior of many cell types of the tumor stroma is influenced by diabetes or hyperglycemia.Noteworthy,all cellular components of tumor stroma express the receptor of advanced glycation end products (RAGE),including tumor cells,and a growing body of pieces of evidence supports the role of the RAGE/advanced glycation end products (AGEs) (RAGE/AGEs) axis on tumor growth.This important modifier of the TME will be covered in the cell signaling disturbances section.
Gastric epithelial homeostasis is maintained by long-lived stem cells surrounded by a supportive niche.Therefore,GC may arise from mutated stem cells that have been accumulating gene mutations during cell half-life,and the subsequent expansion of mutated clones[41].In this context,the chronic inflammation induced byH.pyloriinfection may then damage gastric epithelial mucosa,followed by the recruitment of bone marrow-derived cells (BMDCs),which may then lead to tissue remodeling,transformation,and potential progression to malignancy[42].
Of note,the diabetic condition has been described to force BMDCs to express tumor necrosis factor-alpha (TNF-α) and thus they become a contributor to fuel inflammation instead of repairing the damaged gastric mucosa[43].Tumor-associated mast cells are also part of the cell stroma in GC[44].These infiltrating cells play crucial roles in remodeling the TME[45],by the release of large amounts of preformed and preactivated inflammatory mediators through degranulation,and supporting tumor progression,immunosuppression,and angiogenesis[46,47].
Hyperglycemia and advanced glycation end-products are known to activate mast cells and increase the expression of proinflammatory cytokines such as TNF-α and favor the degranulation of mast cells[48].
Cancer-associated fibroblasts (CAFs) are prominent components of the TME,and play important roles in GC,such as tumor growth and progression,matrix remodeling,promoting angiogenesis as well as fueling inflammation[49].
CAFs are crucial cells in the production of a desmoplastic stroma,characterized by the formation of dense fibrosis and increased remodeling and deposition of ECM components.CAFs not only produce fibrillar collagens and other interstitial ECM components but also release matrix metalloproteinases[49,50].
Of note,desmoplasia is commonly found in patients with diabetes,where the hyperglycemic condition activates fibrogenic pathways,not only through direct stimulation of the synthesis of ECM components but also by triggering epithelial and endothelial cell conversion to a fibroblast-like phenotype[51].
Cancer cells can recruit and activate fibroblasts in the TME by the induction of their trans-differentiation into CAFs.Recently,the serine/threonine homeodomaininteracting protein kinase 2,has been reported as a crucial regulator of this process,and its downregulation favors tumor progression[52].Noteworthy,hyperglycemia produces a sustained degradation of this protein[53] and thus favoring the transdifferentiating process.
CAFs play an important role in the progression of GC,by promoting migration and epithelial to mesenchymal transition (EMT) of GC cells,and EMT is fully potentiated by hyperglycemia[54].
Tumor-associated macrophages (TAMs) are crucial cells in sculpturing the TME[55,56].Furthermore,TAMs have a prognostic significance for GC patients,when combined with the TNM staging system[57].
TAMs are crucial cells in tuning the machinery of inflammatory and host immune responses in TME.Once infiltrated,macrophages undergo a polarization process rendering distinct functional phenotypes,and where classically activated (M1) and alternatively activated (M2) macrophages represent two extreme phenotypes[55,58].In GC,TAMs are predominantly bearing an M2 phenotype,which is associated with cancer metastasis and a worse prognosis in patients[56-59].
During diabetes,macrophages and other innate immune cells are known to have a pro-inflammatory phenotype,which is believed to contribute to the pathogenesis of various diabetic complications[60].Noteworthy,hyperglycemia acts in synergy with hypoxia and sensitizes macrophage responses to cytokine stimuli[61],and generates particular M1/M2 cytokine profiles[62].
Recently,hyperglycemia is reported to induce an increased flux through the hexosamine biosynthetic pathway in TAMS resulting in an upregulation of OGlcNAcylation,which in turn favors the alternative M2 polarization of TAMs and reduced anti-tumor immunity[63].
Tumor-infiltrating neutrophils are very abundant in the GC microenvironment,where they promote GC cell migration and invasion as well as the induction of EMT through the interleukin (IL)-17-mediated JAK2/STAT3 signaling activation,indicating that neutrophils may play an important role in GC metastasis[64,65].
Hyperglycemia is reported to impair granulocyte-colony stimulating factor secretion,thereby hindering the mobilization of antitumor neutrophils,which in turn,leads to increased survival of disseminated tumor cells and consequently increasing the metastatic burden[66].
Obesity is a common comorbidity of diabetes,and some authors have associated obesity with an increased risk of GC[67].Obesity can impact the TME both locally,and systemically through many signals associated with visceral adipose tissue inflammation,as reported for adipokines,growth factors,and cytokines[68,69].In this context,the activation of the STAT3 gastric signaling pathway,which is crucial in promoting the malignant transformation of epithelial cells[70],is induced by leptin and IL-6 in obese subjects[71,72].
Modification of extracellular matrix
ECM is known to be a complex non-cellular network composed mainly of glycosaminoglycans and fibrous proteins,such as collagens,fibronectin,elastin,and laminin,which give structural support to tissues and regulate diverse cellular functions such as survival,growth,migration,adhesion,and differentiation[73].
During these cellular-ECM interactions,a complex network of signaling pathways is activated through mechanotransduction receptors,which are capable of sensing changes in the stiffness of the ECM[74,75].At present,ECM is considered a highly dynamic element that continuously undergoes remodeling induced by several conditions[76].
Compelling evidence support that tumor-associated ECM remodeling and stiffening,are key elements behind the TME of highly invasive phenotypes of several neoplastic cells[77-79].Strikingly,tumor-associated ECM remodeling and the subsequent stiffening play a critical role in the behavior of cancer cells in the TME[80],and thus,supporting cancer cell survival,progression,and metastatic invasion[81].
Fibronectin and type I collagen are the most common and abundant fibrillar ECM proteins found in cancer-associated ECM[82,83].Their increase is a result of excessive fibrotic remodeling,also referred to as desmoplasia,which is largely mediated by alpha-smooth muscle actin-expressing myofibroblasts[83,84].
Most ECM fibrous proteins are long-live potential targets for the higher rate of AGEs formation observed in DM and chronic hyperglycemic-state[85].AGEs crosslinks of load-bearing protein lead to ECM stiffening which favors not only tumor cell survival,but also high rates of proliferation,and metastatic cancer cell interaction with the endothelium[86,87].
Furthermore,the chronic hyperglycemia state not only mediates mechanical changes in ECM but also can generate a reservoir of AGEs with the potential to trigger a multitude of RAGE-dependent mechanisms[85].
In addition,in vivostudies support that these posttranslational modifications of ECM produced during hyperglycemia also favor cancer cell invasion by activation of mechanotransduction-dependent epidermal growth factor receptor (EGFR) signaling pathway[88,89].The interplay between highly glycated-ECM and increased EGFR activity could be a key element in the enhanced cancer cell invasion in DM patients.
In this context,lysyl oxidase (LOX) plays a central role in modulating the formation of molecular cross-linkages of ECM components[90].LOX is known to play a significant role in the GC microenvironment[91].Interestingly,the DM milieu favors the overexpression of LOX[92],which is associated with increased ECM modifications and high invasion activity of GC in DM patients[93,94].
Cellular signaling disturbances
One of the earliest pieces of evidence supporting that DM represents an active landscape for cellular signaling disturbances,coming either from the complex network of mechanisms underlying diabetes complications and the altered insulin sensitivity observed in obesity and type 2 diabetes[95,96].
At present,a growing body of evidence supports that the hyperglycemic condition can activate different signaling mechanisms,such as the polyol pathway,the advanced-glycation end-product formation,and the subsequent activation of the RAGE/AGEs axis,as well as the activation of Protein Kinase C and hexosamine pathway.All these activated pathways may then lead to the over-expression of reactive oxygen species (ROS),activation of the transcriptional factors nuclear factorκappa beta,and consequently the increased production of proinflammatory mediators,the activation of leukocytes,as well as increased apoptosis,and a desmoplastic reaction[97,98].
These pro-inflammatory signaling pathways have supported the new concept called meta-inflammation,which is characterized by a low-grade systemic and chronic inflammation and is associated with the pathogenesis of diabetic complications[99,100].
Hyperglycemia results in calpain-1 upregulation in the mitochondria,which in turn leads to a reduction in ATP synthase activity and increased mitochondrial ROS formation[101].Mitochondrial ROS is crucial for the stabilization of hypoxia-inducible transcription and thus leading to an activation of a transcriptional profile supporting tumor angiogenesis[102].Additionally,ROS is a well-known driver of myofibroblast differentiation,through the activation of the TGF-B pathway[103],and myofibroblasts are recognized as a major source of the CAFs[104].
Chronic hyperglycemia is the hallmark of DM.This condition leads to an accelerated formation of AGEs,a heterogeneous group of compounds resulting from the non-enzymatic reaction of reducing sugars with the free amino group of proteins lipids and nucleic acids[105].
The high rate of AGEs formation in DM patients favors the overexpression of RAGE,and the hyperactivation of the RAGE/AGE axis[98,106,107].This receptor is involved not only in the adhesion of H.pylori to gastric epithelial cells but also in the inflammatory response to infection[108].
The activation of this signaling pathway is an important contributor to inflammation-related tumorigenesis through different signaling mechanisms,including the resistance to apoptotic insults and hypoxia,interfering with antitumor immunity,stimulating angiogenesis,and supporting invasiveness[109].
Interestingly,hyperglycemia can disturb cell cycle regulation not only in normal cells[110] but also in cancer cells[111].High levels of glucose and insulin are reported to enhance cyclin D,cyclin-dependent kinase 4 (Cdk4),and Cdk2 expression and suppress cyclin-dependent kinase inhibitors p21 and p15/16[112].
On the other hand,the protein O-linked β-N-acetylglucosamine (O-GlcNAc)modification is a dynamic post-translational modification affecting a wide variety of proteins involved in cell cycle regulation,and this modification is considered as a major contributor to the deleterious effects of hyperglycemia[113].This post-translational modification relies on the addition of a single N-acetyl-glucosamine molecule to the OH residues of serine or threonine by the action of the O-GlcNAc-transferase,and where some oncogenic factors,such as p53,Myc,and β-catenin,as well as other cell cycle regulators are O-GlcNAcylated[114,115] and is increased in many human neoplasias,including GC[116,117].
A growing body of evidence supports the role of the Wnt/β-catenin pathway in the development,progression,and metastasis of GC[118,119].High glucose levels can produce profound effects on Wnt/β-catenin signaling in cancer cells,leading to an increased expression of WNT target genes[120] by either a sustained increment of the p300 acetyltransferase activity or decreased sirtuin 1 deacetylase activity.In this way,hyperglycemia renders high levels of β-catenin acetylation,which in turn,allows nuclear accumulation and transcriptional activation of Wnt-target genes[121].
An overactive TGF-β1 signaling pathway has been reported in diabetes patients[122] and its role as a critical profibrotic factor in the progression of chronic kidney disease in diabetes is widely documented[27,123].The role of TGF-β in the biology of GI cancers has been extensively studied showing crucial roles in regulating processes such as tumor progression,evasion of growth suppressors,and resistance to cell death,angiogenesis,invasion,and metastasis[124].
TGF-β1 is overexpressed in GCs and the stromal tissues surrounding the cancer cells[125,126].The main source of TGF-β1 is stromal cells,such as fibroblasts,lymphocytes,and macrophages[127],and therefore,the coexistence of the burden due to diabetes reinforces its impact on the TME.
Another key element in the signaling network in the gastric TME is the EGFR family,which consists of four related receptor tyrosine kinases (ErbB1 to ErbB4)[128],and all members of the family are expressed in gastric tumors[129].
On the other hand,diabetic kidney disease is a common microvascular complication of DM and the leading cause of end-stage renal disease[130].Activation of the EGFR signaling pathway is linked to the onset and progression of renal damage in DM,by promoting cell proliferation,inflammatory processes,and ECM modification[131].Interestingly,these signaling pathways can be activated by several ligands,but also by other biological mediators such as ROS,TGF-β,and PKC,all of which are upregulated in DM[122,132,133].
The COX-2/PGE2 signaling pathway plays a critical role in the inflammatory nature of gastric tumors.COX-2 is upregulated in GC and its precursor lesions,and it provides valuable clinical information as a prognostic factor[134].Of note,the high levels of COX-2 expression are an earlier event reported during theH.pyloriinfection of gastric mucosa[135].
Many PGE2-mediated mechanisms supporting tumor growth,new vessel formation,and enhancing metastasis have been described[136].Additionally,COX-2/PGE2-mediated signaling pathways are key contributors to many diabetes complications[137,138].
Noteworthy,the activation of the RAGE/AGEs axis,which is highly expressed in diabetic tissues and cells,can significantly increase both COX-2 messenger RNA and protein expression,together with a rise in PGE2 Levels[139].
Hyperinsulinemia is strongly associated with type 2 diabetes and it has been recently postulated to be a risk factor for GC[140] Emerging data suggest that insulin may be a crucial regulator in some human neoplasias,including GC[141-145].
In addition,hyperinsulinemia also increases the hepatic production and systemic bioavailability of IGF1 but also reduces the hepatic protein production of the insulinlike growth factor binding proteins 1 (IGFBP-1) and 2 (IGFBP-2).These two coordinated actions may,in turn,hyperactivate the Ins-R/IGF1-R system,and thus triggering their proliferative and anti-apoptotic programs in cancer cells[142].Additionally,the activation of the RAGE/AGEs axis also upregulates the expression of both IGF1 mRNA and protein levels[143].
Disturbances in microRNAs network
MicroRNAs (miRNAs) are a class of small non-coding RNAs,which act as posttranscriptional regulators of gene expression[146],and are involved in several cellular activities such as cell growth,differentiation,development,and apoptosis in many cancer types[147].Notably,recent research has demonstrated that dysregulation in the miRNAs network has crucial consequences in the cellular behavior of neoplastic cells[147],by modulating multiple signaling pathways,especially within the gastric TME[148].
Noteworthy,hyperglycemia,and hyperinsulinemia in DM patients are two conditions that induce major changes in miRNA expression profile,especially those that are involved in gastric carcinogenesis[149].
DM patients have a particularly pro-tumoral miRNA profile,characterized by downregulation of tumor suppressor miRNAs such miR-497,miR-495p,and miR-203,which can inhibit tumor cell proliferation and migration[150,151],and its decreased expression has been associated with poor prognosis in GC patients[152,153].
In addition,the expression of some members of the family of Let-7 miRNAs,which are known by their roles in regulating oncogenes and controlling cellular differentiation and apoptosis[154],is often downregulated in several cancers,and thus derepressing some relevant oncogenic targets in GC,such as K-ras,and c-Myc[155].Noteworthy,Let-7 miRNAs are downregulated under DM conditions not only by hyperglycemia but also by insulin resistance[151].
Conversely,a diabetogenic milieu and chronic hyperglycemia favor the overexpression of some oncomiRs,such as miR-17-5p[150,156].Furthermore,recent research support that increased stiffness of ECM significantly induces the expression of miR-17-5p and thus rendering a loop towards the support of tumor growth and invasion[157].
Altered exosomes production and cargo
Exosomes are submicron-sized extracellular vesicles that are involved in cell-to-cell and organ-to-organ communication[158].Recently,exosomes are involved in the pathogenesis of various disorders,including inflammatory diseases and cancer[159].
In addition,these small vesicles are now emerging as important modulators of the interchange of bioactive molecules within the TME[148].
At present,a growing body of evidence supports that either chronic hyperglycemic or hyperinsulinemic state alters not only the molecular cargo of exosomes but also their production in DM patients.These changes consequently induce critical changes in cellular function that enhance the cross-talk communication between neoplastic and non-neoplastic stromal cells[9,160].
Noteworthy,recent studies suggest that exosome release from individuals with diabetes expresses a skewed profile of pro-inflammatory molecular cargo[160,161],which influences the neoplastic transformation and favors cancer cell dissemination,especially in gastric tumors[162].
In addition,the altered exosomes-profile in a hyperglycemic milieu drives an increased expression of pro-angiogenic factors such as VEGF and HIF-1[160,161],which give rise to an increased intercellular cross-talk between endothelial cells,and subsequently leading to a highly angiogenic gastric microenvironment phenotype,thus promoting cancer growth and progression[163].
Besides the alterations in the molecular cargo of exosomes in the DM milieu,the increased release rate of exosomes due to hyperinsulinemia could enhance the exosome-dependent molecular transfer associated with the peritoneal dissemination of GC[160,161,164],and thus affecting the prognosis of DM patients who develop GC[165].
Altered metabolism
The Warburg effect refers to the enhanced glucose uptake and lactate production observed in cancer cells,even in the presence of oxygen and fully functioning mitochondria,also known as aerobic glycolysis[166].Aerobic glycolysis provides glycolytic intermediates,which function as important precursors required for the synthesis of carbohydrates,fats,and proteins by cancer cells[167].
In the context of hyperglycemia,all these requirements are covered;however,hyperglycemia also promotes glycolysis by inducing the expression of glycolyticrelated genes[168-170].However,the metabolic effects of hyperglycemia on cancer cells can go further than the Warburg effect,considering that the activation of some oncogenes can subsequently proceed to an increase in ATP production[171].
Hyperglycemia also enhances the expression of the carbohydrate-responsive element-binding protein (ChREBP) in cancer cells,a well-known promoter of lipogenesis[172].This is particularly interesting considering many tumor cells producede novoalmost the total of the monounsaturated and saturated fatty acids required,which are used in many cellular events crucial for tumor growth and progression[173].
In tumor cells,high glucose levels can promote HIF-1α expression under both normoxic and hypoxic conditions[168,174].In the GC microenvironment,the HIF-1 complex activates the transcription of crucial target genes in conferring the adaptation to the hypoxic milieu and its expression correlates with an aggressive tumor phenotype and a poor prognosis[175].
In summary,DM contributes through a myriad of molecular mechanisms to the remodeling of the GC microenvironment and thus renders crucial phenotypical changes,which in turn generates tumors that are more aggressive (Figure 2).
CONCLUSION
At present,a compelling body of evidence supports the contribution of DM not only to higher cancer incidence but also to an increased mortality rate of DM patients who develops GC.
In recent years,considerable efforts in experimental research have allowed elucidating the molecular mechanisms underlying this association.In this regard,growing data suggest that the mechanical alterations induced in ECM by AGEsmediated cross-linking and the profound changes in stromal cell behavior influenced by diabetes are pivotal elements in supporting tumor growth and progression.
Furthermore,the underlying pro-inflammatory signaling supporting the metainflammation in DM patients,favors several disturbances in intra- and intercellular signaling pathways,which ultimately converge in favor of the development,progression,and dissemination of GC.In addition,the chronic metabolic dysregulation observed in DM patients favors crucial changes in tumor cell metabolism that will ultimately contribute to highly hypoxic-resistant neoplastic cells as well as to a more aggressive tumoral phenotype.
Although in recent years crucial advances have been made in the knowledge of the mechanisms induced by DM in generating a TME that is supportive of tumor growth and spread,the strengthening of clinical research is essential to achieving a better understanding of the mechanisms underlying this epidemiological association.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Management of obstructive colon cancer:Current status,obstacles,and future directions
- Role of endoscopic ultrasound in anticancer therapy:Current evidence and future perspectives
- Mesenchymal stem cell-derived exosomes for gastrointestinal cancer
- Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated
- Pancreatic intraductal papillary mucinous neoplasms:Current diagnosis and management
- Combined treatments in hepatocellular carcinoma:Time to put them in the guidelines?
