Solid extraintestinal malignancies in patients with inflammatory bowel disease
Anastasia Mala,Kalliopi Foteinogiannopoulou,Ioannis E Koutroubakis
Anastasia Mala,Department of Medical Oncology,University Hospital of Heraklion,Heraklion 71110,Crete,Greece
Kalliopi Foteinogiannopoulou,Ioannis E Koutroubakis,Department of Gastroenterology,University Hospital of Heraklion,Heraklion 71110,Crete,Greece
Abstract Malignancies constitute the second cause of death in patients with inflammatory bowel diseases (IBD),after cardiovascular diseases.Although it has been postulated that IBD patients are at greater risk of colorectal cancer compared to the general population,lately there has been evidence supporting that this risk is diminishing over time as a result of better surveillance,while the incidence of extraintestinal cancers (EICs) is increasing.This could be attributed either to systemic inflammation caused by IBD or to long-lasting immunosuppression due to IBD treatments.It seems that the overall risk of EICs is higher for Crohn’s disease patients and it is mainly driven by skin cancers,and liver-biliary cancers in patients with IBD and primary sclerosing cholangitis.The aims of this review were first to evaluate the prevalence,characteristics,and risk factors of EICs in patients with IBD and second to raise awareness regarding a proper surveillance program resulting in early diagnosis,better prognosis and survival,especially in the era of new IBD treatments that are on the way.
Key Words:Extraintestinal malignancies;Crohn’s disease;Ulcerative colitis;Thiopurines;Anti-tumor necrosis factor
INTRODUCTION
Inflammatory bowel diseases (IBD),encompassing Crohn’s disease (CD) and ulcerative colitis (UC),are chronic diseases of unknown etiology.It has been proposed that in genetically predisposed patients,several environmental factors and altered gut microbiota interact,resulting in immune system dysregulation and finally to chronic intestinal inflammation[1].IBD mainly affects the gastrointestinal tract but not exclusively,as 50% of patients experience at least one extraintestinal manifestation.In Western countries,IBD is not uncommon as the prevalence of CD is 246.7/100000 and UC is 286.3/100000[2].
Cancer is the second cause of death in IBD patients,after cardiovascular diseases[3]and 30% of IBD patients are diagnosed with malignancy at some point during the course of IBD[4].A multicenter European study showed that the prevalence of cancer in IBD patients,during a 15-year follow-up period,was 9.1%,the majority of them were extraintestinal cancers (EICs) and the overall cancer frequency was equal to the background population[5].
However,it was recognized that patients with IBD are at increased risk of certain EICs and was also shown in other reports[6,7].
The risk of colorectal cancer (CRC) in IBD patients is 1.7-fold higher than that in the general population[3].Lately,a reduction in the incidence of CRC in IBD has been reported that could be attributed not only to successful inflammation control due to new IBD treatments,but also to better surveillance strategies,colonoscopy techniques and implementation of guidelines supporting colectomy for high-grade dysplasia[3,4,8].On the other hand,there is evidence that IBD patients have a higher risk of certain EICs[9-11].EICs in IBD patients could be related either to chronic inflammation or to longstanding immunosuppressive treatment.As the incidence of EICs is increasing among IBD patients,awareness should be raised for more scrupulous surveillance for early diagnosis and curative treatment.
The aim of this review was to evaluate the prevalence,characteristics,and risk factors of EICs in patients with IBD,and to provide possible measures for prevention and early diagnosis.
METHODOLOGY
A review of the literature in PubMed using the MESH terms Inflammatory Bowel Disease AND neoplasms NOT colorectal cancer NOT intestinal neoplasms was conducted.Only studies in English language involving humans were included.
HEAD AND NECK CANCERS
Head and neck cancers (HNCs) include mainly squamous cancers of the oral cavity(OSCC),oropharynx (OPSCC),nasopharynx,hypopharynx and larynx (LSCC).Approximately 75% of HNCs are caused by alcohol and tobacco use and the remaining are mainly human papilloma virus (HPV) 16 related (HPV + HNCs)[12-17].
Only a few studies on HNCs and IBD have been published[18-21].Danish cohorts showed no increased risk for OSCC and OPSCC[22,23],while a recent US retrospective cohort by Katsanoset al[18] showed for the first time an increased risk for OSCC[standardized incidence ratio (SIR) 9.77 (95% confidence interval (CI):5.14-16.98)] in IBD patients.The risk was more pronounced in females (12-fold increase) compared to males (8-fold increase) and for tongue cancer (22-fold and 17-fold increase in females and males,respectively).A population-based cohort study by Mosheret al[24] also showed an increased 20-year risk of OSCC and OPSCC [20 yr relative risk (RR) 2.00(0.95-4.22)] but not of LSCC [20 yr RR 0.48 (0.07-3.43)].Indeed,a retrospective casecontrol Dutch study showed that IBD patients had a higher rate of HPV + HNCs(52.2%) compared to the general population and an impaired OSCC survival (P=0.018),not related to immunosuppression[19].Older age and UC diagnosis were risk factors for OSCC and OPSCC.A recent Dutch study showed that older age at IBD diagnosis (P<0.001) as well as male sex (P<0.001) were risk factors for LSCC in UC patients.On the other hand,tobacco use (P<0.001),structuring (P=0.006) and penetrating (P=0.008) disease were identified as risk factors in CD patients who developed LSCC,while immunosuppressive medication did not influence survival[20].Currently,studies on the prevalence of HNCs,risk factors and outcome in IBD patients are limited.Education on modifiable risk factors,such as smoking cessation,and safe sexual behavior,is the cornerstone of prevention of HNCs.In addition,given that IBD patients may have reduced immunosurveillance resulting in persisting HPV infections and cancer,oral screening and prophylactic HPV vaccines could be considered.
THYROID CANCER
Thyroid cancer (TC) is the most common endocrine cancer,and is more common in Caucasians,and young adults,predominantly females.Thyroid dysfunction,radiation exposure at a young age and heredity are known risk factors.Over 80% of all cases are of the papillary type[25].
A recent prospective multicenter Italian study showed that the prevalence of TC in IBD patients was 5.2%[26].Several studies,have shown that TC risk was not significantly higher in IBD patients compared with the healthy population[27-29].On the other hand,two studies reported an increased risk of TC in UC patients,the first in both sexes and the latter only in males[30,31].A recent meta-analysis of 8 case-control studies with 334015 patients,confirmed that patients with UC had an increased risk of TC,while patients with CD did not,independent of sex and race[32].On the contrary,a case-control study in Ohio,United States with 289935 IBD patients[33] and an Italian cohort study of 3664 IBD patients[9],reported a higher risk for TC only in CD patients[odds ratio (OR) 2.3 (1.06-5.1),P=0.034 and SIR 5.58 (95%CI:2.41-11.00),respectively].
Regarding the risk of immunosuppressive treatment for the development of TC in IBD,data are conflicting,with two studies reporting an increased risk in patients treated with immunosuppressants[29,31] and another two studies did not observe this relationship[28,32].
Most of the evidence is based on population-based studies[9,30-32] with many potential confounding factors,and limited studies are focused especially on the risk of TC in IBD patients[29,32,33].There is not enough evidence to show that IBD is an independent risk factor for TC,but IBD might provide an appropriate inflammatory environment and promote development of the cancer.Further well-adjusted and population-based studies should be conducted to confirm this speculation,in order that screening strategies for TC can be recommended.
LUNG CANCER
Lung cancer is one of the most common cancers and the major cause of mortality worldwide.Several population-based studies[10,34-36] and a recent meta-analysis by Lo in 2020 showed an increased risk [incidence rate ratio (IRR),1.53 (95%CI:1.23-1.91),P=0.00][37] of lung cancer in CD patients.Masalaet al[38] also reported increased mortality only in CD patients [SMR 4.00 (95%CI:1.60-8.24)].On the contrary,in UC patients no significant risk was found in any study[31,34,36,37,39] and actually a significantly lower risk of lung cancers was reported in two studies[29,40].The difference between CD and UC could be explained by the different association of the two diseases with smoking.In conclusion,further studies focused on lung cancer are needed and efforts should be made to guide patients to quit smoking,an important risk factor for both lung cancer and CD.
CHOLANGIOCARCINOMA
Cholangiocarcinoma (CCA) is a relatively rare EIC that mainly affects IBD patients in the context of primary sclerosing cholangitis (PSC)[41].PSC is a chronic inflammatory,immune mediated,liver disease causing fibrosis of the intrahepatic and extrahepatic bile ducts finally leading to strictures[42].The mechanisms of carcinogenesis in PSC are not well understood but it seems that CCA is the result of DNA damage caused by chronic biliary inflammation and bile acids in IBD patients with altered DNA repair ability[39].
Several studies have shown that IBD patients have an increased risk of biliary cancer (Table 1).A meta-analysis that included 17052 IBD patients showed that CD patients had a borderline significant increased risk of liver-biliary cancer (SIR 2.47,95%CI:0.95-6.46),while UC patients are at significantly increased risk (SIR 2.58,95%CI:1.58-4.22) and this is attributed to the occurrence of PSC in patients with IBD[10].The incidence of CCA in PSC patients ranges between 0.5-1.5/100 person-years[42,45,46].The risk of CCA is increased by 160-fold and PSC patients have a cumulative life-time risk of 5%-10%[44,47].Approximately 30%-50% of CCAs are diagnosed within the first year of PSC diagnosis[45,46,48].In another meta-analysis,it was shown that IBD patients were at increased risk of CCA (RR 2.63;95%CI:1.47-4.72,CD 2.69,95%CI:1.59-4.55 and UC 3.40,95%CI:2.50-4.62).In addition,further analyses concerning the site of CCA revealed that IBD patients are notably at increased risk of intrahepatic (RR 2.61,95%CI:1.72-3.95) and to a lesser extent extrahepatic CCA (RR 1.47,95%CI:1.10-1.97)[49].
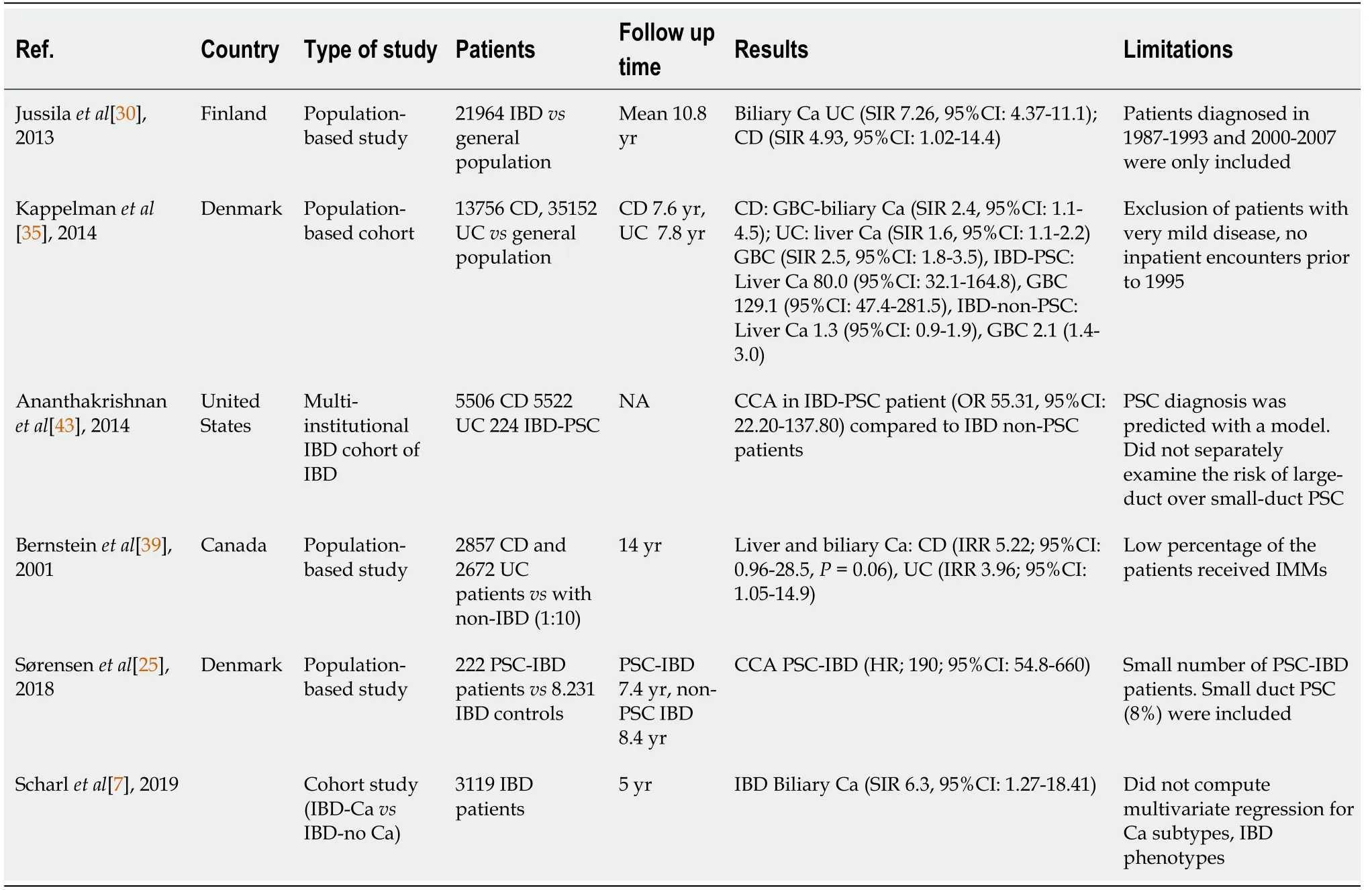
Table 1 Studies on cholangiocarcinoma in patients with inflammatory bowel disease
As for the risk factors of developing CCA in patients with PSC,those with prolonged IBD duration and those who underwent colectomy due to CRC or colonic dysplasia have an increased risk of CCA[50-52].On the other hand,in a Scandinavian cohort,although IBD duration was associated with increased risk of CCA,colectomy and CRC were not[48].CCA develops in chronic inflammation in patients with PSCIBD and particularly in those with dominant biliary stenosis,something that seems to be a predisposing factor[41].The definition of a dominant stenosis (DS) in PSC patients is a stricture less than 1.5 mm in diameter in the common bile duct or less than 1 mm in the left or right main hepatic duct[53].Approximately 10%-62% of PSC patients develop a DS at some point during their disease course[54,55].In a 25-year study of 128 PSC patients in the United Kingdom,the mean survival of the patients with DS was worst (13.7 years) than for those without a DS (23 years) and this difference was related to a 26% risk of CCA,which developed only in patients with DS.In 50% of patients with CCA,the diagnosis of CCA was made within 4 mo of the diagnosis of PSC[54].A German study of 171 PSC patients,who were followed prospectively for 20 years,confirmed that the presence of DS in PSC is associated with a worse prognosis due to increased risk of CCA and CRC.Furthermore,this risk was directly related to the presence of underlying IBD.In total,97 patients had DS,20 at entry and 77 developed DS over the follow-up period.In patients with DS without IBD,no CCA developed and the survival free of transplantation was 77.8% at 18 years.On the contrary,the 18-year survival was only 23% in the PSC with a DS and IBD.On the other hand,the presence of IBD had no impact on survival in those without a DS[56,57].The finding that the risk of CCA is related to the presence of IBD was not confirmed in a cohort of 241 Dutch PSC patients followed for a mean of 6 years[58].As far as small duct PSC is concerned,it has not been associated with an increased risk of both malignancies (CRC,CCA)[59].
CCA is considered to result in a poor prognosis and the surveillance strategy has not been proven to be beneficial.However,it has been suggested that patients with IBD and PSC should undergo magnetic resonance cholangiopancreatography (MRCP)annually,serum CA 19-9 testing periodically and annual colonoscopy as they also have a higher risk of CRC.
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) mostly occurs in IBD on the background of PSC liver cirrhosis[60] or other established chronic liver diseases such as viral hepatitis.The annual incidence of HCC in cirrhotic patients is 5%,and justifies the 6-mo surveillance with liver ultrasound ± alpha-fetoprotein.Nevertheless,there have been sporadic case reports of HCC in non-cirrhotic IBD patients,mostly in CD patients treated with azathioprine[61].Furthermore,it is of note that HCC develops more frequently in PSC-IBD patients compared to IBD non-PSC [hazard ratio (HR),21.00][62].However,most population-based studies address the risk of liver cancer without separating the histologic type (HCC or CCA) under the term liver or liver-biliary cancers.
GALLBLADDER CANCER
The risk of gallbladder cancer (GBC) mostly affects IBD patients in the context of PSC.Many studies have confirmed that PSC patients are at increased risk of GBC[62-70],and the prevalence is 3%-14%vs0.35% in the general population[63].The 10-year cumulative risk was 3% (95%CI:1-7) in PSC-IBD compared to 0% in non-PSC IBD patients[45].Furthermore,a study that evaluated 72 gallbladders from 100 liver explants-o (OLT) due to PSC showed that GBC was associated with intrahepatic bile duct dysplasia (P=0.001),CCA (P=0.023),and IBD (P=0.03) and gallbladder dysplasia was associated with hilar/intrahepatic bile duct dysplasia (P=0.0006),CCA(P=0.028),IBD (P=0.0014),and older age at OLT (P=0.007)[71].
A Danish study showed that CD patients had a significant increased risk of liver-GBC and UC patients had a more notable risk (SIR 2.5,95%CI:1.8-3.5) compared to the general population.Furthermore,this was profound for UC-PSC (SIR 129.1;95%CI:47.4-281.5) than in those without PSC (SIR 2.1;95%CI:1.4-3.0)[35].This indicates the need for annual surveillance with ultrasound of the gallbladder in PSC-IBD patients.
PANCREATIC CANCER
Pancreatic cancer (PC) is the seventh leading cause of cancer death worldwide with increasing incidence and mortality,and is considered to have an unfavorable outcome and prognosis.
In a recent large Scandinavian study with 161926 IBD patients,442 (0.27%) were diagnosed with PC compared with 3386 (0.21%) of the 1599024 IBD-free individuals.The 20-year cumulative incidence was 0.34% (95%CI:0.30-0.38)vs0.29% (95%CI:0.28-0.30),while the IR was 22.1 (20.1-24.2)/100000 person-years in IBD patients.The overall HR was 1.43 (1.30-1.58) [CD 1.44 (1.18-1.74),UC 1.35 (1.19-1.53),IBD unclassified was 1.99 (1.50-2.64)],whereas in IBD-PSC it was 7.55 (4.94-11.5)[72].In a Korean study,the risk was only increased in females with CD (SIR 8.58;CI:1.04-31.00)[30].On the contrary,a previous meta-analysis and several population-based cohort studies have shown that the risk of PC is non-significantly increased in IBD patients [pooled SIR 0.51 (0.06-4.57) and 0.75 (0.30-1.87) accordingly][7-10].IBD-PSC patients should be referred separately since they are at a higher risk of PC[62].A Swedish study,which included PSC patients (79% had concurrent IBD),showed that they had a 14-fold greater risk of PC than the background population[46].In another study of 224 PSCIBD patients,a significant increased risk was confirmed (OR 11.22,95%CI:4.11-30.62)compared to IBD non-PSC patients[43].Given that higher CCA risk in PSC is well established,we could assume that the increased PC risk might be due to misdiagnosis of periampullary cancer.
GASTRIC MALIGNANCIES
The cause of gastric cancer (GC) in IBD patients is uncertain.GC usually develops on the background of intestinal metaplasia and dysplasia due toHelicobacter pylori(H.pylori) infection and chronic inflammation[73].Nevertheless,the prevalence ofH.pyloriin IBD patients is low[74],while a possible causative relation could be attributed to the upper gastrointestinal involvement of CD.A previous review and meta-analysis study,found that CD patients had a significantly increased risk of cancer of the upper gastrointestinal tract (SIR 2.87,95%CI:1.66-4.96) and of the stomachper se(SIR 2.05,95%CI:1.06-3.97)[10].However,most recent population-based studies[7,11,30,35,39,75]did not confirm this finding.With regard to the risk factors and survival of IBD patients with GC,a study that included 59 GC cases in IBD individuals,showed that UC was more frequent among these patients (69.5%vs51.4%;P<0.01) compared with 177 IBD controls,and IBD patients with GC showed reduced survival (P=0.035;HR 1.385,CI:1.023-1.875) compared with 1534 individuals of the general population with the same malignancy[76].Studies on GC in IBD patients are limited and no secure conclusions can be drawn.
BREAST CANCER
Breast cancer (BC) is the second most common malignancy diagnosed worldwide and its mortality is decreasing over time due to screening protocols and improved therapy.Increasing age,prolonged estrogen exposure,obesity in postmenopausal women,reproductive and genetic factors,western life-style,smoking and alcohol are known risk factors[77].
Limited data are available on the pathogenesis of BC in IBD[78].IBD patients seem to have a shorter period of estrogen exposure than the general population,with later onset of menarche and earlier menopause,that could be related to a lower BC risk[78,79].
No studies primarily investigating the risk of BC in IBD are available.Data,generally not adjusted for confounding factors,can be derived mainly from population-based cohort studies investigating the general and type-specific risk of malignancies[6,15,30,36,39,75,80-88].In 2012,Hemminkiet al[84] showed a decreased risk for BC only for CD patients (SIR 0.85,95%CI:0.75-0.97) and a decreased mortality rate (HR 0.75,95%CI:0.58-0.98),and similar results were seen in the Dutch IBD-SL cohort (n=1157;SIR 0.11 (95%CI:0.00-0.64)[15] and the TREAT registry cohort study [n=2975;SIR 0.28 (95%CI:0.08-0.72)][86].On the other hand,a Swedish cohort study conducted in 21788 CD patients showed a 30% higher BC risk in hospitalized patients between 45 and 64 years,possibly reflecting the advanced risk at older age or the exposure to IBD treatment or unregistered confounders[83].Similarly,a retrospective Taiwanese cohort study by Tsai in 2014 indicated that IBD is not associated with increased BC risk (aHR 0.95 (95%CI:0.66-1.36);however,there was an association between the frequency of IBD-related hospitalizations and BC risk in patients less than 65 years old (aHR 8.45;95%CI:4.64-15.4)[87].An increased risk of BC in both UC and CD was reported by the IBSEN study in 2016[88].A large cohort study[36],a Finish register study[30],and two meta-analyses,by Pedersenet al[10] and by Loet al[37],showed no difference in the occurrence of BC in both UC,CD and the general population,but data on treatment modalities were lacking.
No association between the use of thiopurines and BC has been reported by cohort studies and small series[22,75,89-93].The correlation between BC and the use of biologics in IBD patients has been addressed in several reports[94-98].A large nationwide Danish register-based cohort study with 56146 IBD patients and a long followup,showed no increased BC risk for patients treated with infliximab,adalimumab or certolizumab[94].In the TREAT Registry cohort study consisting of CD patients,a decrease in the occurrence of BC was observed in both patients exposed and nonexposed to infliximab [SIR 0.50 (95%CI:0.24-0.92) and SIR 0.32 (95%CI:0.12-0.70),respectively],compared to the SEER database of the general United States population[86].No association for vedolizumab was also reported in a study involving 2830 IBD patients with a follow-up period up to 5 years[95].In addition,no increased risk of BC in IBD patients exposed to adalimumab monotherapy or combination therapy with thiopurine or methotrexate was suggested in a pooled analysis of 1594 CD patients[96].Similar results were shown in a pooled analysis of 5 small studies and 5 landmark trials comprising 2385 IBD patients[99] and by a meta-analysis,involving 22 randomized controlled trials comparing anti-tumor necrosis factor (TNF) therapyvsplacebo,even if only 4 trials had a low risk of bias[100].
So he went out from the feast, where they all sat drinking and making merry, and he walked alone beside the sea in the dusk of the evening, at the place where the great chest, with himself and his mother in it, had been cast ashore10
In conclusion,the risk of BC is probably decreased in CD patients and seems to be similar to the background population in UC patients.IBD and anti-TNF therapy do not seem to affect BC incidence;however,for thiopurines and combination therapy,this is still uncertain.Future studies on the pathophysiological association between BC and IBD may help identify populations at high risk for BC,who might therefore benefit from close surveillance.
CERVICAL NEOPLASIA
Cervical neoplasia (CN) consists of dysplasia or cervical intra-epithelial neoplasia(CIN) and invasive cervical cancer (CC)[101].High-risk HPV infection (types 16,18,45,31) is considered the causal agent of CN,whereas smoking,sexual,socioeconomic,and immunologic co-factors may contribute to persistent infection[102].Most low-grade lesions regress spontaneously but most high-grade dysplasia and CIN 2/3 do not[103,104].While HPV vaccines and Papanicolaou (Pap) smear have substantially reduced CC incidence and mortality,these are still high in developing countries and CC remains the second most common cancer in women worldwide[105-107].
The risk of CN in patients with IBD remains controversial and the role of immunosuppressants,a known risk factor for cervical dysplasia in other immunemediated diseases,is not clear[108,109].It may be hypothesized that the underlying immunologic changes in IBD or immunosuppressive drugs may reactivate HPV from a latent status or decrease HPV clearance and CIN regression or make HPV vaccination less effective.
Data on the association between IBD,treatment and CN are conflicting[31,75,91,110-119] (Table 2).Also,non-conclusive are the results of two meta-analyses.Allegretti in 2015,in a meta-analysis of 8 studies with 77116 IBD patients but also heterogeneity,found that IBD patients had an increased risk of high-grade dysplasia/cancer compared to healthy controls (OR 1.34,95%CI:1.23-1.46) and the risk was greater with the use of immunomodulators,corticosteroids and 5-aminosalicylic acids,but not with anti-TNF[120].A recent meta-analysis by Loet al[37] in 2020 reported no statistically increased risk for CC in IBD patients.

Table 2 Studies on cervical neoplasia in patients with inflammatory bowel disease
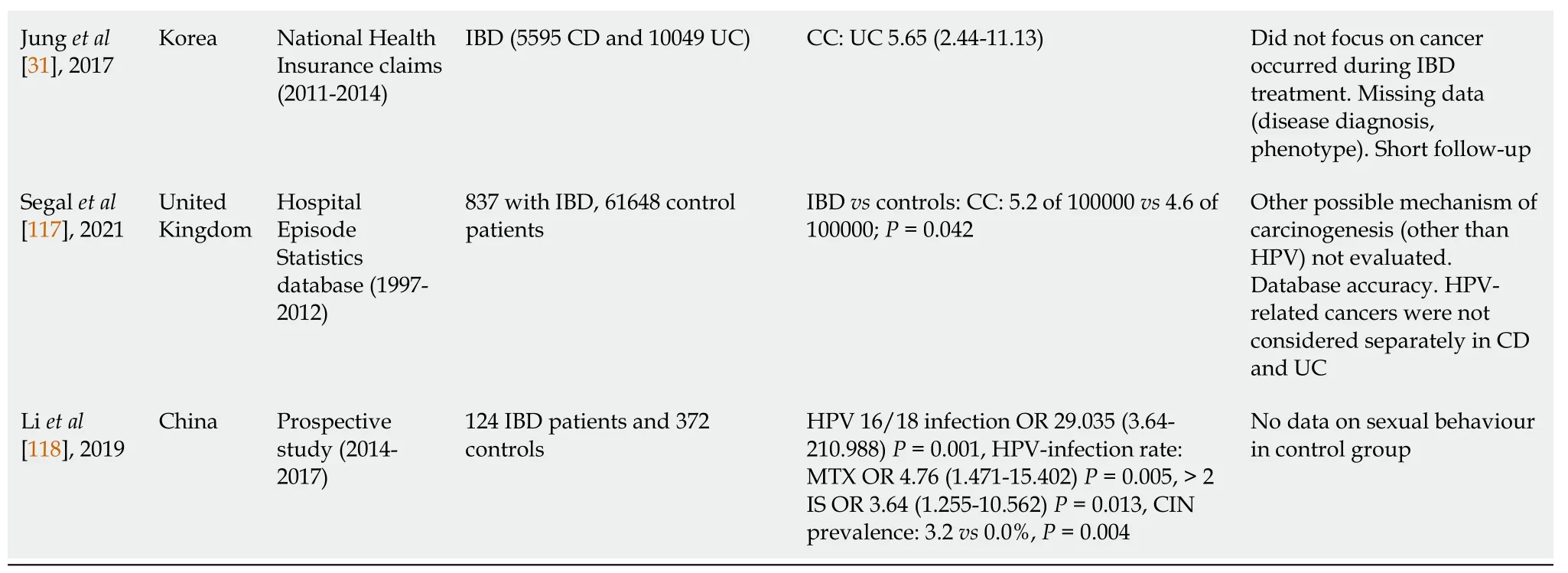
aP:Compared to controls.bP:Compared to non-exposed.IBD:Inflammatory bowel disease;AZA:Azathioprine;Pap:Papanicolaou;CC:Cervical cancer;SIR:Standardized incidence ratio;IFX:Infliximab;6-MP:6-Mercaptopurine;UC:Ulcerative colitis;CD:Crohn’s disease;OR:Odds ratio;aOR:Adjusted odds ratio;CI:Confidence interval;IS:Immunosuppressant;TP:Thiopurine;MTX:Methotrexate;ASA:Amino-salicylate;IMMs:Immunomodulators;anti-TNF:Anti-tumour necrosis factor;SID:Systemic inflammatory disease;HPV:Human papilloma virus;HR:Hazard ratio;IRR:Incidence rate ratio;CIN:Cervical intraepithelial neoplasia.
Regarding screening adherence some studies indicated that this is influenced by various factors such as the state system,increased age and use of immunosuppressants and there is no significant difference with non-IBD patients[121-123].Singh showed that women with IBD in Canada had a low (54%) screening adherence and older age,lower socioeconomic status,lower intensity of healthcare utilization,CD and exposure to immunosuppressant medications were independent predictors of lower use of Pap testing[121].In the United States,Longet al[122] and Xuet al[123] showed higher adherence (70%).
In many health care systems,it is not clear who has the primary responsibility for CC prevention.As incidence and mortality of CN are highly dependent on screening and treatment of precursor lesions,an inter-specialist cooperation is suggested to guide IBD patients to follow all preventive measures such as HPV vaccination,between 9 and 26 years and prior to the initiation of the sexual activity,in both men and women,annual screening testing for chronically immunocompromised patients and smokers starting at age 21,safe sexual practices and smoking cessation[124].
OTHER GYNAECOLOGICAL CANCERS
No studies primarily investigating the incidence and mortality risk of endometrial,ovarian,or vulvar-vaginal carcinoma in IBD have been identified.Data extracted from large cohort studies investigating the general risk of malignancies,an older metaanalysis and a recent meta-analysis in 2020 showed no increased risk of these cancers even when immunosuppressive medication was used[10,29,31,34,35,37,39,40,80-86,125-127].
URINARY TRACT CANCERS
The urinary tract cancers (UTC) include bladder cancer,that occurs at advanced age,predominantly in males,smokers or in association with chronic inflammation,and renal cell carcinoma (RCC),that is related to smoking,obesity,and hypertension[128-131].Elevated levels of TNF,a key mediator of cancer-related inflammation,have been reported in the early stages of RCC due to loss of the Von Hippel-Lindau tumorsuppressor gene[132,133].

Table 3 Studies on urinary tract cancer in patients with inflammatory bowel disease
With regard to bladder cancer,an increased risk in IBD patients was clearly demonstrated by a meta-analysis of eight population-based cohort studies with 17052 IBD patients in 2010 (SIR 2.03,1.14-3.63)[10] and a prospective Spanish cohort study[85].Moreover,long-standing IBD and use of immunosuppressive medication were found to be associated with the development of bladder cancer[137].It is noteworthy that a reversible increased risk of UTC in patients treated with azathioprine has been reported by two large studies[22,138].
Cancer risk assessment in patients under anti-TNF therapy is problematic due to frequent combined treatment with thiopurines.Anti-TNF monotherapy does not increase either UTC risk,according to the results of a population-based prospective cohort nationwide Danish study of more than 56000 IBD patients[94] or RCC risk,independent of diagnosis age,type,and duration of IBD or coexistence of known RCC risk factors[139].
There are currently no screening guidelines for UTC in any population,while incidental detection of RCC has been identified as a positive prognostic index[140,141].Whether patients with a long IBD duration may require different surveillance programs for preventing UTC needs to be confirmed,but all CD patients should be encouraged to quit smoking as tobacco could be a key risk factor[128,142,143].Limited evidence is available on the role of immunosuppressive therapy in the development of UTC;however,elderly men on thiopurine therapy,should be closely evaluated for UTC.
PROSTATE CANCER
Prostate cancer (PC) is the second most common cancer in men worldwide,with screening tools available for early diagnosis[144].The most important risk factors are age,African American ethnicity,genetic and possibly dietary factors.
Some studies have indicated an increased risk of PC in IBD,especially in UC,while others did not confirm this[24,29-31,35,36,39,40,75,83,84,144-147] (Table 4).Intensive surveillance in IBD patients with digital rectal and prostate examination may be a reason for the high incidence of PC,but also potential pathophysiological mechanisms of IBD and PC association have been recognized.In principle,the distinct microbiome level between UC and CD may be related to the elevated risk of PC in UC[148-150].In addition,simultaneous elevation of IBD-related inflammatory markers,such as Creactive protein,and of prostate-specific antigen (PSA),known prostatic inflammation or PC index has been reported[151-155].Receptors of pro-inflammatory cytokines,such as interleukin 6 (IL-6)[156-158],and folate hydrolase 1 (FOLH1)/prostate specific membrane antigen (PSMA) are up-regulated in both IBD and PC and in fact FOLH1/PSMA appears overexpressed in cases of biochemical recurrence with an increase of PSA and metastatic disease[159-163].
A meta-analysis of 9 studies involving 17052 IBD patients by Pedersenet al[10] in 2010 showed no association between IBD and PC [IBD:SIR 1.16 (0.88-1.52),UC:SIR 1.14 (0.85-1.42) CD:SIR 0.77 (0.41-1.45)].These results were not confirmed by recent studies and on the other hand,an increased risk of PC in patients with UC was reported (Table 4).Actually,an increased risk worldwide only for UC patients was shown in another meta-analysis of nine population-based studies by Geet al[164] in 2020,with a large sample and a long follow-up of patients [Cohort studies:IBD RR 1.33 (1.03-1.71);UC RR 1.58 (1.08-2.30);CD RR 1.12 (0.97-1.31);Case control studies:RR 1.81 (1.43-2.29)].In addition,a recent meta-analysis of eight studies by Chenet al[165]in 2020 reported two interesting results.For the first time a higher risk was also found for CD patients [IBD RR 1.78 (1.32-2.41);UC RR 1.76 (1.06-2.91);CD RR 1.29 (1.04-1.61)]and a higher risk in Asian patients with IBD compared to Caucasians was shown [RR 3.02 (95%CI:2.03-4.48) and 1.6 (95%CI:1.17-2.19),respectively],in contrast with the known lower risk of PC in the Asian general population[165].It is noteworthy that two other meta-analyses in 2020,by Carliet al[166] [IBD RR 1.71 (1.16-2.51)P=0.007;UC RR 1.21 (0.98-1.51)P=0.07;CD RR 1.10 (0.98-1.25)P=0.12] and by Loet al[37] [UC SIR 1.08 (0.9-1.3);CD SIR 1.04 (0.8-1.35)] did not demonstrate a higher risk of PC in the subgroup analysis of UC or CD.

Table 4 Studies on prostate cancer in patients with inflammatory bowel disease
In conclusion,data for PC risk in IBD patients are conflicting.Further prospective studies are needed to evaluate the impact of concomitant medications on the risk of developing PC and the possible utility of screening programs for early diagnosis of PC in patients with IBD.
SKIN MALIGNANCIES
Several population-based studies have shown an increased prevalence of skin malignancies among patients with IBD[10,167-169].Furthermore,the incidence of skin cancer among those receiving immunosuppressants is rising over time[170].On the contrary,other population-based studies showed that there is noper seincreased risk of skin cancer in IBD patients[34,39,75].The skin cancers include melanoma (MSC) and non-melanoma skin cancers (NMSC) with the latter further divided into squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).It is well established that anti-TNF exposure is associated with the development of MSC[169],whereas NMSC is associated with thiopurines exposure (azathioprine,6-mercaptopurine)[171].With regard to the mechanism by which thiopurines may predispose patients to skin cancer the most prominent theory supports that thiopurines increase the vulnerability to UVA radiation.In detail,they cause the accumulation of 6-thioguanine in the DNA of patients and further UVA radiation causes production of reactive oxygen species that leads to DNA mutations and oxidative stress,resulting in oncogenesis[172].On the other hand,MSC is an immunogenic aggressive tumor,in which suppression of the immune response allows these tumors to grow and metastasize[173].For this reason,immunosuppressants may cause down-regulation of the tumor surveillance mechanisms,increased susceptibility to infection with oncogenic viruses (e.g.,melanoma associated retroviruses),or direct pharmacologic effects of these medications on DNA metabolism[174].
There is evidence from several studies of an increased risk of NMSC in IBD associated with thiopurines use (Table 5).These findings were not confirmed by two other studies that found no increased NMSC risk among IBD thiopurine users[89,175].A meta-analysis that pooled 60351 IBD patients to assess the risk of NMSC in those treated with thiopurines,showed that the risk was only modestly elevated (aHR 2.28;95%CI:1.50-3.45)[184].There is also evidence of an increased risk of MSC in IBD and this risk is associated with anti-TNF use (Table 5).In a review and meta-analysis that included 172837 IBD patients,179 MSC cases were reported (pooled crude IR 27.5/100000 person-years;95%CI:19.9-37.0) and a 37% increased risk of MSC,independent of biologic therapy[185].On the contrary,a Danish Cochrane review and meta-analysis (56146 IBD patients 8.1% anti-TNF exposed) did not show increased risk of MSC in those treated with anti-TNF (HR 0.62;CI:0.08-4.57)[94],which was further confirmed by a recent systematic review and meta-analysis (comprising 34029 biologic-treated and 135370 biologic-naive patients),where biologics did not significantly increase the risk of MSC[186].In a recent study that included IBD patients with MSC,IBD extent was found to be a risk factor,both in UC (pancolitis OR:3.09;95%CI:1.670-5.727) and CD (ileocolonic disease:OR:1.98;95%CI:1.009-3.882),corticosteroids were risk factors in UC (OR:1.41-3.72) whereas anti-TNFs were protective factors in UC (OR:0.15-0.88) and CD (0.27-0.92),but this was attributed toin-situmelanoma only.Moreover,no association between survival of IBD-MSC patients and anti-TNF or immunosuppressants after MSC diagnosis was found[181].

Table 5 Studies on skin cancer in patients with inflammatory bowel disease
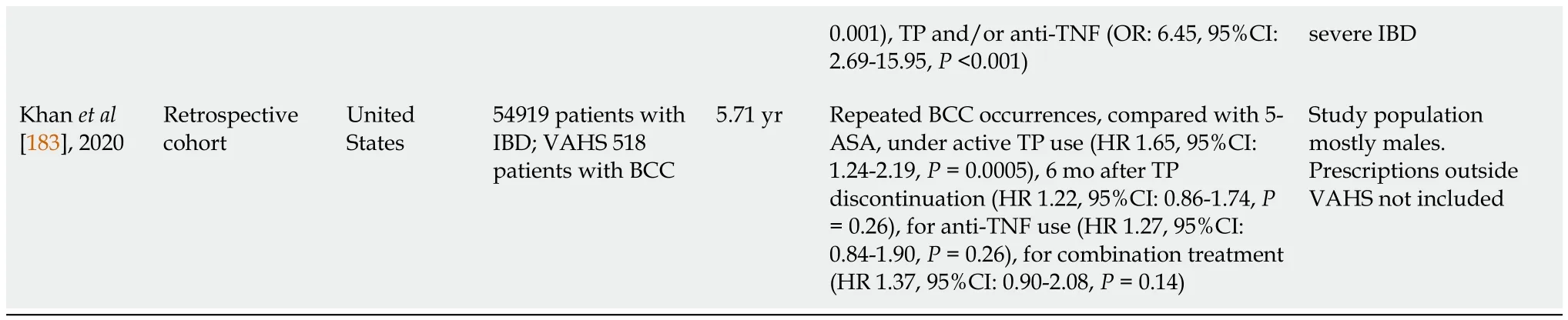
0.001),TP and/or anti-TNF (OR:6.45,95%CI:2.69-15.95,P<0.001)severe IBD Khan et al[183],2020 Retrospective cohort United States 54919 patients with IBD;VAHS 518 patients with BCC 5.71 yr Repeated BCC occurrences,compared with 5-ASA,under active TP use (HR 1.65,95%CI:1.24-2.19,P=0.0005),6 mo after TP discontinuation (HR 1.22,95%CI:0.86-1.74,P=0.26),for anti-TNF use (HR 1.27,95%CI:0.84-1.90,P=0.26),for combination treatment(HR 1.37,95%CI:0.90-2.08,P=0.14)Study population mostly males.Prescriptions outside VAHS not included
The known risk factors for the development of skin cancers include smoking,older age,male sex,fair skin type,cumulative sun exposure (mostly for NMSC),sun-burn(mostly for MSC),family history of skin cancer,Caucasian race,geographical area and certain genetic factors (e.g.,p53 polymorphisms).All these factors should be taken into account when immunosuppressant therapy is being considered for a patient with IBD.Given these findings,drugs other than anti-TNFs might be safer for use in patients after a melanoma diagnosis or in those at high risk for these tumors,whereas thiopurines should also be avoided in patients with a history of SCC,multiple BCCs,or premalignant skin lesions (e.g.,solar keratosis)[187].
With regard to the maintenance of immunosuppressive treatment after the initial diagnosis of skin cancer,the results from various studies have been controversial.A large cohort study showed that thiopurines did not increase the risk of second NMSC when used in combination treatment compared with anti-TNF monotherapy (HR 0.79,95%CI:0.30-2.08)[180].In addition,a previous study by Beaugerie concluded that exposure to immunosuppressants did not increase the risk of new or recurrent malignancy in individuals with previous cancer[188].On the contrary,another recent cohort study with 54919 IBD patients,of whom 518 developed BCC and maintained their treatment,it was shown that repeated BCC occurrences were associated with active thiopurine use[183].In line with this,a large meta-analysis that included 3706 IBD patients with 10332 person-years of follow-up after a cancer diagnosis showed,in a subgroup on skin cancer alone,that the risk was significantly higher in immunosuppressant-users (71.6 per 1000 person-year,95%CI:58.9-84.2,P=0.035) compared to those not receiving immunosuppressants (50.8 per 1000 person-year,95%CI:43.7-57.8)and numerically higher than anti-TNFα therapy (55.5 per 1000 person-year,95%CI:44.7-66.3,P=0.22)[189].
With regard to prevention,a recent study showed that only a small proportion of IBD patients (8.3% during the 7-year study period) were seeking dermatologic care[190].
In conclusion,it seems that there is an increased risk of NMSC and a slightly lower risk of MSC in IBD patients,which justifies the recommendations for routine screening for skin malignancies.Although there is some evidence of second NMSC after an index diagnosis with azathioprine,overall data are conflicting regarding maintaining immunosuppressive treatment after the diagnosis of a skin malignancy and the risk of recurrence or a second skin malignancy,so the decision should be individualized.Conclusively,primary prevention measures,such as sun-protection,annual dermatologic examination and smoking abstinence should be encouraged in all IBD patients,regardless of thiopurine and/or anti-TNFα use.
CONCLUSION
Cancer is the second cause of death in patients with IBD,after cardiovascular disease.Lately there has been evidence that the incidence of intestinal cancers is decreasing over time in IBD patients,as a result of the implementation of a tight screening strategy.Although recent data supporting that there is no excess overall risk of extraintestinal cancers,it seems that IBD patients are in higher risk of certain extraintestinal cancers.This indicates the need for close monitoring,as it seems that chronic systemic inflammation but also other factors such as older age,smoking,specific virus infections (e.g.,HPV) and concurrent diseases (PSC) play a role in carcinogenesis.The IBD treatments,for both CD and UC,are aimed at clinical improvement,endoscopic remission and sustained mucosal healing.Although immunosuppressants,including azathioprine,methotrexate and anti-TNFs,constitute the cornerstone of IBD treatment,these drugs might have a carcinogenic effect either by directly altering cellular DNA or reducing tumor immunosurveillance.Lately there are more treatment choices available,such as anti-integrins,anti-IL-12/23 and JAK inhibitors.Although these new treatments are considered to have a better safety profile as far as cancer is concerned,data are limited and future studies are required.
Specialists who deal with the management of IBD patients must bear in mind the possibility of extraintestinal malignancies,especially in those with long standing immunosuppressive therapies and persistent systemic or local inflammation.Guidelines and recommendations should incorporate all the preventative measures[sun protection,dermatologic examination,vaccinations (HPV,hepatitis B virus),annual Pap smear,mammography,PSA testing,annual MRCP where appropriate]and applied in everyday clinical practice to diminish the risk of IBD-related extraintestinal malignancies,achieve an early diagnosis and improve the prognosis and overall outcome.
 World Journal of Gastrointestinal Oncology2021年12期
World Journal of Gastrointestinal Oncology2021年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Management of obstructive colon cancer:Current status,obstacles,and future directions
- Role of endoscopic ultrasound in anticancer therapy:Current evidence and future perspectives
- Mesenchymal stem cell-derived exosomes for gastrointestinal cancer
- Gender differences in the relationship between alcohol consumption and gastric cancer risk are uncertain and not well-delineated
- Pancreatic intraductal papillary mucinous neoplasms:Current diagnosis and management
- Combined treatments in hepatocellular carcinoma:Time to put them in the guidelines?
