Composite intestinal adenoma-microcarcinoid: An update and literature review
Zhi-Yan Fu, Michel Kmeid, Mahmoud Aldyab, Stephen M Lagana, Hwajeong Lee
Zhi-Yan Fu, Michel Kmeid, Mahmoud Aldyab, Hwajeong Lee, Pathology and Laboratory Medicine, Albany Medical Center, Albany, NY 12208, United States
Stephen M Lagana, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY 10032, United States
Abstract Composite intestinal adenoma-microcarcinoid (CIAM) is a rare intestinal lesion consisting of conventional adenoma and small, well differentiated carcinoid [microcarcinoid (MC)] at its base.The incidence of CIAM is 3.8% in surgically resected colorectal polyps.While its pathogenesis is unknown, studies support the role of Wnt/β-catenin pathway in the tumorigenesis of CIAM.CIAMs have been primarily reported in the colon wherein they present as polyps with well-defined margins, similar to conventional adenomatous polyps.MC is usually found in adenomatous polyps with high-risk features such as large size, villous architecture, or high grade dysplasia.Histologically, the MC component is often multifocal and spans 3.9 to 5.8 millimeters in size.MC is usually confined within the mucosa but occasional CIAM cases with MC extending to the submucosa have been reported.MC of CIAM demonstrates bland cytology and inconspicuous proliferative activity.The lesional cells are positive for synaptophysin and 60% to 100% of cases show nuclear β-catenin positivity.MC poses a diagnostic challenge with its morphologic and immunohistochemical resemblance to both benign and malignant lesions, including squamous morules/metaplasia, adenocarcinoma, squamous cell carcinoma, sporadic neuroendocrine tumor and goblet cell adenocarcinoma.CIAM is an indolent lesion with a favorable outcome.Complete removal by polypectomy is considered curative.Awareness and recognition of this rare entity will help arrive at correct diagnosis and improve patient care.Currently, CIAM is not recognized as a subtype of mixed neuroendocrine-nonneuroendocrine neoplasm by WHO.
Key Words: Composite; Adenoma; Microcarcinoid; Composite intestinal adenomamicrocarcinoid; Wnt/β-catenin; Mixed neuroendocrine-non-neuroendocrine neoplasm
INTRODUCTION
Composite intestinal adenoma-microcarcinoid (CIAM) is a rare intestinal lesion consisting of conventional adenoma and associated microscopic well-differentiated neuroendocrine cell clusters [microcarcinoid (MC)] at its base.The adenoma component presents as a typical polyp, which is removed either endoscopically or surgically[1-3].The MC component does not form grossly evident nodules or masses[1,3] and is typically located at the base of the polyp, usually within the mucosa.Occasional cases of CIAM with the MC component extending into the submucosa have been reported[1,4,5].As MC occupies only a minute area and forms small nests or clusters microscopically, the overall architecture of the polyp is preserved[2,3].
CIAM was first described by Moyanaet al[6] in 1988.In this report, the authors described two adenomas co-existing with carcinoids: One was in the center of a domeshaped polyp, and the other was at the base of a sessile villous adenoma.The authors also noticed a transition zone between the two components.It is unclear how much of the lesion was composed of carcinoid component in their report.However, based on the illustrations provided in the report, the carcinoid components do not appear subtle[6].Since its first description, CIAM have been sporadically documented as case reports or small case series[2,5,7,8].
Although CIAM is a rare entity, endocrine cell “differentiation” is not uncommon in colorectal adenomas, wherein the cells of neuroendocrine phenotype are considered to originate from the endoderm[9,10].For example, argyrophil cells have been reported in 59% to 85% of adenomatous polyps[10,11].In Iwashita’s study, argyrophil cells and argentaffin cells were found in 76.4% and 60.4% of 212 colorectal adenomas, respectively.These cells were usually located in the lower third portion of the adenomatous glands[9].In 8% to 10% of these cases, the density of the neuroendocrine cells may be higher than usual[9,10].Therefore, it is not surprising that endocrine cell neoplasia may arise within adenomas and that it localizes preferentially at the base of the adenoma[2].
CIAM is distinct from mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN).MiNEN is an umbrella term referring to a neoplasm with both neuroendocrine and non-neuroendocrine components[4,12].It is required that each component constitutes at minimum 30% of the neoplasm to qualify for MiNEN[12-14].The terms “low grade” MiNEN and mixed adenoma well-differentiated neuroendocrine tumor (MANET) have been interchangeably used in the literature for a subset of CIAM meeting the required criterion of 30% for each component[4,12].However, not all CIAMs described in the literature are necessarily low grade MiNEN.Moreover, recent WHO did not officially endorse a composite tumor consisting of an adenoma (a precursor of invasive adenocarcinoma) and well-differentiated neuroendocrine tumor as a subtype of MiNEN in the gastrointestinal tract and hepatopancreatobiliary organs[14].
Although this rare entity is not recognized by the current WHO classification, its recognition will allow for more efficient pathological diagnosis and more detailed clinicopathologic studies, thus leading to better patient care.CIAM may be underrecognized given its rarity and occasional morphologic subtlety.Moreover, it can resemble other benign and malignant lesions and can be mis-diagnosed.Its prognosis is vastly different from that of malignant composite tumors with expansile growth.We summarize the current state of knowledge on CIAM and provide an overview on its pathogenesis, microscopic features, differential diagnosis, as well as prognosis and treatment options.The differences in terminologies–CIAM, collision tumor and MiNEN–are also briefly discussed.
DEMOGRAPHICS
CIAM is identified in middle-aged to elderly patients, with a reported mean age of 60 years[1-4].Slight male predilection has been reported[1,4,15], while another study found no gender predilection[3].It is unknown whether there is a demographic divergence between CIAM and typical adenomatous polyps.
INCIDENCE
Recently we reported that the incidence of CIAM is 3.8% in surgically resected colorectal polyps.Our cohort consisted of consecutive, surgically resected 158 colorectal polyps from one tertiary care center over a span of 16 years[1].Its incidence in endoscopically removed polyps is unknown.
To date, the largest series of colorectal CIAM has been reported by Kimet al[3] in South Korea, consisting of 24 cases.In their series, the polyps were excised endoscopically (91.7%) or surgically (8.3%) over a span of 7 years[3].In the United States, the largest series of intestinal (to include 4 cases in the duodenum) CIAM was reported by Estrellaet al[15] in a Cancer Center, consisting of 25 cases over a span of nearly 18 years[15].However, the incidence of CIAM was not reported in these studies.
ASSOCIATED CONDITIONS
Colorectal MC is likely exceedingly rare and no minimum size criterion is currently available.MC has been observed in patients with chronic colitis, such as diversion colitis[16] and inflammatory bowel disease (IBD), especially in ulcerative colitis[17-21].Likewise, Weyantet al[22] described a case of colonic MC and diffuse neuroendocrine cell hyperplasia following long-term cystoplasty[22].These associations suggest that MC may represent an exaggerated proliferative response of gut mucosa to chronic inflammation.
On the other hand, it is largely unknown whether these patients with inflammatory conditions actually have a higher incidence of CIAM.Most reported CIAMs are sporadic, and it appears to be a much rarer condition than solitary MC[3].Sigel and Goldblum[17] described a well differentiated neuroendocrine tumor adjacent to high grade glandular dysplasia in the setting of IBD.The authors postulated that the neuroendocrine tumor might have originated from multipotential dysplastic cells in the adjacent mucosa[17].Alternatively, the MC component may reflect a metaplastic phenomenon related to chronic injury of the overlying adenomatous component[7].
Genetic predisposition may account for some cases of CIAM.Carcinoids at the base of duodenal adenomas have been reported in association with familial adenomatous polyposis (FAP)[15,23].These observations support a role of the adenomatous polyposis coli (APC)/β-catenin pathway in the pathogenesis of CIAM (to be discussed below), although the risk of CIAM is probably explained by the risk of adenoma in this cohort.
PATHOGENESIS
The mechanism for the development of MC component in CIAM is not well understood.Earlier, authors postulated that CIAM represents a form of collision tumor wherein the two components arise from two different clones and they coincidentally occur adjacent to one another[8].However, evolving knowledge regarding the multipotent stem cells in the gut and their role in tumorigenesis has shed light on the possible histogenesis of tumors with different histologic components such as CIAM.Indeed,in vitrostudies of the ileal epithelial cells (IEC-18) of rat have shown that these cells can transform into differing cell types with one type showing neuroendocrinelike morphology and expressing serotonin receptor gene, and the other with adenomalike mRNA transcription and protein expression[24].
Likewise, a morphologic “transition zone” has been observed in several studies of CIAM[2,4,6,25].In Pulitzeret al[2]’s study, the MC appeared to arise directly from the basal epithelium of adenomatous crypts, penetrating the basement membrane and infiltrating the lamina propria[2].La Rosaet al[4] also observed numerous cells with both morphologic and immunohistochemical neuroendocrine differentiation along the base of the adenomatous glands.In addition, these cells demonstrated the same mutational and microsatellite instability profile as the adenomatous components, further supporting the hypothesis that these two components most likely represent divergent differentiation of a common precursor[4].Interestingly, unlike conventional adenomas without MC, noKRASmutation was identified in either component of CIAM.These findings suggested that the adenoma component of CIAM may develop through an alternativeKRAS-independent pathway[4].
The finding of CIAM in FAP patients suggests the involvement of the Wnt/βcatenin pathway in the tumorigenesis of CIAM, as expected based on the canonical pathway by which normal mucosa becomes adenomatous.The MC components of CIAMs frequently display strong and diffuse nuclear β-catenin reactivity by immunohistochemistry[1,2,7,15].In Estrellaet al[15] study, the level of nuclear β-catenin expression was higher in the MC component of CIAM when compared with either the sporadic neuroendocrine tumors without associated adenoma, or neuroendocrine carcinomas associated with adenoma.Moreover, there was no difference in the level of β-catenin expression between CIAM patients with and without FAP[15].
This plausible hypothesis, though, requires confirmation by additional molecular studies as neither the presence nor absence of nuclear β-catenin expression by immunohistochemistry appears to be a true reflection of an activated Wnt signaling pathway[15,26-29].For example, Suet al[29] found that carcinoid tumors can show nuclear β-catenin immunohistochemical staining without mutations in theβ-cateninandAPCgenes[29].
In summary, CIAM appears to represent a true composite tumor with a common origin for the MC and adenoma components, and is not a collision tumor.Further molecular studies are needed to better understand the mechanisms driving its tumorigenesis.
PRESENTATION
CIAMs have been reported in the stomach, duodenum, ileum, colon, and rectum[4].They are predominantly found in the colon, usually in the cecum and right colon[1-3].They present as polyps with well-defined margins, similar to conventional adenomatous polyps.The reported mean size of the polyps is 2.4 cm[3].As the MC component is microscopic, it is incidentally found during the pathologic examination of otherwise typical adenomatous polyps.
To the best of our knowledge, no definite clinical symptoms related to the MC component of CIAM have been established, however, one case report of rectal “collision tumor” consisting of adenoma and carcinoid tumor presented with carcinoid syndrome (elevated serum serotonin and chromogranin A, elevated urine 5-hydroxyindoleacetic acid level, and moderate tricuspid regurgitation).The patient’s symptoms subsided following the endoscopic removal of the polyp with wide margins[30].It is unclear whether this case represents a composite tumor (CIAM) or a collision tumor, as the author did not provide detailed histologic examination and classified the lesion as “collision” tumor[30].
MICROSCOPIC EXAMINATION AND IMMUNOHISTOCHEMISTRY
Adenomas with a MC component are usually high-risk adenomas (size ≥ 10 mm, villous components and/or high grade dysplasia)[1,3,5,7,15].Therefore, the adenomatous components of CIAM tend to be large.For example, the mean size of polyps was 24 mm in Kimet al[3]’s study.In our study, the average size of the polyps was 42 mm (probably because our cohort consisted of surgically removed polyps that were deemed endoscopically unresectable), all of the adenomas showed villous components and 50% had high grade dysplasia (Figure 1).However, no statistically significant differences in terms of polyp size, polyp location (rightvsleft) or the frequency of associated high grade dysplasia between the adenomas with and without MC were found[1].In contrast, in Kimet al[3]’s study where most of CIAMs were detected in endoscopically resected polyps, 86% of CIAMs had conventional adenoma with low grade dysplasia[3].In Salariaet al[7]’s study, high grade glandular dysplasia was seen in 4 (36%) of 11 CIAMs[7].
Microscopically, the MC component is found at the base of full-thickness adenomatous glands.The background lamina propria is myxoinflammatory with sometimes conspicuous eosinophils.The MC components are oftentimes connected to the overlying glandular components[3].These small nests, irregular cords or clusters of neuroendocrine cells are sparsely distributed and do not form grossly evident nodules or masses (Figure 1).Occasional acinar structures may be seen[1-3,7].
In Salariaet al[7]’s study, the MC component extended over an average length of 3.9 mm.Also 64% (7/11) of the MCs were multifocal[7].In Kimet al[3]’s and La Rosaet al[4]’s studies, the mean size of the MC components was 4.7 mm and 3.2 mm, respectively[3,4].In our study, MCs were distributed over a mean area of 5.8 mm and were multifocal in 83% of the cases.In a majority of CIAMs, the MC components are confined within the mucosa, though extension into the submucosa can be seen[1,4,15] (Figure 2).
Cytologically, the neuroendocrine cells constituting MC are bland and monotonous (Figure 1).The cells show scant to abundant granular or eosinophilic cytoplasm and round central nuclei with salt and pepper-pattern chromatin.They are devoid of nuclear atypia, hyperchromasia, nuclear pleomorphism, conspicuous mitotic activity, and apoptosis.In other words, they are typical well-differentiated neuroendocrine cells.
By immunohistochemistry, the MC components are positive for synaptophysin (Figure 2B), supporting their neuroendocrine differentiation[1,3,15].Chromograinin-A and CD56 show variable staining[4,5].Variable immunolabeling with squamous markers such as p63 and CK5/6 can be seen[1,7].They are well-differentiated with a low Ki-67 proliferation index (usually < 1%-2%) (Figure 2D), although sometimes the total number of neuroendocrine cells in MC may be insufficient (< 500 cells in total) for reliable Ki-67 index measurement[1,3,7].The MC component shows nuclear β-catenin positivity (Figure 2C) in 60% to 100% of the cases, suggesting the role of Wnt/βcatenin pathway in the CIAM tumorigenesis[1,7,15].
MOLECULAR ANALYSIS
La Rosaet al[4] carried out mutational analysis forKRAS, BRAF, PIK3CAand microsatellite instability analysis on 6 CIAMs.No mutations were identified, and all cases were microsatellite stable in both adenoma and MC components[4].
DIFFERENTIAL DIAGNOSIS
MCs in CIAM may pose diagnostic challenge and may lead to misdiagnosis or overdiagnosis.MC can resemble squamous morules/metaplasia, invasive adenocarcinoma, squamous cell carcinoma (SCC), sporadic neuroendocrine tumor, and goblet cell adenocarcinoma (GCA).Awareness and recognition of this entity is crucial for accurate diagnosis and patient care.
Squamous morules/metaplasia
Squamous morules/metaplasia is an incidental histologic lesion that can be seen in colorectal adenomas[13,31].The reported incidence of squamous morules in colonic adenoma is about 0.4%[11,32,33].In our study, the incidence of squamous morules was 5.1% in surgically resected large colonic polyps[1].
Microscopically, squamous morules are characterized by a proliferation of immature squamoid or spindled cells forming nests and nodules without definitive keratinization or intercellular bridges[11,13,32].Usually the nests protrude into the lumen of adenomatous glands (Figure 3), or may be identified at the base of the polyps especially in the cases of torsion and prolapse[1,13,32].Immunohistochemically, squamous morules are positive for pan cytokeratin, CK5/6, cyclin D1 and β-catenin (nuclear staining)[1,13,34,35] (Figure 4) and show variable staining for p63[15,32].Focal synaptophysin and chromogranin positivity can be seen[32].
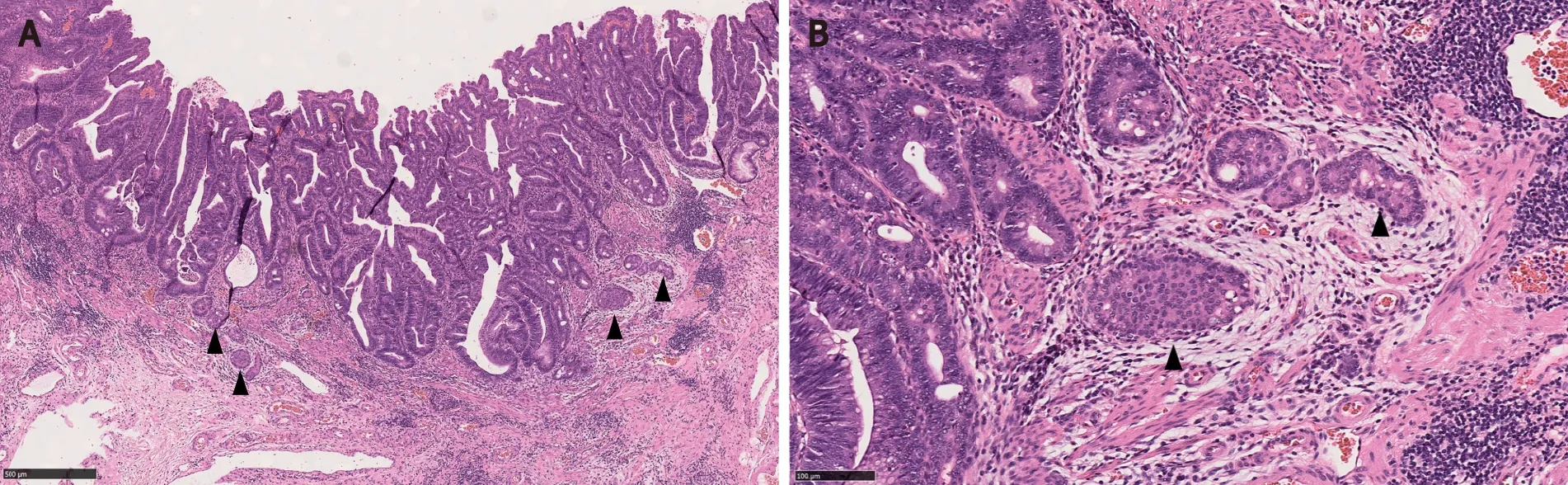
Figure 1 Composite intestinal adenoma-microcarcinoid consisting of tubulovillous adenoma with high grade dysplasia and microcarcinoid components (arrowheads) at its base.
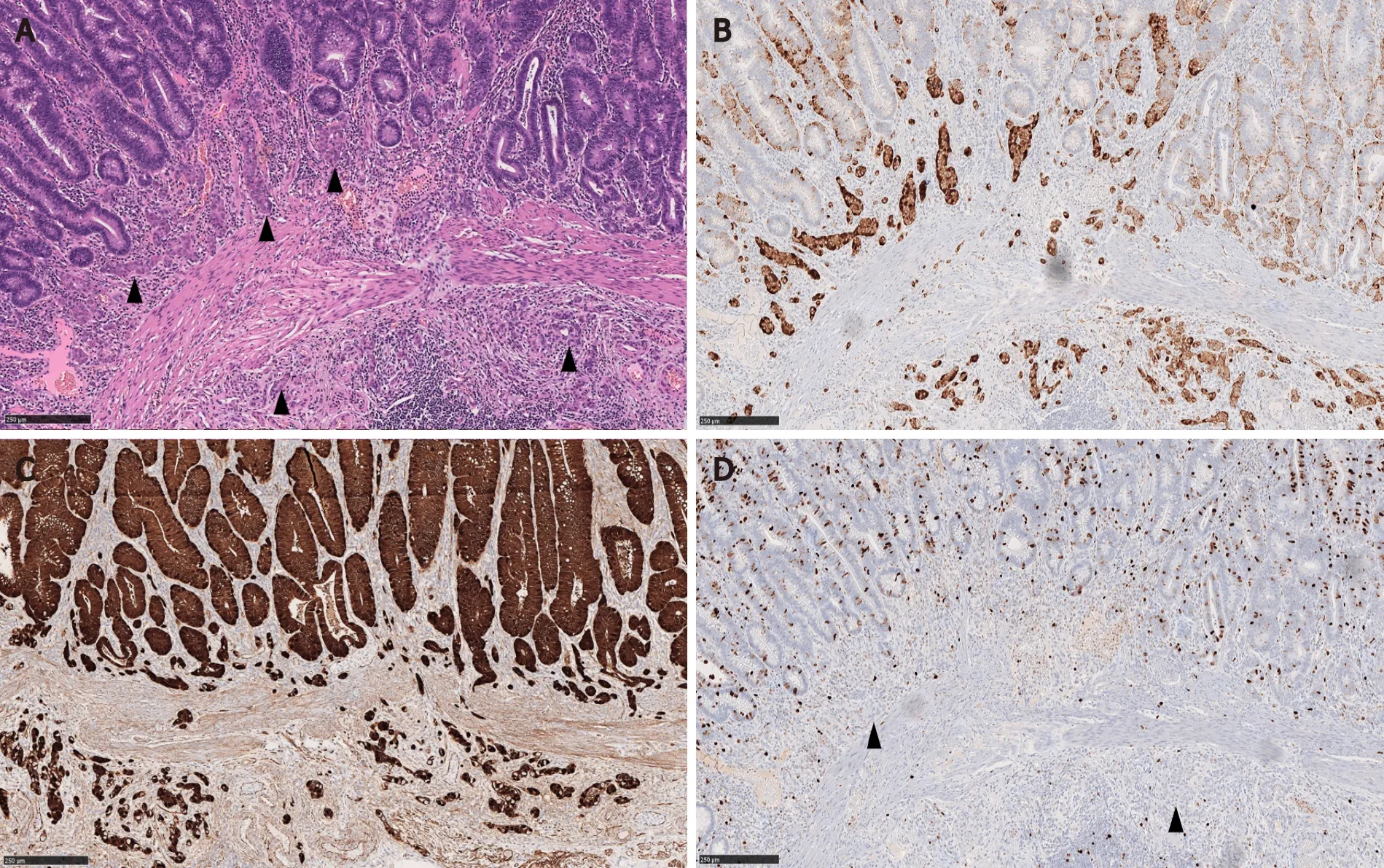
Figure 2 Composite intestinal adenoma-microcarcinoid with submucosal invasion of the microcarcinoid component.
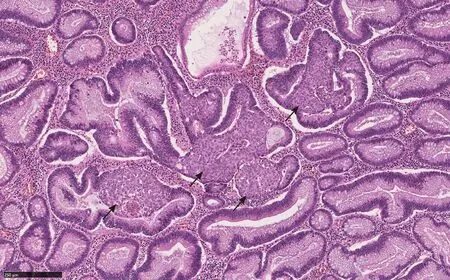
Figure 3 Squamous morules (arrows) with associated tubulovillous adenoma (Hematoxylin and eosin, 100 ×).
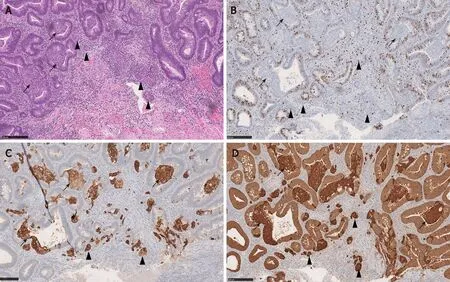
Figure 4 Composite intestinal adenoma-microcarcinoid with associated squamous morules (arrows).
There can be significant histomorphologic overlap between the MC component of CIAM and squamous morules.Both can present as solid nests around the bottom of adenomatous glands or myxoinflammatory stroma[1,32].Indeed, in Kimet al[3]'s study, 6 CIAM cases were initially diagnosed as adenoma with squamous morules/metaplasia[3].In Pulitzeret al[2]'s study, one CIAM was originally interpreted as adenoma with focal squamous metaplasia owing to the presence of abundant eosinophilic cytoplasm in MC[2].In Salariaet al[7]'s study, MC was initially interpreted as squamous morules in 5 of 10 CIAMs[7].
In addition, there is immunophenotypic resemblance between the MC component of CIAM and squamous morules.Squamous morules may show focal positivity for neuroendocrine markers such as synaptophysin and chromogranin[32].Conversely, the MC components of CIAM are variably immunoreactive with p63 and/or CK5/6 (Figure 4), suggesting squamous differentiation.In Salariaet al[7]’s study, 2 of 6 MC were focally positive for p63, and 5 of 6 MC were positive for CK5/6[7].
Given the morphologic and immunohistochemical overlap between squamous morules and the MC component of CIAM, we hypothesized that these two entities may be related.Interestingly, 33.3% (2 of 6) of CIAM showed concurrent squamous morule (Figure 4), compared to 4.0% (6 of 152) of adenomas without MC in our cohort, suggesting shared pathogenesis between the two (P< 0.05)[1].Similarly, Estrellaet al[15] reported that 4 (16%) of 25 CIAMs had squamous metaplasia in the adjacent adenomatous component[15].
Nevertheless, given that squamous morules/metaplasia is benign and the MC of CIAM is likely indolent, misdiagnosing MC as squamous morules/metaplasia may not have a significant clinical impact.In fact, it may be nearly impossible to distinguish these two in some cases.
SCC
As stated above, 16 to 33% of CIAMs can co-exist with squamous morules/metaplasia[1,15].Moreover, MC components can demonstrate squamous differentiation with variable p63 and/or CK5/6 immunoreactivity (Figure 4) in a myxoinflammatory background mimicking desmoplasia.Therefore, SCC is considered a differential consideration for MC component of CIAM.
Primary colorectal SCC is a rare malignancy with an incidence of 0.1%-0.25%[36].To date, less than 100 cases of colorectal SCC have been reported in the literature[37].
Usually, SCC of colon presents late in the disease course and shows an aggressive behavior with early metastasis and poor overall survival[38,39].Thus, it is important not to overdiagnose the MC of CIAM as SCC.It will be helpful to be aware that MC can show immunohistochemical squamous differentiation to avoid this misinterpretation.
Invasive adenocarcinoma
MC components of CIAM may be misdiagnosed as invasive adenocarcinoma or tumor budding.Possible and reasonable explanations for this are: First, MC may show infiltrative or single-cell patterns at the polyp base, mimicking invasive disease[2] (Figure 5).Second, the background myxoinflammatory lamina propria associated with MC may resemble the edema and fibroblastic proliferation of desmoplasia that is usually associated with invasive disease[5,7].Third, MC is commonly found at the base of full-thickness adenomatous mucosa frequently with high grade glandular dysplasia[1,5].In fact, one of the CIAM cases reported by Linet al[5] had been initially misinterpreted as adenocarcinoma[5].
Awareness of this entity and the recognition of bland cytoarchitecture and negligible mitotic activity of MC will be helpful to avoid misclassification[2].Confirming neuroendocrine differentiation can be a useful diagnostic tool in challenging cases[7] (Figure 5).
Conventional sporadic neuroendocrine tumor
CIAMs and sporadic neuroendocrine tumors are treated differently.The MC components in CIAMs are usually situated at the polyp base in the mucosa, therefore complete polypectomy may suffice to remove the MC component with negative margin.On the other hand, the usual epicenter of sporadic neuroendocrine tumors is the submucosa.Therefore, additional surgery may be required to achieve complete resection with negative margin when the initial endoscopic biopsy shows sporadic neuroendocrine tumor[3].
For example, sporadic rectal neuroendocrine tumors are relatively common and oftentimes present as nodules or polyps on endoscopy[14,40-43].They are usually small (over 50% of the cases < 1.0 cm in diameter), low grade, and located in the mucosa or submucosa[14] (Figure 6).Moreover, 79% to 84% of rectal neuroendocrine tumors are L-cell type that is known to be associated with rather indolent biologic behavior[44,45].Therefore, rectal neuroendocrine tumors have an excellent overall prognosis especially after an endoscopic resection[41,42,45].However, tumor stage and grade are still important prognosticators[41,43,46].Large tumor size [(≥ 1.0 cm), high grade (WHO grade 2 to 3)], and the presence of muscular and lymphovascular invasion are often associated with metastatic disease, requiring aggressive treatment[43].
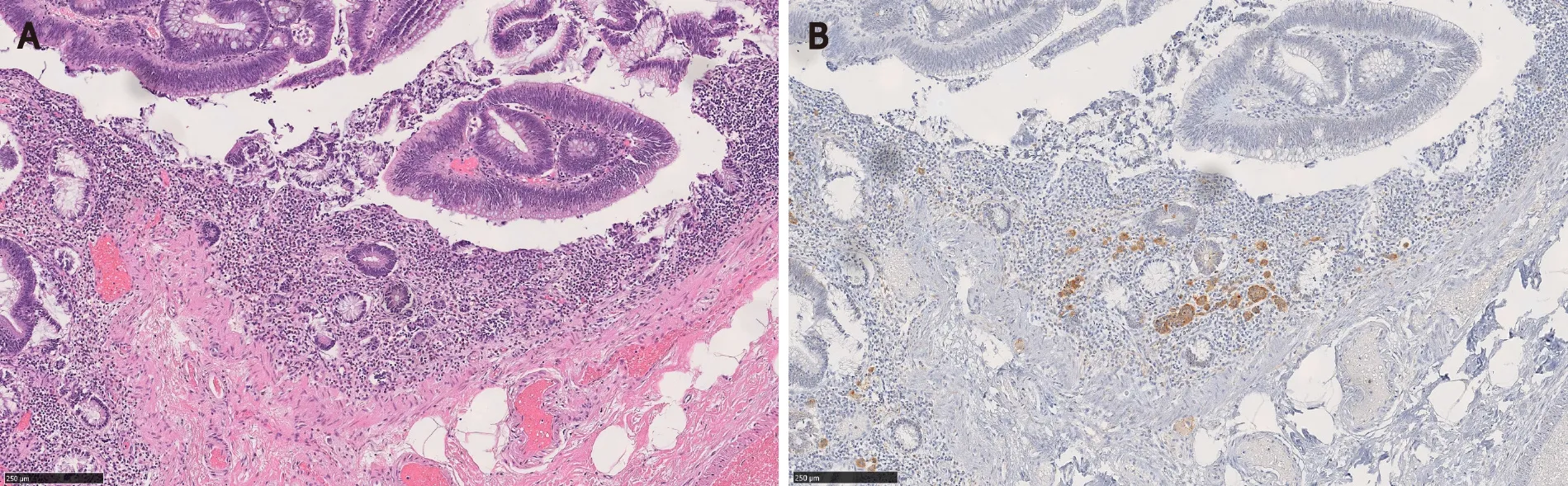
Figure 5 Microcarcinoid component of composite intestinal adenoma-microcarcinoid may mimic invasive adenocarcinoma.
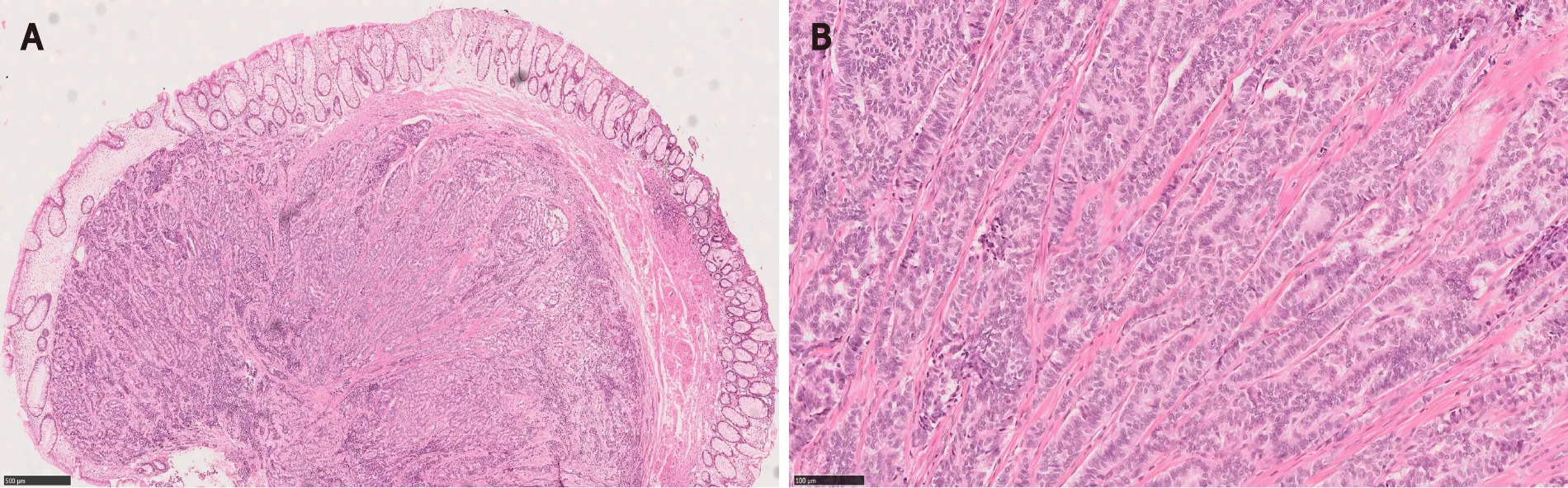
Figure 6 Rectal neuroendocrine tumor forming a nodule/polyp.
Nevertheless, MCs of CIAMs may also invade the submucosa[1,4,5,15].Thus, to ensure complete removal of the MC component, further surgery may still be required following polypectomy[47].Therefore, from a management standpoint, the tumor size and depth appear to be more relevant than their classifications.
Few studies have explored the biological differences between the MC components in CIAMs and sporadic intestinal carcinoid tumors without associated adenomatous components.Estrellaet al[15] observed significantly higher β catenin expression score in CIAMs compared with sporadic neuroendocrine tumors, suggesting that CIAM may developviaa distinct pathway from the latter (i.e., the adenoma pathway).In this study the overall 3- and 5-year survival of CIAM patients was significantly lower than those with sporadic NET[15].This likely is due to the co-existing adenoma in CIAM as no CIAM patients died of neuroendocrine tumor in this study.
GCA
GCA, previously known as goblet cell carcinoid, adenocarcinoid, crypt cell carcinoma and microglandular carcinoma, is a subtype of appendiceal neoplasm.GCA is a mixed tumor with both glandular and neuroendocrine elements, and contains goblet cells (Figure 7).The tumor nests stain positively for neuroendocrine markers and mucin[14].Despite its mixed phenotype, GCA is officially recognized as a subtype of adenocarcinoma in the current WHO given its aggressive biologic behavior that is akin to adenocarcinoma[14,48].GCA may co-exist with adjacent cecal adenoma[49].Therefore, it is possible that cecal adenoma with underlying GCA may be interpreted as CIAM.Indeed, based on the provided illustrations, some authors raised a possibility that one of Linet al[5]’s CIAM cases with lymph node metastasis may represent GCA with overlying adenoma[3,50].GCA is an aggressive tumor and often presents with metastatic disease[51-53].Further surgical management and chemotherapy are commonly required[53].
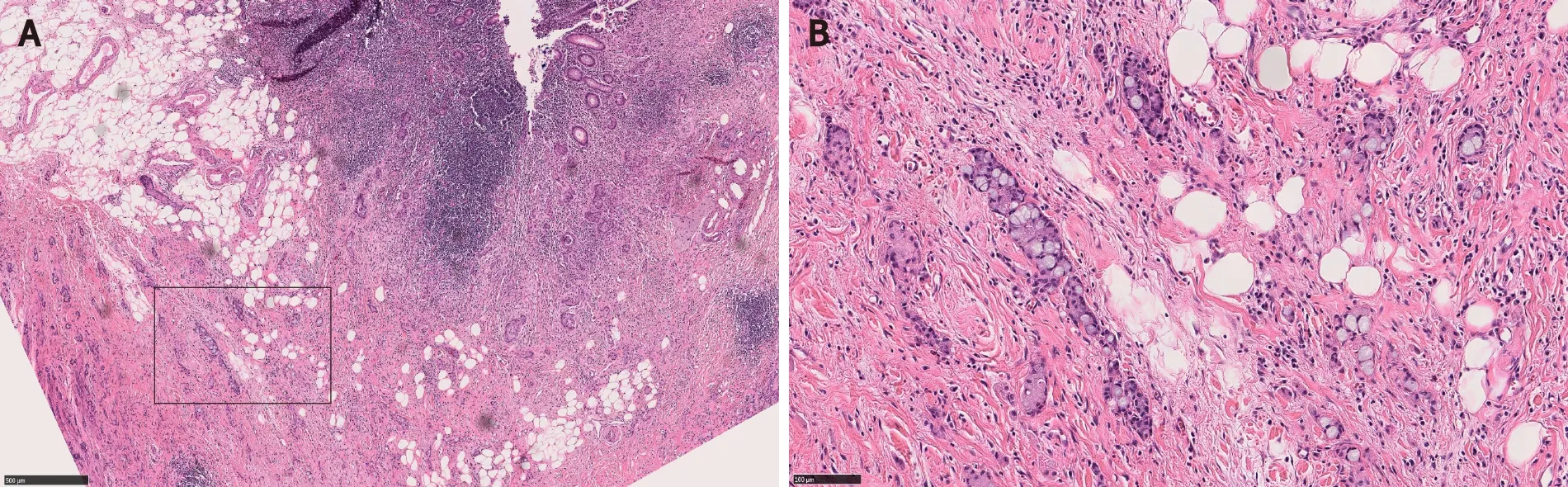
Figure 7 Appendiceal goblet cell adenocarcinoma.
CIAM VS COLLISION TUMOR VS MINEN
Composite tumor, such as CIAM, is considered pathogenetically distinct from collision tumor.MiNEN is a broader category than CIAM.
Collision tumor
Lewin[54] first proposed to separate composite tumor and collision tumor when neoplastic endocrine cells and nonendocrine epithelial cells are admixed.In a composite tumor, glandular and neuroendocrine components are intermingled, and both components may share common origin.Whereas in a collision tumor, the two elements “collide” but are pathogenetically independent of each other.One of the two elements may represent a metastasis from another primary site[14,54].
Recently, Schizaset al[55] carried out a literature review on collision tumors of the digestive system.In this review, the authors defined collision tumors as those consisting of two or more independent neoplasms without intermingling (thus without transition zone).In colon, adenocarcinoma was the main component of collision tumors, found in 78.6% of the cases, followed by carcinoid, seen in 35.7%[55].Collision tumors are often high grade with early metastasis and a shorter survival[56-58].
Traditionally, collision tumors have been believed to represent “double primaries” though a few studies challenged this concept[56,58,59].For example, Minaya-Bravoet al[58] reported a case of colonic collision tumor consisting of adenocarcinoma and large cell neuroendocrine carcinoma without identifiable transition zone.Three years later, the tumor metastasized to the retroperitoneum.Interestingly, both components metastasized, suggesting that both components of this collision tumor may have originated from the same clone[58].Similarly, Pecorellaet al[56] reported a cecal collision tumor consisting of adenocarcinoma and high grade well-differentiated neuroendocrine tumor (reported Ki67 proliferation index was 36%).There was focal positivity for CEA in the neuroendocrine tumor component without clear transition zone between the two components.The authors concluded that some mixed tumors cannot be precisely classified.
MiNEN
MiNEN is a recently introduced umbrella terminology referring to a neoplasm demonstrating a mixture of neuroendocrine and non-neuroendocrine components[4,12,14].The terms “low grade” MiNEN and MANET have been proposed to describe mixed tumors with adenomatous components and well-differentiated neuroendocrine tumors (to include WHO grades 1 to 3)[4,12].However, neither low grade MiNEN nor MANET has been officially recognized as a subtype of MiNEN in the current WHO[14].In fact, in the gastrointestinal tract and hepatopancreatobiliary organs, WHO limits the use of the MiNEN term only to the mixed tumors with malignant nonneuroendocrine components[14] (Figure 8).
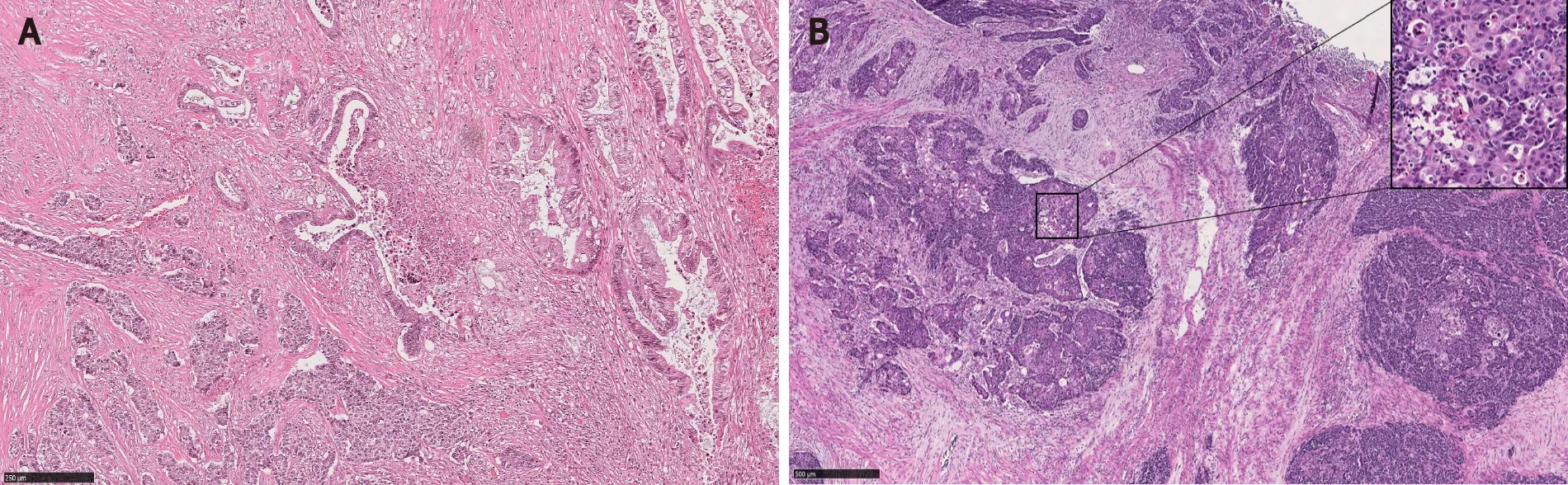
Figure 8 Mixed neuroendocrine-non-neuroendocrine neoplasm.
Even if low grade MiNEN (MANET) were to be recognized by WHO, there are differences between CIAM and low grade MiNEN.In MiNEN, each component should represent at least 30% of the total volume of the neoplasm.Therefore, some CIAMs with minor MC components would not meet the 30% cutoff criterion for low grade MiNEN.As many studies on CIAM did not specify the amount of MC components relative to the tumor volume, it is difficult to assess how many of the reported CIAM cases had MC components that occupied over 30% of the total tumor volume[2,5,7].In our study, all 6 CIAM cases had minor MC components constituting much less than 30% of the tumor volume[1].In addition, most of the MC components in CIAM are low grade with a negligible ki67 proliferation index, whereas low grade MiNEN can have grade 2 and 3 levels of proliferation in the neuroendocrine components[4].Typical MiNEN with malignant non-neuroendocrine component mixed with neuroendocrine carcinoma is an aggressive neoplasm with a median overall survival of 13.2 mo.The ki67 proliferation index of the neuroendocrine component may drive the prognosis of these tumors[60].
PROGNOSIS
CIAM is an indolent disease with a favorable outcome.One study found that after mean follow-up of 6 (range 0.5 to 27) years, none of the patients had recurrence of CIAM or metastasis after endoscopic or surgical treatment[4,15].In our study, after mean follow-up of 53 mo, all patients were free of CIAM.In addition, all the lymph nodes retrieved during the surgical resection were devoid of adenocarcinoma or neuroendocrine tumor.Our two patients with MC components extending into the submucosa were followed for 14 and 15 mo, respectively.There was no evidence of recurrence or metastasis of neuroendocrine tumor at the end of the follow-up[1].In La Rosaet al[4]’s study, one CIAM case had MC in the submucosa.The patient was followed for 12 years without evidence of disease[4].No tumor-related death has been reported in the literature.
The size of MC component appears to have no bearing on the outcome[3].This is likely due to the fact that the MC component tends to be small, and is usually confined in the mucosa.Likewise, the lesional cells constituting MC are bland with low proliferative activity.
TREATMENT
Given its indolent course, complete removal of both adenoma and MC by polypectomy is considered curative[4].Additional radical surgeries should be reserved for cases with adverse histologic features such as deep submucosal extension or increased proliferative activity of the MC component[3].
CONCLUSION
CIAM is a rare intestinal lesion consisting of a conventional adenoma and a well differentiated MC component at its base.CIAM is considered to represent a true composite tumor wherein both adenoma and MC appear to share a common origin and developviathe Wnt/β-catenin pathway.MC in CIAM poses diagnostic challenges with its morphologic resemblance to other benign and malignant lesions.CIAM is an indolent lesion with a favorable outcome.Complete removal of both adenoma and MC by polypectomy is considered curative.Raising awareness of this rare entity will lead to correct diagnosis and appropriate management.
 World Journal of Gastrointestinal Endoscopy2021年12期
World Journal of Gastrointestinal Endoscopy2021年12期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Application of robotic technologies in lower gastrointestinal tract endoscopy: A systematic review
- Efficacy and tolerability of high and low-volume bowel preparation compared: A real-life single-blinded large-population study
- Role of endoscopic ultrasound guided fine needle aspiration/biopsy in the evaluation of intra-abdominal lymphadenopathy due to tuberculosis
- When should we perform colonoscopy to increase the adenoma detection rate?
- Large polyps: Pearls for the referring and receiving endoscopist
- Primary prophylaxis of variceal bleeding in patients with cirrhosis: A comparison of different strategies
