Use of graphene-based materials as carriers of bioactive agents
Wing-Tk
a Ciechanover Institute of Precision and Regenerative Medicine, School of Life and Health Sciences, The Chinese University of Hong Kong (Shenzhen), Shenzhen 518172, China
b Department of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hong Kong, Special Administrative Region, China
Keywords: Graphene Carbon Nanomaterial Loading Controlled release
ABSTRACT Graphene possesses a large specific surface area,a high Young’s modulus,high fracture strength,high electrical conductivity,and excellent optical performance.It has been widely studied for biomedical use since its first appearance in the literature.This article offers an overview of the latest advances in the design of graphene-based materials for delivery of bioactive agents.To enhance the translation of these carriers into practical use,the toxicity involved is needed to be examined in future research in more detail.In addition,guidelines for standardizing experimental conditions during the evaluation of the performance of graphene-based materials are required to be established so that candidates showing higher practical potential can be more effectively identified for further development.This can streamline the optimization and use of graphene-based materials in delivery applications.
1.Introduction
Carbon is a group IV element.It exists in multiple forms (including graphite,fullerene,diamond,and amorphous carbon) [1].Although each crystalline form of carbon has its unique physical properties,all crystalline forms possess a common 2D structure,namely graphene,which is a single layer of sp-hybridized carbon atoms arranged in a honeycomb crystal lattice.In graphene,each carbon atom is connected to three carbon atoms,with the C–C bond length and the bond angle being around 1.42 ˚A and 120 °[2],respectively.Because the carbon skeleton of graphene consists ofσ
-bonds with paired electron clouds above and below the skeleton,this bonding system is similar to that observed in benzene.Graphene is,therefore,regarded as a polycyclic aromatic hydrocarbon.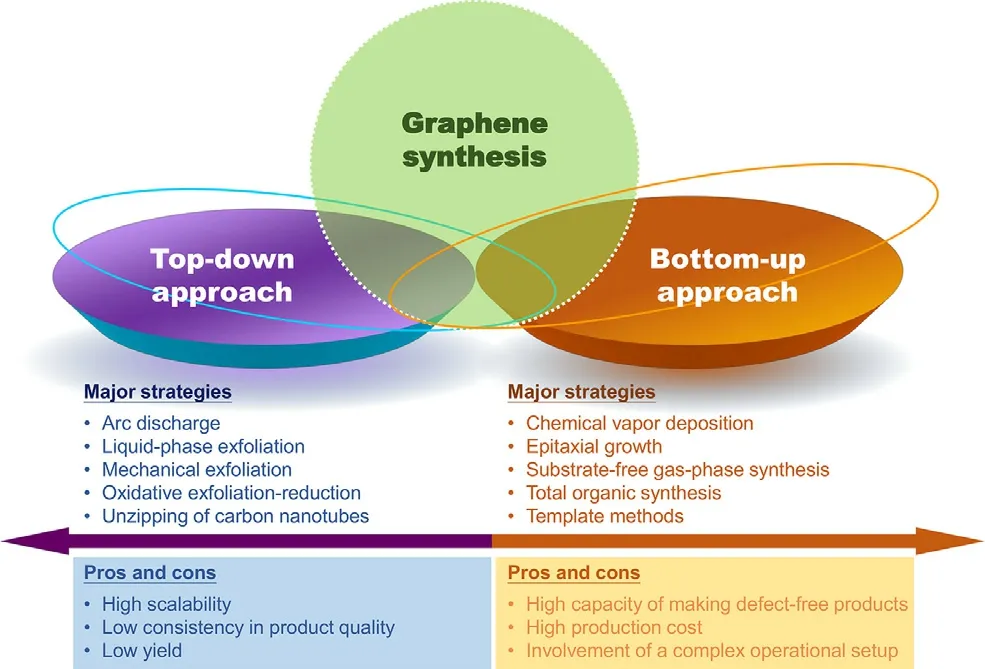
Fig.1–Overview of major strategies for the generation of graphene.
Graphene has been widely studied for biomedical use since its first appearance in 2004 [3],partly owing to its high Young’s modulus,high fracture strength,high electrical conductivity,and excellent optical performance.Graphene also possesses a large specific surface area for adsorption of bioactive compounds and has high optical absorption efficiency in the near-infrared (NIR) region.Compared to other types of carriers (such as polymeric nanoparticles [4,5],hydrogels [6–9],and liposomes [10,11]) which display very few functionalities,if any,unless further manipulation is performed,graphene-based carriers intrinsically enable not only delivery of bioactive agents but also the execution of physical treatments such as photodynamic therapy [12,13].On the other hand,compared to metal particles which are deficient of functional groups for direct functionalization [14,15],graphene-based materials possess a large number of reactive groups for chemical modification,enabling more precise optimization of chemical properties as carriers.The unique properties of graphene as mentioned above render graphene and its related materials attractive in delivery research.
2.Generation and use of graphene-based carriers for agent loading
Graphene can be generated by using multiple methods (Fig.1),including mechanical exfoliation [16],oxidative exfoliation-reduction [17],arc discharge [18],total organic synthesis [19],liquid-phase exfoliation [20],template routes [21],epitaxial growth [22],substrate-free gas-phase synthesis [23],and chemical vapor deposition (CVD) [24].Because 2D graphene can be manipulated to form 0D structures [e.g.,fullerenes and graphene quantum dots (GQDs)],rolled into 1D structures (e.g.,carbon nanoribbons and nanotubes),or stacked into 3D structures (e.g.,graphite),it is the building block of carbon-based materials with diverse dimensions.Upon the generation of graphene,graphene oxide (GO) can be obtained by oxidizing carboxyl groups and hydroxyl groups on the graphene plane.Oxidation increases the solubility of graphene;however,due to van der Waals interactions between GO nanosheets,aggregation of the nanosheets easily occurs in the physiological environment.This partially accounts for the occurrence of toxic effects during biomedical use of GO.
To use graphene and its derivatives as carriers,the bioactive agent has to be first loaded into the materials.This can be achieved by first dispersing the agent in an aqueous GO dispersion,followed by the addition of a reducing agent [25].Compared to pristine graphene,more active groups are available on the surface of GO.These active groups cannot only provide sites for covalent binding of the agent to the GO surface,but also allow for structural modification of GO.The latter is exemplified by the success of modifying the GO surface with polyethylene glycol (PEG) and poly(ethylenimine) (PEI) via nucleophilic substitution,generating nanocarriers showing high dispersibility for bioactive agent delivery [26,27].Apart from the covalent means,non-covalent interactions can be adopted to load various agents into graphene-based materials and to manipulate the properties of GO.For example,due to the availability of carboxyl groups,polyelectrolytes can be adsorbed on the GO surface via electrostatic forces [28].Through physisorption,doxorubicin (DOX) has also been loaded into gelatin-functionalized graphene nanosheets for delivery purposes [29].More recently,chitosan-functionalized GO has been used as a carrier of ibuprofen and 5-fluorouracil [30].The drug loading process is mediated largely byπ
–π
stacking and hydrophobic interactions.The adoption of such non-covalent interactions,indeed,can prevent changing or destroying the intrinsic structure and physical properties of the loaded agent.3.Methods of manipulating the properties of graphene-based carriers
Manipulation of the physicochemical properties of graphene-based carriers can modify the pharmacokinetic profile achieved [31,32].Because graphene is the building block of 0D,1D and 3D structures,manipulating the size and shape of graphene-based nanosheets may help control the size and shape of the derived carriers.
3.1.Size manipulation
Size is one of the important factors affecting the performance of a carrier [6,33–35].In general,carriers having a size of 5 nm or less are easily excreted from the body in urine [36].Those having a larger size may tend to accumulate in the heart [37],bone marrow [38],kidney [39,40],stomach [41],spleen [42],and liver [43].To manipulate the size of a graphene-based nanosheet (and hence the size of the carrier derived),one of the simplest approaches is to use the separation method,which has previously been used to obtain ultrasmall nano-graphene oxide (NGO) [44].Generation of NGO begins with the activation of GO using chloroacetic acid to produce GO–COOH,which is subsequently grafted with 6-arm branched PEG molecules and finally undergoes density gradient ultracentrifugation to obtain the product [26,44].Apart from using density gradient ultracentrifugation,GO with different dimensions can be separated based on pH-dependent amphiphilicity [45,46].This method provides a facile strategy to control the size distribution of graphene-based nanosheets for subsequent carrier fabrication.
Graphene-based nanosheets can be etched to specific dimensions directly by using electron-beam lithography,local electrochemical etching,reactive plasma etching,and Oplasma etching with templates,too [47–49].The use of these methods in large-scale production of graphene-based carriers is,however,limited due to the high production cost.This problem may be alleviated by the emergence of the solution-based cutting approach [50],which has been reported to generate a 0D structure with an average diameter of 9.6 nm from GO.Another method to solve the problem involved in conventional etching is to control the process of bottom-up synthesis.The feasibility of this has been suggested by an earlier study [51],which has fabricated nano-sized N-doped GO by cutting and unzipping N-doped carbon nanotubes (CNTs).During synthesis,N-doped CNTs are first fabricated via CVD [51].After that,acid treatment is performed to generate nano-sized N-doped GO.The size and thickness of the product are negatively related to the length of the oxidation time [51],and hence can be manipulated by controlling reaction conditions.
3.2.Shape manipulation
The shape of the graphene-based nanosheet is another parameter to be considered during the fabrication and optimization of a graphene-based carrier because it may ultimately affect the kinetics of diffusional mass transport of the loaded agent [52,53],cellular uptake efficiency [54,55],and blood retention time [54,55].Graphene-based nanosheets with different shapes can be made by nanocutting [56],in which nickel nanoparticles are adopted as a knife to cut nanosheets with high precision.Via methane CVD on a copper surface,equiangular hexagon-shaped graphene flakes with either an armchair edge or a zigzag edge can also be produced [57].Along with the success of obtaining both single-layered and multi-layered graphene flakes [57],the possibility of engineering the shape of graphene-based carriers has been enhanced.
More recently,circular and elliptical GQDs have been successfully produced by using GO nanosheets [58].During synthesis,GO nanosheets are first generated from graphite powder,followed by deoxidation at 250 °C to produce reduced GO (rGO) powder.After oxidation of the powder in an acidic environment,the acid is removed.Purified GO powder is subjected to heating at 250 °C and is dispersed in distilled water to form a suspension,which is filtered through a nanoporous membrane to obtain a brown solution in which GQDs are present.The shape of the GQDs has been found to link with the size (Fig.2) [58].When the GQDs have a diameter of around 5–10 nm,they are largely circular and elliptical in shape.When the diameter is increased to 15 nm,mainly elliptical GQDs are found.When the diameter reaches 20 nm,most of the GQDs are hexagon-shaped,with around 25% of them deformed with curved sides.At the diameter of 35 nm,GQDs take the form of parallelogram-type rectangles with rounded vertices.Although this method of shape manipulation can only be applied to GQD production,it provides a route to easily fabricate graphene-based carriers with diverse shapes when GQDs are adopted as the starting material.
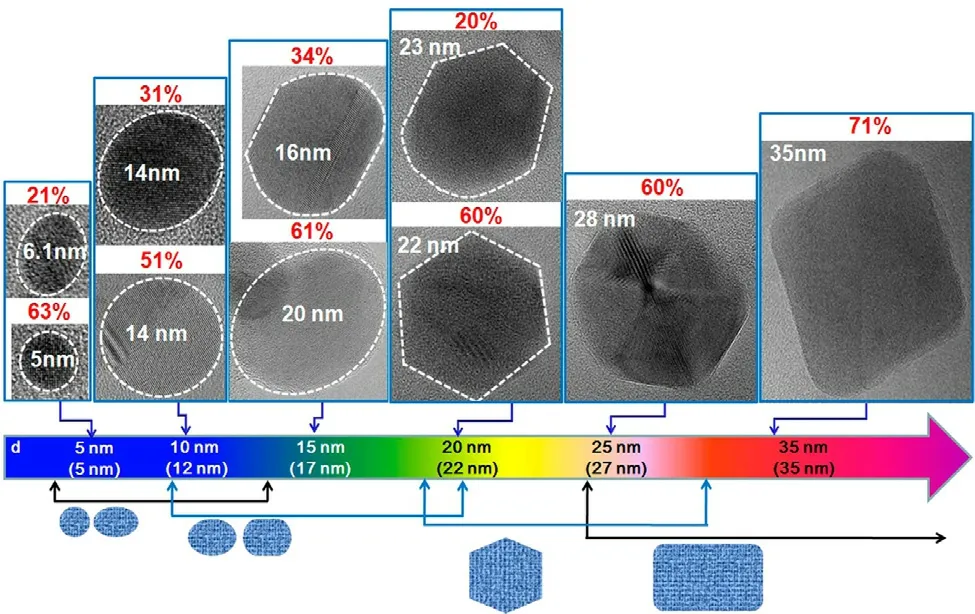
Fig.2–High-resolution transmission electron microscopy (HRTEM) images of GQDs showing changes in the shape of the quantum dot when the average size increases.The dotted line shows the region of a GQD.The connected arrow shows the range of the average size in which GQDs with a specific shape are obtained.The p value shows the percentage of GQDs with a specific shape.The average size is indicated in the parentheses at the bottom of the figure.Reproduced from [58]with permission from American Chemical Society.
4.Roles of graphene-based materials in bioactive agent delivery
As far as the use of graphene-based materials in agent delivery is concerned,one of the major uses is to apply the materials as modifiers to enhance the delivery performance of other carriers.The feasibility of this has been demonstrated by Chen and coworkers [59],who have fabricated dissolvable microneedles for transdermal drug delivery.In fact,polymeric microneedles are one of the most extensively used systems for transdermal drug administration because of their minimal invasiveness that can lead to high patient compliance [59].Upon GO incorporation,the microneedles display not only improved mechanical strength and higher moisture resistance [59],but also antibacterial and anti-inflammatory properties in thein
vitro
context [59].Importantly,the presence of GO enables the microneedles to mediate near-infrared (NIR) light-activated controlled drug release [59].This demonstrates the potential use of graphene-based materials as enhancers of other delivery systems.Such potential has been further corroborated by Aderibigbe and coworkers [60],who have generated a hydrogel biocomposite from gelation of a mixture containing acrylamide,N,N’
-methylenebisacrylamide,N,N,N,N’
-tetramethylethylenediamine,and potassium persulfate,with rGO serving as a modifier.Compared to the blank gel,the one containing rGO has shown higher release sustainability,and has displayed pH-responsiveness.This evidences the capacity of rGO to modify the hydrogel properties and hence the release pattern of the loaded agent.Besides serving as a carrier modifier,graphene-based materials can function as carriers of bioactive agentsper
se
.The mechanism undertaken by graphene-based carriers in tegafur delivery has been investigated by an earlier study [61],which has performed molecular dynamics simulation to elucidate how tegafur is delivered into cells by using GO nanosheets.At the start of the simulation,the tegafur-GO complex is located at a distance of about 2 nm from the model membrane.During the delivery process,the complex moves from the bulk water region to the 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) bilayer surface,with the nanosheet positioned almost vertically out of the membrane at the first 5 ns [61].GO loses a part of its electrostatic interaction energy with the solvent due to its positioning on the plasma membrane;however,the amount of energy loss with water is compensated by the interaction energy of GO with POPC [61].The complex subsequently touches the surface of the phospholipid bilayer.This is accompanied by a rapid decrease in both the electrostatic and van der Waals interaction energies of GO with the model membrane [61].The tegafur-GO complex fluctuates in the vicinity of the membrane surface until the electrostatic and van der Waals interaction energies get their first minimum values [61].At that time,hydrogen bonds are formed between GO and POPC [61]Formation of these hydrogen bonds can prevent GO from moving far away from the membrane.When GO is inserted partially from water into the membrane,it enhances the membrane permeability of the loaded drug [61],partly because of the high affinity of tegafur to GO during the cellular internalization process and hence the minimal loss of the drug via drug release or passive diffusion.Apart from tegafur,other compounds have been adopted as models to elucidate the interactions between the payload and graphene-based carriers during bioactive agent delivery.One of these is DOX.Molecular dynamics simulation reveals that the strength of the interactions between DOX and a graphene-based carrier is affected by the oxygen density on the graphene surface and by the pH of the surrounding medium [62].In particular,DOX can interact strongly with the surface of pristine graphene via van der Waals interactions due to theπ
–π
stacking of the aromatic group of the drug with the graphene surface [62].A similar observation has also been made in the case of paclitaxel [63],which spontaneously moves towards the carrier and forms strongπ
-π
interactions with the graphene surface (Fig.3).Such interactions,however,may be changed upon surface modification of graphene.In particular,if the number of oxygen-containing functional groups on the graphene surface increases,electrostatic interactions will build up between the drug and the graphene surface while van der Waals interactions will be weakened [62].By using GO with an O/C ratio of around 0.17 as a model for molecular dynamics simulation,the interaction energy of the DOX/GO system has been found to increase as the pH of the surrounding medium changes from 7 to 5 [62].This suggests that drug molecules are more loosely adhered to the carrier surface,leading to an increase in the release rate.Such computational simulation of the processes of agent loading and release may streamline the design and engineering of graphene-based materials as carriers.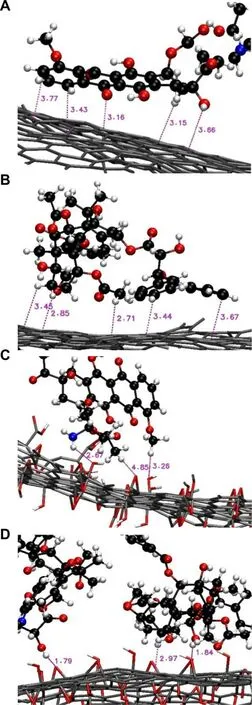
Fig.3–Close snapshots of (A,C) DOX and (B,D) paclitaxel interacting with the (A,B) graphene surface and the (C,D) GO surface.Reproduced from [63]with permission from Elsevier B.V.
5.Design of graphene-based materials for bioactive agent delivery
Owing to the capacity of graphene and its derivatives in enhancing membrane permeability of bioactive agents [61,62],over the years diverse carriers have been generated by using graphene as a building block (Table 1) [25,26,28,64–77].The emergence of these carriers has not only made effective delivery of small molecules and biomacromolecules possible,but has also enabled co-delivery of multiple agents.
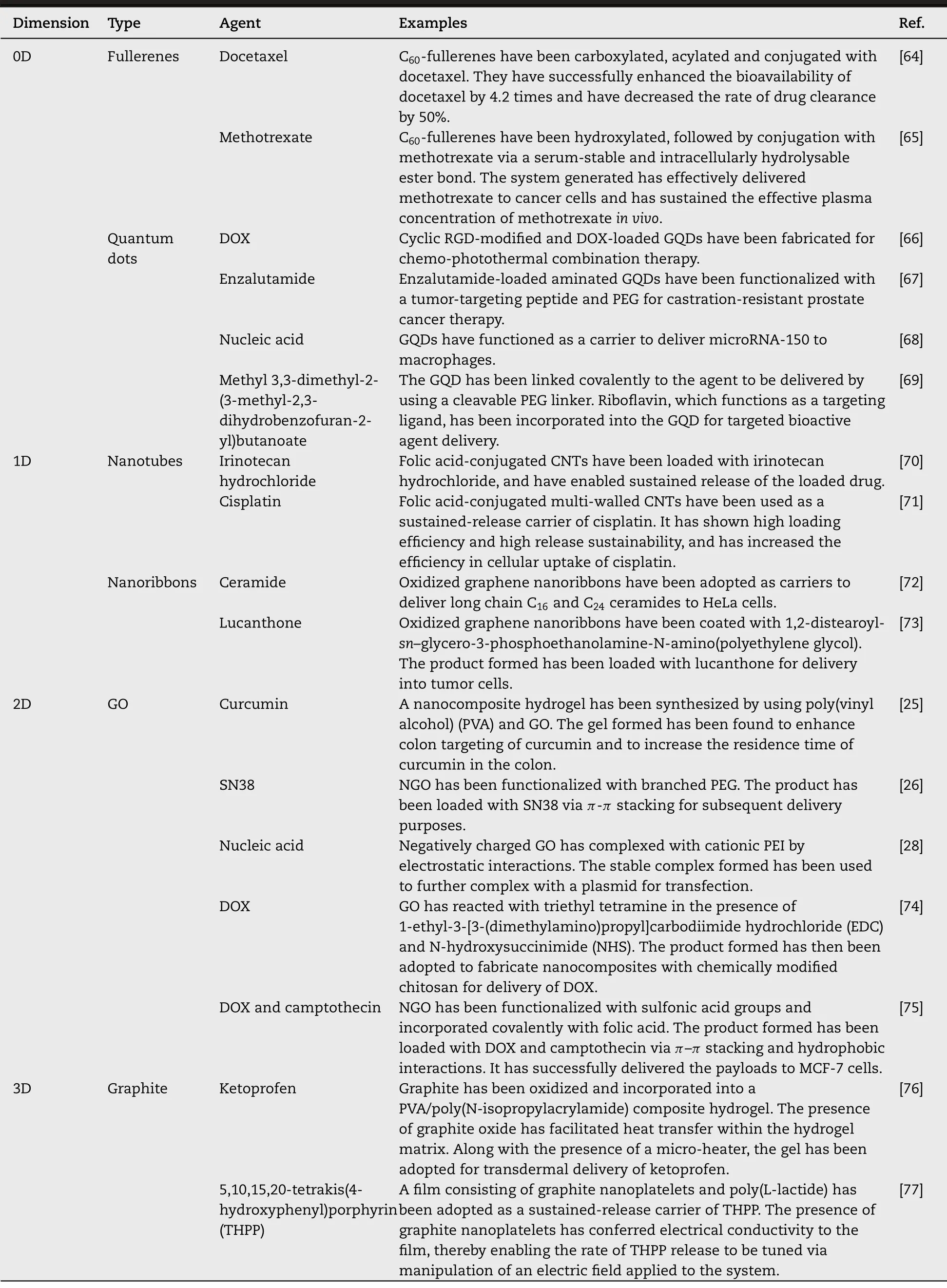
Table 1–Examples of graphene-based materials of different dimensions reported for delivery of bioactive agents.
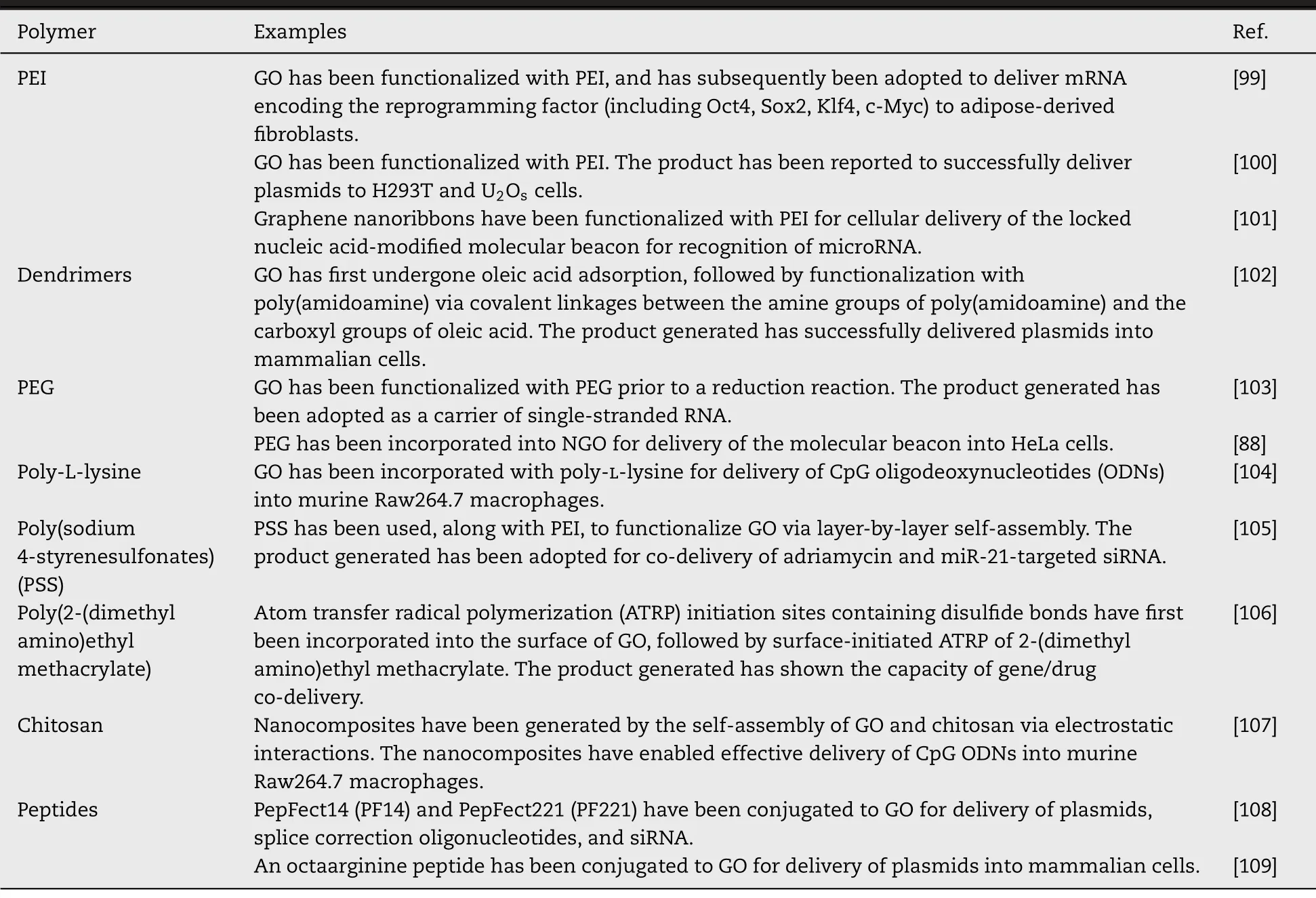
Table 2–Major polymeric modifiers of graphene-based materials for delivery of nucleic acids.
5.1.Delivery of small molecule compounds
During delivery of small molecule compounds,carriers have to interact with the plasma membrane and undergo cellular internalization via endocytosis.Release of the loaded compounds in the cytosolic compartment can be enhanced partly by taking advantage of the pH-responsiveness of some graphene-based carriers.One example of these carriers is the DOX-GO-chitosan-folic acid nanohybrid composite,which enables the release rate of DOX attained at acidic pH (5.3) much higher than that at physiological pH (7.4) [78].Another example is the GO-based polyelectrolyte nanocomposite [74].During synthesis,folic acid is first conjugated to chitosan via N,N-dicyclohexylcarbodiimide coupling [74].After that,ethylene glycol dimethacrylate is adopted as a cross-linker to graft itaconic acid and acrylic acid to the chitosan backbone,followed by the use of potassium peroxydisulfate as an initiator to generate carboxyl groups to form chemically modified chitosan [74].Upon mixing with amine-functionalized GO,the derivative of chitosan forms a nanocomposite,which can be loaded with DOX viaπ
-π
stacking interactions [74].The loading capacity of the nanocomposite has been found to be as high as 95.0%,with the release rate of the loaded drug at pH 5.3 being substantially higher than that at physiological pH [74].Apart from the pH-responsive carriers mentioned above,graphene-based carriers have been designed to respond to other stimuli.For instance,by functionalizing graphene sheets with poly(N-isopropylacrylamide),thermosensitive properties have been observed [79].By linking a detachable PEG shell to NGO via disulfide linkages,redox-responsive drug release has also been achieved [80].The rate of drug release has been found to be 2-fold higher upon intracellular glutathione (GSH) stimulation [80].This demonstrates the technical feasibility of controlling the rate and onset of release of the loaded agent by rendering the graphene-based carrier stimuli-responsive via careful structural design.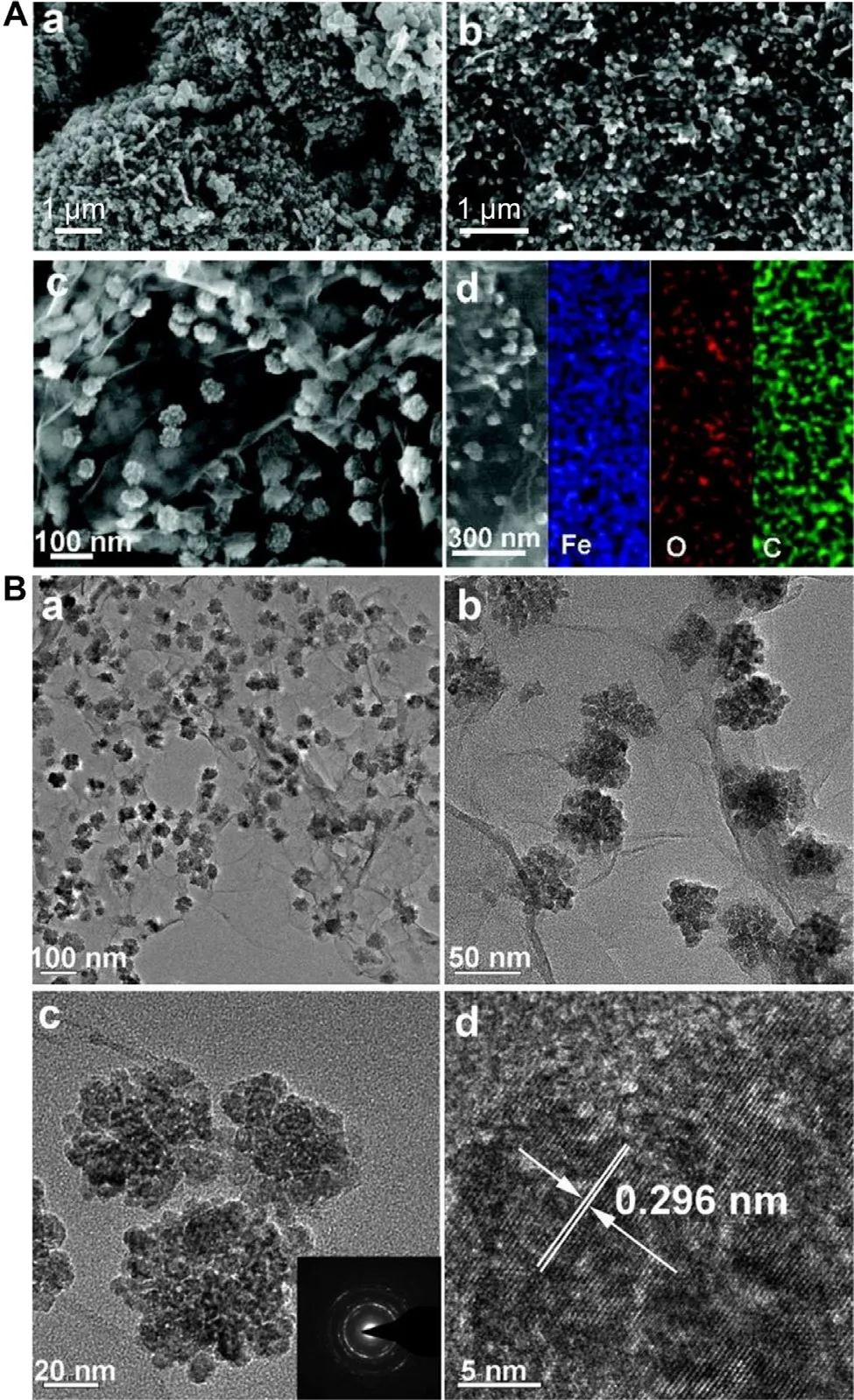
Fig.4–(A) Scanning electron microscopy (SEM) images of (a) bare Fe 3 O 4 nanoparticles,(b,c) Fe 3 O 4/graphene nanosheet composites,and the (d) SEM image attained from the Fe 3 O 4/graphene nanosheet composite with corresponding electron dispersive X-ray spectroscopy (EDS) maps for Fe,O,and C.(B) (a-c) Transmission electron microscopy (TEM) and (d) high-resolution TEM (HRTEM) images of Fe 3 O 4/graphene nanosheet composites.Inset is the selected-area electron diffraction (SAED) pattern of the composite.Reproduced from [82]with permission from American Chemical Society.
To enhance the functionality of graphene-based carriers,few studies have incorporated the carriers with metal nanoparticles,too.One example is the hybrid consisting of GO and iron oxide (Fe 3 O 4) nanoparticles.The hybrid is generated via wet chemical precipitation [81].It can be uniformly dispersed in an aqueous solution before and after DOX loading,but undergoes agglomeration in an acidic environment.This pH-triggered behavior enables controlled release of the loaded agent.Another example is the Fe 3 O 4/graphene composite (Fig.4) [82].Due to the presence of iron oxide,the composite shows higher electrical conductivity as compared to pristine graphene,and displays pH-triggered release of rhodamine-B.More recently,a PEG bis(amine)-functionalized GO/iron oxide nanocomposite has been developed for delivery of methotrexate [83].The nanocomposite has high blood compatibility,and enables sustained drug release [83].Although the role played by the superparamagnetic properties of the nanocomposite in controlling the release pattern of the loaded drug has not yet been extensively exploited,the possibility granted by iron oxide to enable the separation of the nanocomposite from the reaction mixture in the presence of an external magnetic field may facilitate the collection of the nanocomposite upon agent loading.
5.2.Delivery of biomacromolecules
Graphene-based materials can carry not only small molecules but also proteins and peptides.In an earlier study,amine-terminated GO has been loaded with protein therapeutics (ribonuclease A and protein kinase A),and has been found to protect the therapeutics from enzymatic hydrolysis during the delivery process [84].Bone morphogenic protein-2 (BMP-2) has been loaded successfully onto a titanium substrate coated with layers of positively (GO–NH 3) and negatively (GO–COO) charged GO nanosheets [85].Apart from proteins and peptides,nucleic acids can be carried by graphene [86].This is partly attributed to the interactions between graphene and nucleobases.Such interactions have been verified by Varghese and coworkers [87],who have performed isothermal titration calorimetry and have observed that the binding energy between graphene and guanine is the highest,followed by adenine,cytosine and thymine.Because graphene and its derivatives can protect DNA from enzymatic degradation [86,88],they show potential to function as non-viral carriers of nucleic acids.Despite this,contrary to single-stranded nucleic acids which can be easily loaded into graphene-based carriers via hydrophobic interactions andπ
-π
stacking interactions [86],the efficiency in loading double-stranded DNA into graphene-based carriers is generally low because the nucleobases are hidden in the double-helix structure,thereby failing to formπ
-π
stacking interactions with the carriers [89].To enhance the efficiency in loading nucleic acids,graphene-based carriers are often functionalized with polycations.One of the widely used polycations is PEI [90],which on one hand can interact with negatively charged nucleic acids [91–95]and on the other hand can provide active groups for further functionalization to enhance the transfection efficiency and the tissue targeting capacity.This is demonstrated by an earlier study [96],which has functionalized GO with PEI as a gene carrier and has found that the PEI moieties enable the carrier to complex with nucleic acids and to promote the adhesion of the complex to the plasma membrane for enhanced cellular uptake [96].By labeling the nucleic acids with TOTO-3 before complexation with PEI-functionalized GO,PC-3 cells transfected with the complex has exhibited green fluorescence under confocal fluorescence microscopy [90].This provides a route to combine imaging with nucleic acid delivery (Fig.5) [90].Till now,PEI-functionalized graphene-based carriers have been widely adopted in gene therapy of diverse diseases including myocardial infarction [97],osteoporosis [28],and cancer [98].Apart from PEI,other agents (e.g.,dendrimers,chitosan,and peptides) have been adopted to modify graphene-based materials for enhanced transfection (Table 2) [88,99–109].
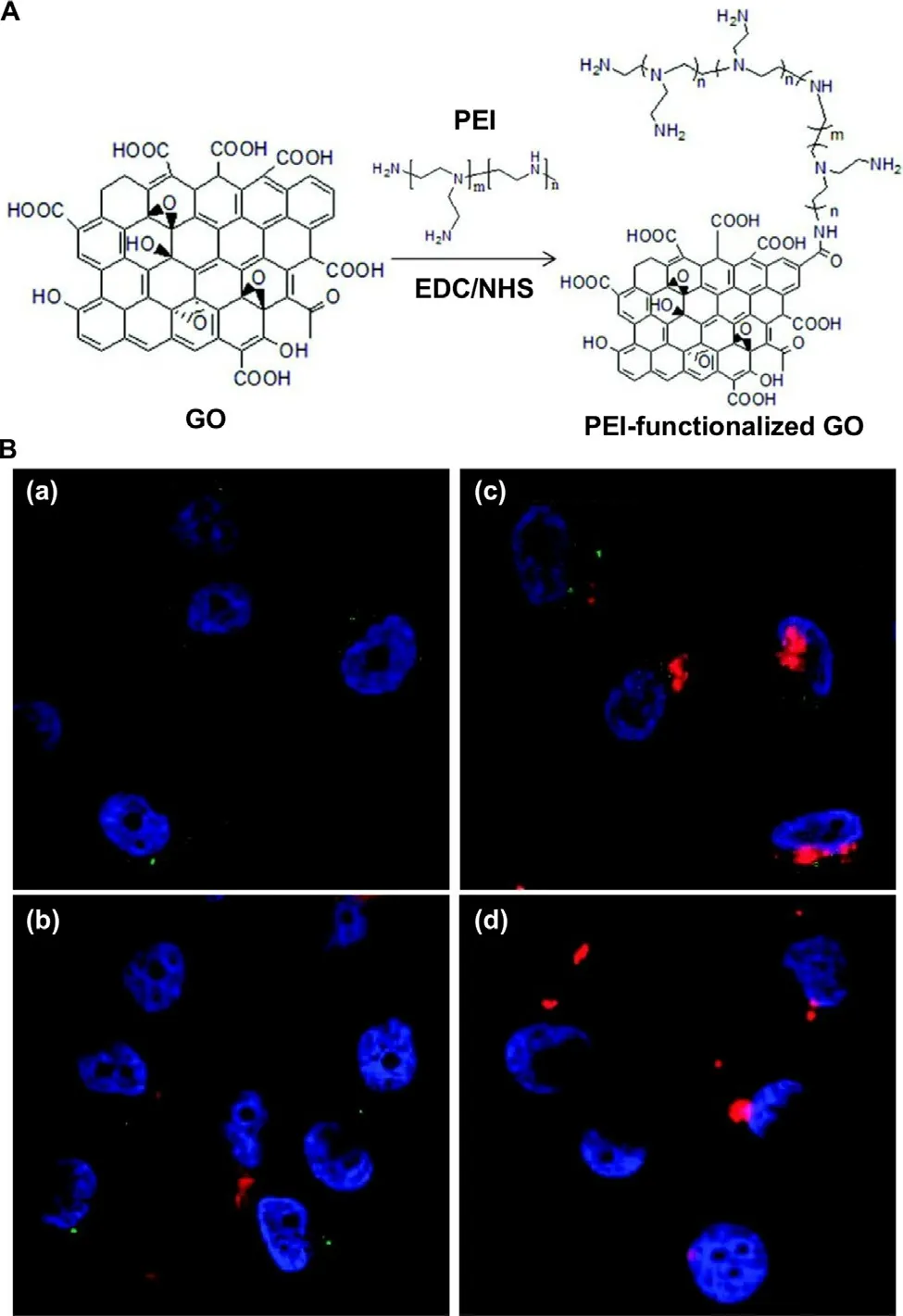
Fig.5–(A) A schematic diagram depicting the route of synthesis of GO functionalized with PEI.Abbreviations:EDC,1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride;NHS,N-hydroxysuccinimide (B) Confocal fluorescence microscopic images of PC-3 cells treated with (a) PEI-functionalized GO alone,(b) complexes formed between GO and TOTO-3-labeled plasmids,(c) complexes formed between PEI-functionalized GO and TOTO-3-labeled plasmids,and (d) complexes formed between PEI and TOTO-3-labeled plasmids.Nuclei were stained with DAPI.Photoluminescence was observed under excitation using a 488 nm laser.Reproduced from [90]with permission from American Chemical Society.
5.3.Co-delivery of multiple agents
While the“single molecule,single target,and single drug" strategy dominates for most of the 20th century [110],multi-drug therapy has attracted increasing interest partly owing to its potential to boost the therapeutic efficiency beyond that of mono-drug therapy [111,112].The possibility of using graphene-based materials to mediate multi-drug therapy has been illustrated by an earlier study [75],in which GO has first been functionalized with sulfonic acid groups followed by conjugation with folic acid [75].The resulting carrier enables co-delivery of DOX and camptothecin,with the toxicity achieved in MCF-7 cells much higher than that achieved by either DOX-or camptothecin-loaded GO.Another example demonstrating the possible use of graphene-based carriers in co-delivery of multiple agents is the chitosan-GO complex,which makes simultaneous delivery of camptothecin and nucleic acids possible [113].Recently,transferrin-targeting hyperbranched poly(amidoamine)-functionalized GO has been adopted to co-deliver docetaxel and matrix metallopeptidase 9 (MMP-9) short hairpin RNA (shRNA) [114].The carrier can deliver the agents to HNE-1 cells,and gives chemotherapeutic sensitization effects [114].By combining drug/gene co-delivery,chemotherapeutic sensitization,and active targeting into one single platform,this carrier warrants further development for use in cancer treatment.
Apart from letting the co-delivered agents to be released from the carrier naturally,by making use of the photothermal effect of irradiated NIR,controlled release of the co-delivered agents can be achieved.This has been suggested by the case of PEG-and PEI-functionalized rGO,which has been used to deliver DOX [115].Upon cellular internalization of the carrier,NIR irradiation has been applied to successfully trigger DOX release [115].That carrier has also shown potential to enable photothermal control of the nucleic acid delivery process [116].In PC3 and NIH/3T3 cells,no cytotoxicity from the carrier alone has been observed [116].Upon NIR irradiation,the carrier has induced heat to facilitate endolysosomal escape,leading to enhanced transfection [116].Despite the fact that at the moment studies directly demonstrating the success of photothermally controlled release of multiple co-delivered agents are lacking,the successful use of PEI-functionalized rGO in delivering,though separately,both small molecule compounds and nucleic acids has pointed to this possibility.Future efforts directing to verifying this will be of immense practical value.
6.Conclusions and outlook
Graphene-based materials have been rapidly developed in recent years and,as presented in sections above,have shown high practical potential in delivery of bioactive agents.Despite this,extensive future efforts to verify the biocompatibility of these materials will be needed if their use involves either direct contact with body tissues or injection into the blood circulation.Clarification of the metabolic fate and biodistribution profile upon injection of those graphene-based materials is also in dire need before the attainment of the US Food and Drug Administration (FDA)’s approval.The legitimacy of this need has been evidenced by the capacity of GO to alter cell morphology and to damage the plasma membrane to trigger apoptosis [117].In macrophages,necrotic cell death elicited by GO is partly linked to the initiation of the toll-like receptor 4 (TLR4) signaling pathway [118].In addition,graphene nanosheets have been found to induce cellular damage by increasing the levels of various cytokines and their corresponding soluble receptors,especially interleukin-33 [119].In thein
vivo
context,graphene nanosheets have led to signs of thromboembolism,inflammation,and immune activation in mice upon intravenous administration [119].In fact,the toxicity of graphene-based carriers can be affected by diverse factors.As shown by the case of 1,2-distearoyl-sn
-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)]-wrapped oxidized graphene nanoribbons,graphene-based materials display different levels of toxicity in different cell lines in dose-and time-dependent manners [120].The forms of graphene used may also affect the toxic effects attained.This is supported by the observation that,upon injection of various forms of graphene (including GO,aggregated graphene,and dispersed graphene) into the lungs of mice,severe lung injury has been noted only in those treated with GO.Those treated with aggregated and dispersed graphene have experienced no significant lung injury [121].The safe use of graphene-based carriers,therefore,has to be evaluated in a case-by-case basis.Apart from the need of proper toxicological evaluation,efforts should be directed to deciphering the interactions between GO and body cells,as well as the subsequent cellular response.This can facilitate the prediction and optimization of the performance of graphene-based carriers in cellular internalization [122].In addition,partly due to the lack of standardization of experimental procedures (particularly in terms of the use of controls and testing methods),fair and direct comparison among data from different studies can hardly be made,leading to the difficulty of identifying more promising candidates,among a plethora of reported graphene-based materials,for bioactive agent delivery [123].Solving this problem requires collaborative efforts among researchers in the field.Raising the awareness of the necessity of procedural standardization is,therefore,a challenge to be settled in the forthcoming decades.
Conflicts of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors acknowledge the financial support received from the Chinese University of Hong Kong,Shenzhen (PF01001421 and UDF01001421),Natural Science Foundation of Guangdong Province (2018A030310485),and Research Grants Council of the Government of Hong Kong Special Administrative Region (C5012 -15E).
 Asian Journal of Pharmacentical Sciences2021年5期
Asian Journal of Pharmacentical Sciences2021年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Commentary of the mRNA vaccines COVID-19
- Regulatory role of long non coding RNAs (lncRNAs) in neurological disorders:From novel biomarkers to promising therapeutic strategies
- Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility:What can we learn from the literature
- Role of the cation-chloride-cotransporters in the circadian system
- Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy
- Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles:rhIFN α-1b example
