Role of the cation-chloride-cotransporters in the circadian system
Frh Azln Sundy Josih Zhijun Wng Zhng
a Institute of Biomedical and Clinical Sciences, Medical School, College of Medicine and Health, University of Exeter, Hatherly Laboratories, Exeter EX4 4PS, UK
b Department of Neurology, Institutes of Brain Science, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institute of Biological Science, Zhongshan Hospital, Fudan University, Shanghai 200032, China
Keywords: GABAergic Na + -K + -2Cl −cotransporter 1 (NKCC1) K + -2Cl −cotransporter 2 (KCC2) WNK3-SPAK/OSR1,Chloride (Cl −) homoostasis;Suprachiasmatic nucleus (SCN) Circadian rhythms
ABSTRACT The circadian system plays an immense role in controlling physiological processes in our body.The suprachiasmatic nucleus (SCN) supervises this system,regulating and harmonising the circadian rhythms in our body.Most neurons present in the SCN are GABAergic neurons.Although GABA is considered the main inhibitory neurotransmitter of the CNS,recent studies have shown that excitatory responses were recorded in this area.These responses are enabled by an increase in intracellular chloride ions [Cl−]i levels.The chloride (Cl−) levels in GABAergic neurons are controlled by two solute carrier 12 (SLC12) cation-chloride-cotransporters (CCCs):Na +/K+ /Cl− co-transporter (NKCC1) and K+ /Cl− co-transporter (KCC2),that respectively cause an influx and efflux of Cl− .Recent works have found altered expression and/or activity of either of these co-transporters in SCN neurons and have been associated with circadian rhythms.In this review,we summarize and discuss the role of CCCs in circadian rhythms,and highlight these recent advances which attest to CCC’s growing potential as strong research and therapeutic targets.
1.The circadian system
The circadian system refers to our near 24-hour biological clock that plays a crucial role in the daily control of physiological and behavioural processes [1–3].Essential aspects in our body such as hormonal secretions,body temperature and sleep/wake cycles rely on regulation from our circadian system [1].Research done in mammals reveal that the circadian system is composed of a stratified assemblage of biological clocks that are supervised by a master circadian clock known as the suprachiasmatic nucleus (SCN) [1,4].The primary role of the SCN is to generate and harmonise the circadian oscillations to a near 24-h cycle matching the environment [3,4].Evidence to support the role of the SCN in controlling circadian rhythmicity stemmed from numerous experiments such as disrupting circadian rhythmicity by introducing lesions in the SCN [5]or restoring the circadian system through SCN transplant [6].
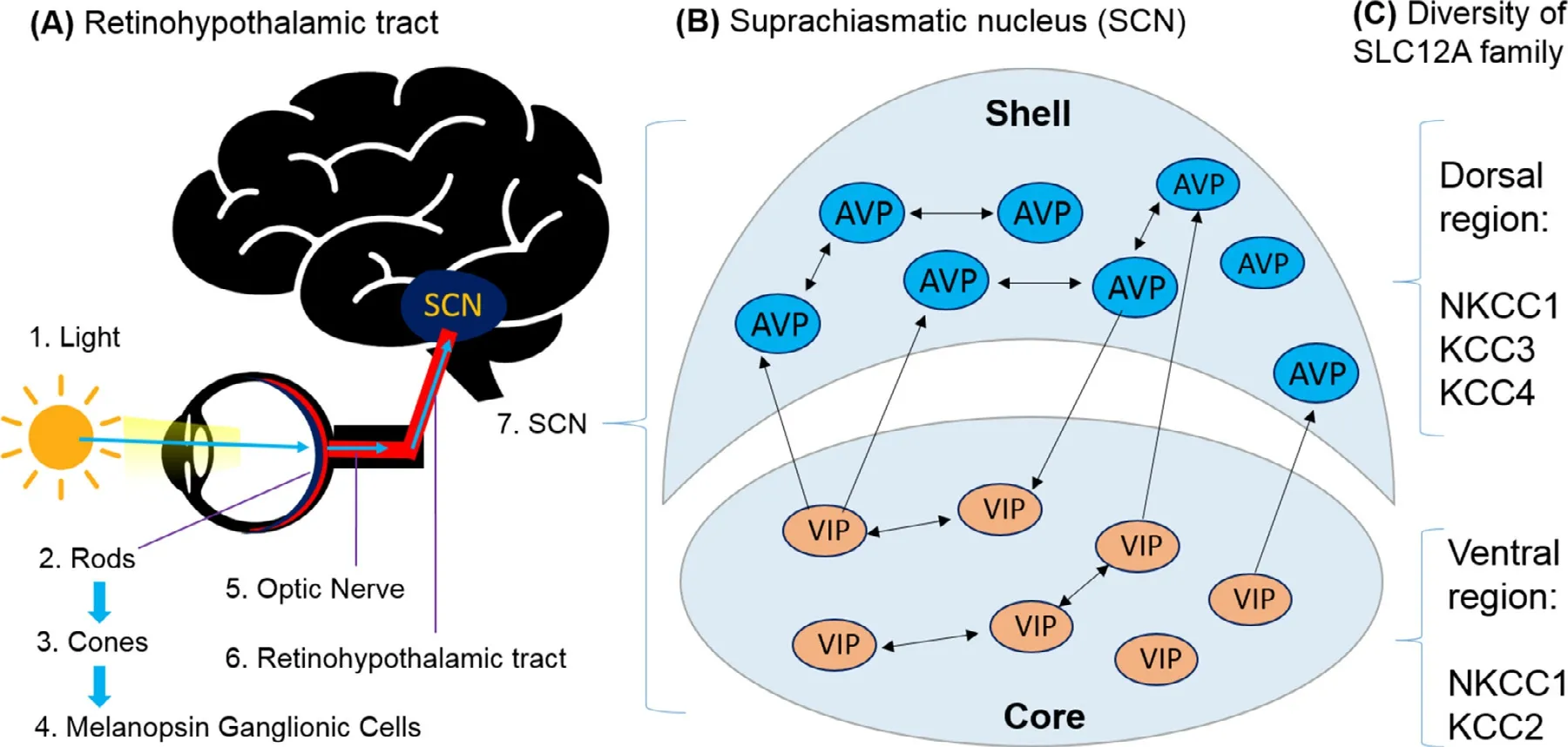
Fig.1–Illustration of the retinohypothalamic tract and the suprachiasmatic nucleus. (A) illustrates the pathway where light cues are transmitted from rod,cones and melanopsin ganglion cells through the optic nerve that goes into the retinohypothalamic tract to reach the master clock,the Suprachiasmatic Nucleus.(B) SCN of the hamster can be divided into a ventrolateral core and a dorsomedial shell,both densely packed with somata of small neurons.VIP is predominantly expressed in the ventral core region and the AVP is predominantly expressed in the dorsal shell region.Most neurons contain additionally GABA.(C) Diversity of SLC12A family in SCN neurons,NKCC1,KCC3 and KCC4 cotransporters were mainly found expressed in SCN neurons in Dorsal region,whereas the NKCC1 and KCC2 cotransporters in SCN neurons in Ventral region.
The SCN is located in the anterior part of the hypothalamus,and it is composed of approximately 20 000 neurons,referred to as clock cells,that communicate between each other to monitor,regulate and stabilise the circadian system [2,7].Through studies of the SCN,researchers were able to identify the different anatomical regions [2,3].Indeed,the SCN can be divided into two parts,the dorsal that is often referred to as the shell and the ventral part,also known as the core region [1,3](Fig.1 B).The core region of the SCN is mainly composed of vasoactive intestinal peptide (VIP) expressing neurons while the shell region is predominantly formed of arginine vasopressin (AVP) expressing neurons [8](Fig.1 B).It must be noted that although VIP and AVP expressing neurons concentrate in different regions of the SCN,their anatomical separation is not absolute [9].However,for the purpose of this review,it will be considered that VIP neurons constitute mainly the ventral part while the dorsal part contains,primarily,AVP neurons.
To synchronise with the solar time,the SCN relies on integrating photic signals where information about the presence and intensity of light are transmitted as well as non-photic cues such as the timing of meals [6,10].Cones,rods and photosensitive melanopsin ganglion cells of the retina collect light information that they transmit,through the retinohypothalamic tract (RHT),to the VIP producing neurons in the core region of the SCN [6,10](
Fig.1 A).This assures that the synchronisation is maintained between the clock and the environment [6,10].It must be noted that other parts of the brain and body express their own circadian clock,but are controlled by the master clock through neuronal projections and paracrine signalling [7,11].On a cellular level,the molecular clock in individual cells is regulated through a molecular feedback loop that maintains a near-24 h circadian activity locally in cells [11,12].
There is a diversity of SLC12A family ion-transporters found in SCN neurons (Fig.1 C,discussed in next section).A balance between excitatory and inhibitory signalling must be maintained for a proper function of the brain [13].γ
-aminobutyric acid (GABA) is considered as the main inhibitory neurotransmitter in the nervous system [14].It plays an essential role in different parts of the brain,such as the thalamus [15]and the hypothalamus [16].GABA seems to play a vital role in communications between clock cells in the SCN,evident by GABA expression in almost all SCN neurons [7,17].Models and experiments brought evidence of the significant impact GABA has on plasticity,properties and function of the SCN [18]with a recent study showing that a lack of GABA function led to disruptions of the circadian rhythms [19].Although GABA is often referred to as an inhibitory neurotransmitter,there is a growing number of evidences to support that GABA can also elicit excitatory responses [7,16].In fact,more than 95% of the neurons that enables the mammalian SCN to play its role as the master pacemaker for circadian rhythms are GABAergic neurons [20],which speaks volume of the role of GABA in the regulation of the circadian rhythm in SCN.Recently,Ono and colleagues published review articles on the role of GABA in the regulation of circadian rhythm and how GABAergic mechanisms influences the circadian system [7,21],while He et al.and Chi-Castañeda et al.highlighted the effect of circadian systems on GABA transport in the SCN in their respective review publications [22,23].It is generally accepted that GABA plays the role of an excitatory neurotransmitter during foetal development [24,25].Indeed,GABA stabilises and refines the circadian firing rhythm in the foetal SCN [19].Recently,this GABA-depolarising phenomenon was also observed in multiple areas of a matured brain,including the SCN [26].However,this excitatory theory was recently opposed by Zilberter,as the paper questioned the validity of this theory [26].The commentary highlights the contrast observed betweenin
vitro
andin
vivo
where GABA is respectively excitatory and inhibitory during foetal development.The difference may stem from limitations associated within
vitro
studies or preparation on acute brain slices [26].The exact cause of difference observed was not identified but emphasis was given on the need to conduct more research on this topic [26].Polarity in GABA neurons depends on the GABA equilibrium potential (E GABA).E GABA is regulated by the intracellular concentration of chloride ions [Cl][25].If the [Cl]in an SCN neuron is high,the E GABA will be more positive,and the GABA signalling will result in depolarisation [25].If the [Cl]is low,the E GABA will be more negative,and the response is an inhibitory hyperpolarisation [25](Fig.2).In all these,however,the mechanism of GABAergic signalling is still elusive as controversy persists on its status as an excitatory or inhibitory neurotransmitter [7].Neuronal [Cl]is majorly regulated by two cation chloride cotransporters (CCCs):Na-K−2Clcotransporter 1 (NKCC1) and the K−2Clcotransporter 2 (KCC2) [27].NKCC1 drives Clinto the cell through the Nagradient generated by Na/K/ATPase,and KCC2 extrudes the Clin mature neurons [28].NKCC1 expression is reduced with KCC2 increased during development,this results in high [Cl]in immature neurons,and low [Cl]in mature neurons[29,30].Therefore,mature neurons have low [Cl]causing a shift in E GABA from depolarising to hyperpolarising [29,30].Thus,KCC2 and NKCC1 are crucial regulators of GABA mediated hyperpolarisation:an essential component of synaptic inhibition within the adult brain (Fig.2).
Recently,a lot of research has been conducted on CCCs and SCN in the body;in view of this,the structural aspect of NKCC1 and KCC2 are not dealt with in this review.Thus,the aim of this review is to summarise the current understanding of the role of NKCC1 and KCC2 cotransporters in SCN neurons,and the impact they have on the master clock of the circadian system,and on a broader picture,the regulation of the circadian system.The review will first examine the expression of NKCC1 and KCC2 within the SCN,and the impact different expression has on the polarity of SCN neurons.Then,the relationship between CCC expression and light,a main driver of circadian rhythmicity,will be considered.Lastly,the regulation of NKCC1 and KCC2 by other components of the cell,that may play a critical role in maintaining the circadian system,will be discussed.
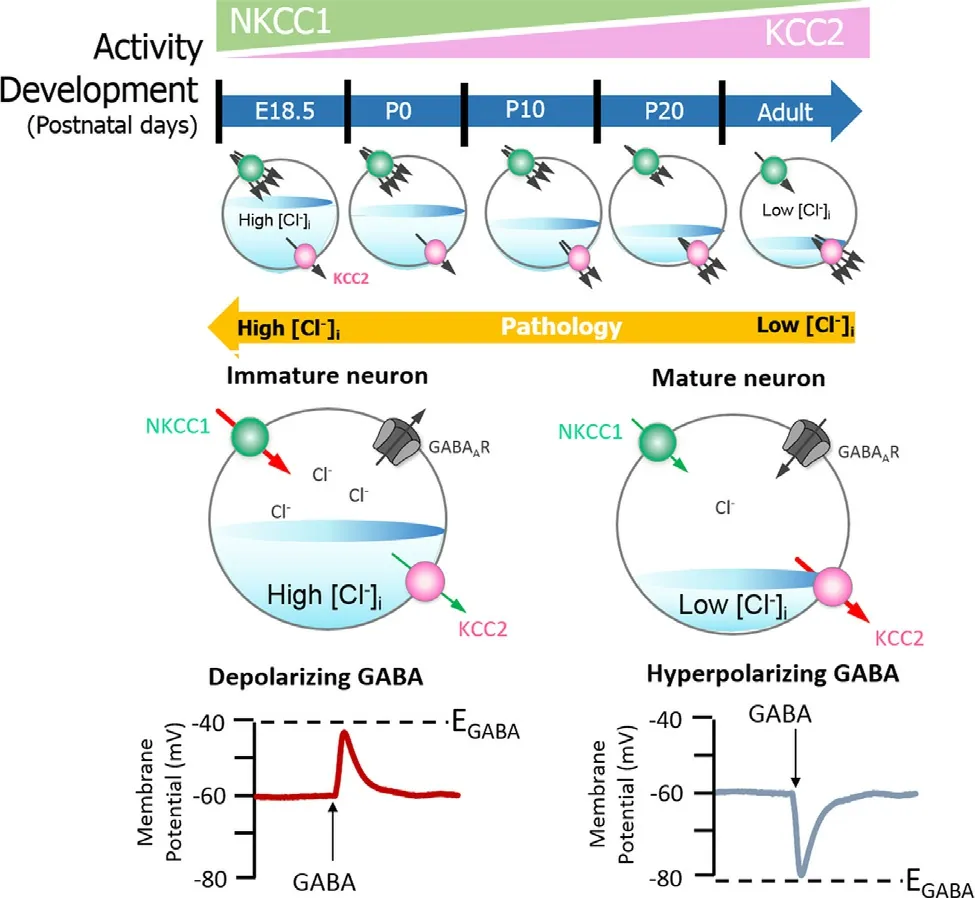
Fig.2–GABAA signalling shifts from depolarizing to hyperpolarising responses is mediated by developmental expression of KCC2 and NKCC1. The differential expression of these channels regulates [Cl −]i,and therefore determines the activity of GABA.NKCC1 pumps Cl −into neurons,its expression is high in the early postnatal period,decreasing as maturation proceeds.The expression pattern for KCC2,responsible for Cl −efflux is directly opposite.In the embryonic and early postnatal periods,[Cl −]i is high and so GABAergic signalling is excitatory (depolarising);as maturation occurs,[Cl −]i decreases,initiating the development hyperpolarising shift,whereby GABAergic signalling becomes inhibitory.Figure elements were taken and modified from Tillman and Zhang [25].
2.Expression of NKCC1 and KCC2 in the SCN
When investigating the role of CCCs in the circadian system and its master clock,the SCN,one approach was to first observe the distribution of NKCC1 and KCC2.Studying the distribution of CCCs will provide vital information on the regulation of [Cl]in the GABAergic neurons of a matured SCN.This section aims to gain insight into the diverse expression of the CCCs in the SCN.Kanaka and colleagues investigated the expression of the CCCs by analysing the messenger RNA (mRNA) of NKCC1 and KCC2 in the nervous system of rats [31].Using five male Wistar rats,they observed that NKCC1 mRNA seemed to be ubiquitously expressed in the nervous system,including the SCN,while KCC2 mRNA was absent in the dorsal part of the SCN [31].This void of KCC2 in the shell region of the SCN was also observed by Belenky and colleagues [9]who noticed that KCC2 was instead highly expressed in the ventral area [9].When comparing both studies,they have similar conclusions;however,Belenky’s paper seems to be more accurate than Kanaka’s for numerous reasons.First,Kanaka and colleagues used only 5 male rats,whereas Belenky used 47 rats;this provides a strong statistical significance for Belenky’s study.Then,the method used by Kanaka was to study the mRNA distribution.One common limitation cited with this method is that although mRNA expression usually translates in the presence of the protein it codes for,in this case CCCs,it is not a certainty.Belenky’s method is considered more reliable as the team employed numerous immunostaining methods that were used alongside a confocal microscope.The method employed by Belenky and colleagues is more reliable as they used antibodies that specifically bind to membrane-bound KCC2 and NKCC1.By binding directly to the CCCs,the antibodies confirm the presence of the co-transporters.The mRNA method is less reliable as mRNA presence might not necessarily mean that they were translated into proteins.
Overall,both papers showed that NKCC1 is expressed across the SCN,while KCC2 is expressed only in the ventral region of the SCN [32].In trying to understand the underlying mechanism of GABA induced excitation in the SCN,Belenky and team uncovered a potential explanation for the void of KCC2 in the dorsal region [32].Using immunostaining on the SCN of 32 adult rats,Belenky’s team observed that while KCC2 is highly expressed in the core region,the dorsal region expresses other isoforms of KCC,KCC3 and KCC4 [32](Fig.1 C).The paper showed that no co-localisation existed between KCC2 and KCC4 [32].Though,we did not discuss the structural characteristic of CCCs in this review but it is important to briefly state that various isoforms of KCC can be differentiatedin
situ
by their specific antibodies.Over the years,data base queries and library screening coupled with molecular techniques such as polymerase chain reaction have been used to identify putative isoforms of CCCs specifically localized in various cells.Payne et al.used KCC2-specific cDNA probe for a northern blot analysis on cell culture prepared from a fresh whole brain of Wistar rats [33].Furthermore,they investigated the KCC2 transcript distribution within the rat brain with a KCC2-specificS-labeled riboprobein
situ
.Pan and colleagues used real time RT-quantitative PCR technique to investigate the expression of mRNAs for various isoforms of KCC in red blood cells of C57/BL6 mice [34].Overall,a combination of NKCC1 and KCC2 is expressed in VIP-expressing neurons of the core region in the SCN while NKCC1 and KCC3/4 is found in the AVP-expressing neurons of the shell region.Interestingly,reports that putatively restrict KCC2 to neuronal tissue [33,35,36],and confirm NKCC1 to be ubiquitous [37–39]both exist.Taken together,these investigations highlight the importance of NKCC1 and KCC2 in the SCN and suggests a role for them in the circadian system.3.Regulation of [Cl −]i by NKCC1 and KCC2 in SCN neurons
NKCC1 and KCC2 are crucial for the regulation of the intracellular concentration of chloride ions [Cl],that is important to determine the polarity of the neurons [25].It is known that during development,an elevated [Cl]i is observed in immature neurons and that when activated,they display a depolarising response [29].This is due to a higher expression of NKCC1 in comparison to KCC2.During maturation,NKCC1 expression gradually decreases and KCC2 expression increases,resulting in an opposite expression pattern [29](Fig.2).When a pharmacological blocker of NKCC1 is applied to immature neurons,it was observed that the polarity shifts from excitatory to inhibitory [29].This exemplify the importance of the NKCC1– KCC2 pair for the regulation of [Cl]and more broadly,for the polarity of the neuron.
Choi and colleagues noticed that NKCC1 expression in matured SCN neurons evolved during a 24-h cycle and was particularly high during the night in the dorsal region of the SCN [40].This increase in expression was associated with GABA-evoked excitatory responses in the shell region that was recorded through a gramicidin-perforated-patch recording [40].This increased NKCC1 expression in the dorsal region of the SCN at night was also observed by Alamilla and colleagues [41].Using a patch-clamp approach,they noticed that the E GABA of the dorsal and ventral regions reversed between day and night [41].Concurrent with an increase in NKCC1 expression at night,the team recorded an E GABA of −30 mV and that when GABA was stimulated,the signalling induces an excitatory response [41].In both experiments,the application of bumetanide,an NKCC blocker,resulted in a dampening of the excitatory responses in SCN neurons [40,41].Alamilla and colleagues observed that bumetanide resulted in a more negative equilibrium potential [41]while Choi and colleagues observed that the application of bumetanide on individual neurons was sufficient to switch the response from excitatory to inhibitory [40].The high expression of NKCC1 in the dorsal region at night,coupled with the excitatory responses observed,display the clear role of NKCC1 in the regulation of [Cl]i in SCN neurons.
Klett and Allen investigated the regulatory mechanism of [Cl]in AVP and VIP neurons by using specific blockers for NKCC1 and KCCs,respectively,bumetanide and VU0240551 (VU) in rodents [42].To quantify the effectiveness of these blockers and study the role of NKCC1 and KCCs in the regulation of intracellular levels of Clions,the team used ratiometric Climaging [42].Consistent with previous studies,the pair reported KCC2 expression in VIP neurons and absence in AVP neurons and KCC3 and KCC4 in AVP neurons.They also observed that [Cl]i levels are higher during the day in comparison to night in both VIP and AVP neurons but bumetanide had little effect on the [Cl]i levels of neurons when compared to VU.This suggests that KCC seems to play a more prominent role in the regulation of intracellular levels of Clin comparison to NKCC1 [42].Klett and Allen also noticed that VU had a more significant effect on VIP-expressing neurons compared to AVP neurons [42].Although VU exhibit selectivity towards KCC2 over NKCC1,VU lack selectivity for a specific KCC isoform.Thus,VU may have acted on KCC3 or KCC4 in AVP neurons and it can concluded that KCCs are the primary regulators of [Cl].These papers have provided evidence that the NKCC1– KCC2 pair is massively involved in the regulation of intracellular Cllevels of SCN neurons.This regulation influences the response of SCN neurons.An upregulation of NKCC1 leads to a higher [Cl]since it allows an influx of Cland thus when GABA is stimulated,causes an excitatory response.However,these papers do not provide sufficient information on elements that modulate the NKCC1 and KCC2 expression in SCN cells.Although these papers provide interesting information on the NKCC1-KCCs pairing in SCN neurons,they do not cover on modulators involved in regulating their expression and activity.Furthermore,more research is needed to understand the relationship between the two CCCs,to categorically conclude if one is more essential than the other in AVP and/or VIP neurons.
As earlier mentioned in this review,KCC2 and NKCC1 are crucial regulators of GABA mediated polarisation shifts.In other words,the progressive difference in [Cl]regulation between immature and mature neurons is mainly caused by a difference in expression/activity of the key chloride transporters NKCC1 and KCC2 [29,30]and it varies across different species [43].Ben-Ari and co-workers documented that a shift in [Cl]in rodents happen during the second postnatal weeks [24].Similarly,Rivera et al.experiment on hippocampal tissue and Dzhala et al.study on cortical tissues of experimental rats both revealed that an increased KCC2 mRNA expression increased after postnatal week 2 while mRNA expression of NKCC1 declined between 14 and 21 postnatal days [44,45].Furthermore,Dzhala and colleagues comparatively carried out the same experiment on human cortex and found out that KCC2 expression increases around 40 d postnatal week,whereas NKCC1 expression reaches matured stage approximately around 50 postnatal days [45].These findings suggest that significant variation in maturation patterns exist among different species;a key factor worth considering when comparing studies using different models.
4.The relationship between light and NKCC1 and KCCs in the SCN
When considering the upregulation of NKCC1 in the dorsal region at night [40,41],one can question if light influences the expression of the CCCs in the SCN neurons.Indeed,this point is compelling as light is missing during night and it is known that SCN neurons integrate photic cues that are collected and transferred from the retina through the RHT (Fig.1).Beyond the simple difference between day and night,variations of light exposure is key during seasonal change.Indeed,the transition between seasons (e.g.winter to spring) is mainly characterised by lengthening or shortening of light exposure [46].For animals,synchronising their biological clocks is,in some case,a do or die situation as these annual changes can threaten their survival [46].Seasonal affective disorder,a form of depression that is sensitive to day length changes,is partially caused by a transition in the photoperiod exposure length [47,48].Understanding if light regulates the expression on NKCC1 and KCC2 to alter the SCN and thus the circadian system is paramount as humans in the modern society are often exposed to artificial light [49].This section of the review aims to explore the potential link of light exposure to the expression of NKCC1 and KCC2 in SCN neurons to further understand the possible consequences on light regulation on the circadian system/rhythm through NKCC1 and KCC2.Although the team did not focus on NKCC1 and KCC2,the work conducted by VanderLeest and colleagues provides precious insight into the role of SCN neuronal network in encoding day lengths [50].They housed mice under long and short photoperiods and recorded SCN activityin
vitro
andin
vivo
.Their results revealed different activity profiles for long-day and short-day conditions [50].In particular,in long days,the animals showed less nocturnal activity than in short-day.This paper provided evidence that the SCN is influenced by day length and that the difference is significance between long and short days [50].The rest of this section will go in focus on the CCCs’ expression in relation to light.4.1.Light and NKCC1
McNeill and colleagues administered the NKCC1 blocker,bumetanide,at different times of the cycle and to varying doses in hamsters to see the effects of NKCC1 on the circadian clock [51].Exposure to light in the early subjective night phase delays the SCN and light in the subjective night phase advance the SCN.This information shows the phase delaying capacity of light that contributes to one of the main functions of the circadian system-the entrainment of day &night cycles.They observed that bumetanide administered during the early subjective night phase reduced the light-induced phase delays but did not alter the circadian phase in the absence of light [51].This paper provides proof that reduction of excitatory GABA,reduces the phase delays in the circadian system triggered by light [51],suggesting NKCC1 activity could be one of the biomarkers for such process.The data gathered by McNeill and colleagues are consistent with models that propose that depolarising response from RHT innervations are associated with neurons having a higher ratio of NKCC1:KCC2 activity [52].
Farajnia and colleagues explored the expression of NKCC1 in SCN under long and short-day photoperiods by recording the neuronal response [47].Using the NKCC1 blocker bumetanide on mice,the team noticed that in mice subjected to long-day photoperiods,40% of cells were excitatory,while 36% were inhibitory.However,for short-day subjects,52% of neurons were inhibitory and 28% excitatory [47].These results support the different phenotypes associated with short-days and long-days,consolidating the idea presented in VanderLeests’ paper.Farajnia’s findings also support the concept that long-day photoperiod exposure induces a polarity switch in GABAergic neurons of the SCN,from inhibitory to excitatory.Treatment with bumetanide reduced the excitatory GABAergic response,suggesting photoperiod modulation of NKCC1 activity or expression [47].
Taken together,the papers highlight the contribution of light to the entrainment of the circadian system and that this role is accomplished via regulatory feedback on NKCC1 activity.Farajnia and colleagues showed that longer photoperiods induced a higher number of SCN neurons to switch polarity and display excitatory activity,thus providing different excitatory and inhibitory ratio profiles for long and short days [47].McNeill and colleagues’ paper showed that NKCC1 was involved in circadian phase delay due to a higher exposure to light [51].Due to the mediatory role of NKCC1 in the SCN neurons,Farajnia et al.[47]suggested the potential clinical use of bumetanide to reduce inappropriate excitatory GABAergic responses.This will enable the modulation of the circadian system for patients exhibiting long-day phenotype to restore it closer to a normal rhythm [47].Although bumetanide-induced reduction of excitatory GABAergic signalling improved the behaviour of autistic children [47],more research is needed to explore the potential clinical use of bumetanide in this scenario.In particular,dosage should be investigated further as bumetanide shows a 500-fold greater affinity to NKCC1 compared KCC2 but at high doses,bumetanide can inhibit Clefflux via KCC2 [51].
4.2.Light and KCC2
Olde and colleagues investigated the role of KCC2 by studying mice that were exposed to short and long photoperiods [14].They studied the responses of GABAergic neurons in the SCN for each photoperiod and compared it to mice given KCC2 blocker,VU0255011 [14].The team observed that in the control groups,mice exposed to short photoperiods exhibit a higher GABAergic inhibitory response (56%) when compared to mice entrained to a long photoperiod (31%).This suggests that the ratio activities of NKCC1:KCC2 differ according to light exposure:KCC2 activity increased with less light exposure.In the VU0255011 groups,Olde and team noticed that inhibitory responses severely decreased to 31% inhibitory responses in short photoperiods and only 15% inhibitory response in long photoperiods [14].This reduction in inhibitory responses and increase in excitatory responses is wholly justified as KCC2 activity was blocked,consequently increasing the [Cl].When looking closely at the data,one can notice that with the use of VU0255011,short photoperiod neurons had a similar percentage of excitatory and inhibitory neuronal response [14].Beyond showing that light influences the excitatory/inhibitory(E/I) balance via the ratio activity of NKCC1 and KCC2,the paper suggests that only blocking KCC2 is enough to change the E/I balance in SCN neurons.
4.3.NKCC1 and KCC2 under light
Although the aforementioned papers provided substantial evidence for the regulation of the CCC in different photic exposure,the researches only focused on one of the CCCs without investigating both under short and long photoperiods [53].Myung and colleagues investigated short and long entrained SCN neurons,that will be discussed below.First,at a transcriptional level,the team examined the transcripts of two proteins,brain and muscle Arnt-like1 (Bmal 1) and period (Per),that are involved in maintaining the near 24 h circadian oscillations in clock cells.They noticed only a little difference between short and long photoperiods,however,when observing NKCC1 and KCC2,they notice an upregulation of both NKCC1 and KCC2 in the long-phase entrained SCN neurons in comparison to the short-photoperiod SCN neurons [53].Furthermore,the team noted that NKCC1:KCC2 expression ratio was significantly higher in the dorsal region for long entrained SCN neurons [53].However,Myung and colleagues used transcripts to obtain these results and mRNA presence does not necessarily translate into protein expression.Thus,there is insufficient evidence to confidently confirm a higher expression of NKCC1.The researchers also explored the effect of different light duration entrainment on the circadian oscillations emitted in the SCN neurons.They noticed excitatory GABAergic response in the dorsal region of the SCN and concluded that this is probably due to an upregulation of NKCC1.The excitatory GABA was also associated with phase advances in circadian oscillations of the dorsal region [53].This phase-advance in the dorsal region causes a desynchronisation with the ventral area [53].Indeed,since the oscillation phases are quicker in the dorsal region,the ventral part has difficulty keeping up.This desynchronisation is further exacerbated by the fact that the VIP signal produced by the ventral SCN become diffuse,thus diminishing the ability of ventral region SCN neurons to synchronise with dorsal region of SCN neurons [53].This study provides evidence that light exposure can alter the expression ratios of NKCC1 and KCC2 in the SCN and adjust the polarity of GABA signalling,resulting in dysregulated circadian oscillations.The idea of using bumetanide as a therapeutic tool,suggested in the Farajnia’s paper [47],can be useful for resetting and resynchronising the two regions of the circadian master clock.Indeed,application of the NKCC1 can reduce the polarity switch to excitatory neurons and thus reduce the phase advance observed in the dorsal region,permitting the resynchronisation of both zones.
Overall,these studies provide evidence that light has an impact on the expression and activity of CCCs,NKCC1 and KCC2 in the SCN.There is substantial evidence that NKCC1 and KCC2 are expressed differently in short and long photoperiods and contributes to the regulation of circadian phases.Overexposure to light can result in alteration and desynchronization of the circadian oscillations that are generated by the SCN regions.This is a cause for concern as failure to maintain synchrony may contribute to pathogenesis as the body does not follow one rhythm.Targeting the CCC in the SCN should be explored as it could yield great potential value.
5.Phosphoregulation of NKCC1 and KCCs
Like all the other CCCs,NKCC1 and KCCs are regulated by a cascade of kinases [54].The primary kinase of interest is the serine-threonine kinase WNK (with no K [lysine]) and its downstream substrates,Ste20/SPS1-related proline-alanine-rich kinase (SPAK) and oxidative stress responsive 1 (OSR1),initiates downstream phosphorylation that directly affect the activity of NKCC1 and KCCs in cells.Susa et al.[55]studied WNK4 in male mice and reported that the activity of the WNK4 cascade displayed a diurnal rhythm in mouse kidneys.Transcript [53]and immunostaining [32]studies identified WNK3 as the most common isoform in the SCN.WNK3 is a chloride sensitive kinase that is involved in the regulation of [Cl]levels and GABA induced excitation [32,53].These kinases may play a vital role in the detection of direction of Clmovement in SCN neurons [32];thus,they are involved in the regulation of [Cl]i levels and in the polarity of GABAergic neurons.WNK3 has important control of the cellular activity of NKCC1 and KCC2.Indeed,NKCC1 activity is increased when it is phosphorylated by WNK3 while KCC2 activity is inhibited [54,56](Fig.3).These characteristics of WNK3 were obtained after researched conducted by Kahle and colleagues [56].They studied the impact of WNK3 regulation on NKCC1 and KCC2 cotransporters inXenopus
oocytes
.One effective result that shows this importance of WNK3 in the activation of NKCC1 is that in the abs-ence of WNK,NKCC1 was inactive or partially active in respectively hypotonic and isotonic conditions,but NKCC1 was only fully activated in hypertonic conditions [56].However,when NKCC1 was co-expressed with WNK3,NKCC1 was fully active in all three states [56].When the team used a mutated version of WNK3,referred to as kinase-dead WNK3,they observed the opposite effect in NKCC1 and KCC2 [56].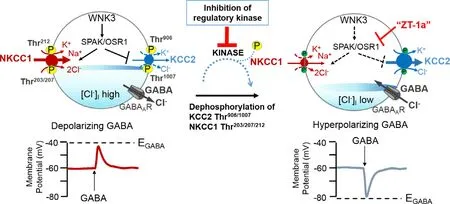
Fig.3–A novel strategy to facilitate neuronal Cl −extrusion by coincident NKCC1 inhibition and KCC2 activation by inhibiting the WNK3-SPAK/OSR1 kinases. In neurons in multiple neuropsychiatric conditions driven by hyperexcitable circuits (e.g.,seizures,neuropathic pain,spasticity,schizophrenia,and others),[Cl −]i are elevated due to increased NKCC1 activity,and/or decreased KCC2 activity,promoting GABA A R-mediated membrane depolarization and excitation.In healthy mature neurons,[Cl −]i is low due to the opposite activity profile of the CCCs,promoting GABA A R-mediated hyperpolarization,which is critical for the proper balance of excitation-inhibition in neuronal circuits.WNK-SPAK/OSR1 inhibition,via the coincident effects of NKCC1 inhibition and KCC2 activation (the main Cl −extrusion mechanism in neurons) might be a potent way of facilitating neuronal Cl −extrusion to restore ionic inhibition in diseases that are characterized by disordered Cl −homoeostasis and GABA disinhibition.ZT-1a,a specific SPAK inhibitor [63].Figure elements were taken and modified from Tillman and Zhang [25].
We recently employed a functional kinomics study,incorporating a kinome-wide siRNA-phosphoproteomic screen and a kinase trapping-Orbitrap MS screen,to show that WNK3-SPAK kinase complex is an essential regulator of both KCC3 Thr991 and Thr1048 phosphorylation (corresponding to KCC2 Thr906 and Thr1007) inin
vitro
cells andin
vivo
mouse brains [57,58].We further demonstrated that WNK1/3-regulated phosphorylation of KCC2 at Thr906 and Thr1007,by SPAK/OSR1,maintains depolarising GABA activity in immature neurons [59,60].Notably,while phosphorylation of KCC2 reduces its activity,the WNK-SPAK/OSR1 kinase pathway also phosphorylates NKCC1,but this increases NKCC1 function.Thus,the same kinase pathway causes opposite effects on the opposing co-transporters,providing a very powerful push-pull regulatory control of [Cl].Previous crystallographic analysis [61]and our recent study usingin
vivo
SPAK mouse model [62],suggest SPAK or OSR1 serves as a bridge to facilitate the signalling cascade between WNKs and CCCs.Furthermore,we have recently developed a novel SPAK binding inhibitor,termed ZT-1a,which specifically blocks this signalling pathway,and subsequently reduces the NKCC1 and KCCs phosphorylation in cultured cells andin
vivo
mouse and rat brains [63].This is promising because ZT-1a may interfere with the SPAK regulation of GABA signalling via NKCC1 and KCC2 through controlling [Cl]in neurons.These studies show that WNK3-SPAK/OSR1 is a key component in the control of [Cl]i,and it accomplishes this role through the phosphorylation of the CCCs.This role can be especially crucial in the polarity shift of GABAergic neurons observed in the SCN.Although these current studies support the role of WNK3-SPAK/OSR1 signalling in the regulation of clock cells in the SCN,this paper does not provide information on whether the WNK3 or SPAK/OSR1 phosphorylation activity presents circadian patterns.More research should be conducted to see if light exposure can influence or work alongside WNK3 or SPAK/OSR1 for the regulation of NKCC1:KCC2 ratio in the SCN.Furthermore,other regulatory mechanisms of NKCC1 and KCC2 should be explored to see if they have any association to our circadian system,as this field remains still fairly novel.Technological advancements can be a driving factor in unveiling the mechanism underlying regulation,as seen with the papers used in this review.
6.Conclusion
NKCC1 and KCC2 are massively involved in regulating the polarity of GABAergic neurons in the SCN.These CCCs enable this through a control of the intracellular Cllevels,that alter the equilibrium potential.This polarity regulation is a key instrument to maintain an appropriate ratio of excitatory and inhibitory GABA response in the SCN.These CCCs expression ratios and activity can be altered by light,resulting in profound changes in the circadian oscillations and rhythmicity of the SCN.NKCC1 and KCC2,and their upstream regulators,WNK-SPAK/OSR1,and new drug like molecular ZT-1a,also present potential therapeutic options for conditions related to circadian disruption.However,more research on this topic is needed to decipher their mechanism of action.Further studies should focus on human’s clinical trials as most of the experiments were completed in animal models.Nevertheless,these experiments provide precious insights into this complex yet important regulation.
Conflict of interest
The authors assert that there is no conflict of interests concerning the publication of this review.
Acknowledgment
This work was in part supported by a Commonwealth PhD Scholarship (S.S.J.),NSFC grants to Y.W.(31771188,31471027),and the University of Exeter Medical School start-up fund (J.Z.) and NIH Grants R01 NS109358 (J.Z.).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2020.10.003 .
 Asian Journal of Pharmacentical Sciences2021年5期
Asian Journal of Pharmacentical Sciences2021年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Spatiotemporally co-delivery of triple therapeutic drugs via HA-coating nanosystems for enhanced immunotherapy
- Investigating the crucial roles of aliphatic tails in disulfide bond-linked docetaxel prodrug nanoassemblies
- A practical strategy to subcutaneous administered in-situ gelling co-delivery system of arsenic and retinoic acid for the treatment of acute promyelocytic leukemia
- Synthetic anti-angiogenic genomic therapeutics for treatment of neovascular age-related macular degeneration
- Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles:rhIFN α-1b example
- Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy
