Growth of high performance coal tar-based carbon film and its application in Joule heating
DU Zhi-ming,LEI Zhi-ping,YU Wen-hao,YAN Jing-chong,LI Zhan-ku,SHUI Heng-fu,REN Shi-biao,WANG Zhi-cai,KANG Shi-gang
(Institute of Material Science &Engineering, School of Chemistry &Chemical Engineering, Anhui Province Key Laboratory of Coal Clean Conversion and High Valued Utilization, Anhui University of Technology, Ma’anshan 243002, China)
Abstract:Conductive carbon film has a wide range of application prospects,especially in the fields of electric heating devices,energy storage devices,and solar cells.Coal tar is an ideal precursor for preparing carbon film.In order to improve the performance of coal tar-based carbon film,it is necessary to study the influence of tar composition on the structure and performance of carbon film.In this paper,a carbon film is prepared using aromatic compounds,heteroatom compounds and tar as carbon sources.It is found that the carrier concentration of aromatic hydrocarbon-based carbon films is higher than 1022 cm−3,but the mobility of the carrier is lower than 1 cm2/Vs.The resistivity and sheet resistance of the aromatic hydrocarbonbased carbon film are lower than that of the coal tar-based carbon film.Naphthalene-based carbon film has the best electrical and thermal properties.The maximum heating temperature of naphthalene-based carbon film at 30 V exceeds 300 °C.The thickness of the carbon film has a decisive influence on the sheet resistance of the carbon film.The performance of the heteroatom compound-based carbon film is significantly lower than that of aromatic compound-based film.
Key words:coal tar;carbon film;Joule heating
In China, high-temperature coal tar production was more than 13 million tons in 2017.The high content of fused-ring aromatic hydrocarbons makes coal tar as a high-quality precursor for the preparation of carbon materials, which could be used for the production of supercapacitors[1,2],sodium-ion battery anode[3],lithium-ion batteries[4],carbon quantum dots[5],Zn–air batteries[6],etc.To improve the performance and control the structure of coal tar-based carbon materials,it is necessary to investigate the relationship between the structure of coal tar and the performance of coal tar-based carbon materials.
The highly conductive carbon film could be used in a wide range of applications,especially in the fields of electric heating devices,energy storage devices,and solar cells[7,8].At present, the researches of highperformance conductive carbon film mainly focus on graphene film.However,there are many problems in the preparation of high-performance graphene,such as complex processes,harsh conditions,large equipment investment, and low yield.Seo et al.[9]prepared a graphene film on the surface of SiO2/Si substrate using coal tar pitch and Ni catalysis in Ar/H2atmosphere.They found that the prepared graphene film could be used as electrodes of bottom-contact pentacene fieldeffect transistors (max.field effect-mobility ~0.36 cm2/Vs).To utilize coal with large reserves,low prices,and rich structural diversity, and to utilize steamcracker tar,which is large in volume and not useful in petrochemical plants,Keller et al[10,11]research group found that the performance of carbon films prepared by coal or steam-cracker tar was equivalent to the performance of reducing graphene.They believed that those carbon films could be used to remove fog/ice on the window glass or aircraft wings,and to manufacture electronic heating devices.Joule heating is an effective,energy-efficient,and real-time electrothermal technique for making high-performance composites.Morris et al[11]found that Joule heating device fabricated from steam-cracker tar showed competitive performance compared to those based on reduced graphene oxides.
Recently,high-performance carbon films for joule heating have been prepared from coal tar.Through varying the preparation conditions,the conductivity of the carbon films can be adjusted by three orders of magnitude[12].As coal tar has a wide range of sources and complex composition,including compounds such as phenol oil,anthracene oil,naphthalene oil,wash oil,asphalt,pyridine,fluorene,and benzothiophene.It is obvious that the structure and composition of tar have a significant effect on the structure-activity relationship of the carbon film.Therefore, to improve the performance of coal tar-based carbon film, it is necessary to study the influence of tar composition on the structure and performance of as-prepared carbon film.To the best of our knowledge,there are almost no reports in this area.
To explore the influence of various compositions in coal tar on the structure and performance of carbon films,some light compositions in coal tar,such as toluene, naphthalene, anthracene, and phenanthrene,and heteroatom compounds, such as pyridine,phydroxybiphenyl,and benzothiophene,were used as coal tar-related model compounds to prepare carbon films under the same condition to simulate the influence of the compositions of coal tar on the preparation and performance of carbon films in this study.
1 Experimental
1.1 Materials and chemicals
Toluene (TL),naphthalene (NA),anthracene (AN),phenanthrene (PH),pyridine (Py),p-hydroxybiphenyl(4-pp),and benzothiophene (BT) were obtained from Sinopharm Chemical Reagent Co.,Ltd..The quartz sheet (20 mm×10 mm×0.5 mm) was purchased from Reinuo Glass Products Co., Ltd..Prior to the preparation of carbon films, the quartz sheet was sequentially washed with acetone and ethanol under ultrasonic.
1.2 Preparation of carbon films
The carbon films directly grew on the quartz glass substrates using negative-pressure chemical vapor deposition (CVD) method.In a typical CVD process,a quartz glass and a small crucible (9 mm in diameter)loaded with 0.16 g sample were placed in the corundum boat on both sides,and then the corundum boat was loaded into a horizontal quartz tube placed inside a high-temperature furnace.Then CVD system was flushed with N2to remove air and pumped to a negative pressure state (about 1000 Pa) prior to the temperature being ramped.The fabrication of carbon film was carried out at 1000 °C for 2.5 h.
1.3 Characterizations
Raman spectra was acquired using a Horiba LabRAM 800 HR spectrometer equipped with a He-Ne(632.817 nm) excitation source.The laser spot on the sample was~532 nm in diameter and had a power of~5 mW at the sample surface.TEM was performed on a Zeiss Ultra55 or a Helios Nanolab 600,both operated at 5 kV.The film thickness and surface roughness of the carbon film were measured using the Alpha-step IQ surface profiler (KLA-Tencor P-7).When measuring the thickness and surface roughness of the carbon film,each sample was measured three times,and finally,the average of the three sets of data was taken as the final result.The sheet resistance of the carbon film was measured by using a four-probe resistance tester.The carrier density,mobility,and resistivity of the carbon film were measured on a Hall Effect tester (HMS-3000) at magnetic field strength 0.55 T and 300 K.Each sample was continuously measured three times in the test to make sure that the contact between the probe and the carbon film was an ohmic contact.Joule heating measurements were performed with a FLIR AX5 camera at emissivity set of 0.93.The specific test device is shown in Figure 1.
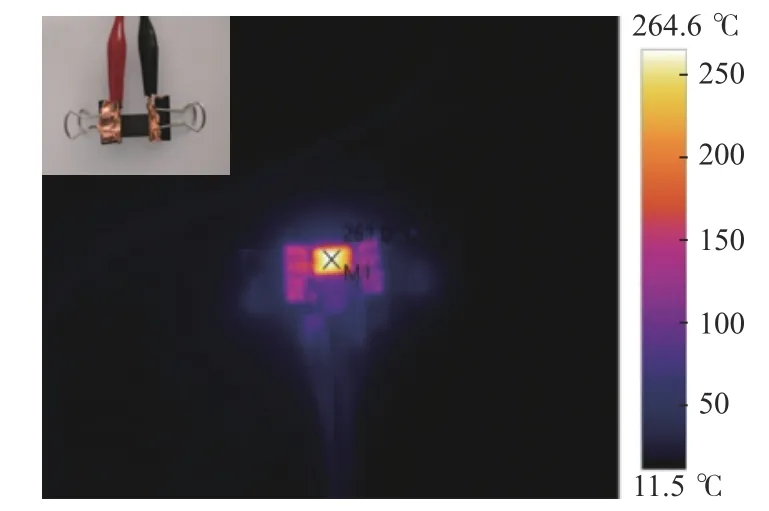
Figure 1 Joule heating devices fabricated from arene or coal tar
2 Results and discussion
2.1 Effect of precursor on characteristics of carbon films
Coal tar mainly contains phenol oil,naphthalene oil, anthracene oil, washing oil and pitch.These fractions have different boiling points and pyrolysis products, which have a significant impact on the composition of as-prepared carbon film.Similarly,trace amounts of nitrogen,sulfur,oxygen and other heteroatoms in the tar will also affect the structure and performance of the carbon film.Therefore,TL,NA,AN,PH,Py,4-pp,and BT as representatives of the above fractions,were used as precursors to prepare carbon films and to investigate the relationship between the structure and properties of tar-based carbon films.The diffraction peak at about 22° (as exhibited in Figure 2(a),(b)) reveals that the tar-based carbon films prepared with different precursors are amorphous materials.As shown in Figure 2,the tarbased carbon films have two Raman characteristic peaks in the range of 1000−2000 cm−1,namely D peak(1370 cm−1) and G peak (1590 cm−1),indicating that the as-prepared tar-based carbon films are all amorphous carbon films.ID/IG(the ratio of peak heights of the D to G Raman bands) and G peak broadening are closely related to thesp2cluster size and the variety and disorder insp2domains[10,11].As exhibited,compared with the tar-based carbon film,thesp2clusters and disorder of the NA-based carbon film are larger,which indicates that the performance of the NA-based carbon film may be better.Among the carbon films prepared by heteroatom compound, BT-based and Py-based carbon films have the largestsp2clusters and the most disorderedsp2domains,respectively,indicating that the type of heteroatom compound precursors may affect the structure of carbon film.
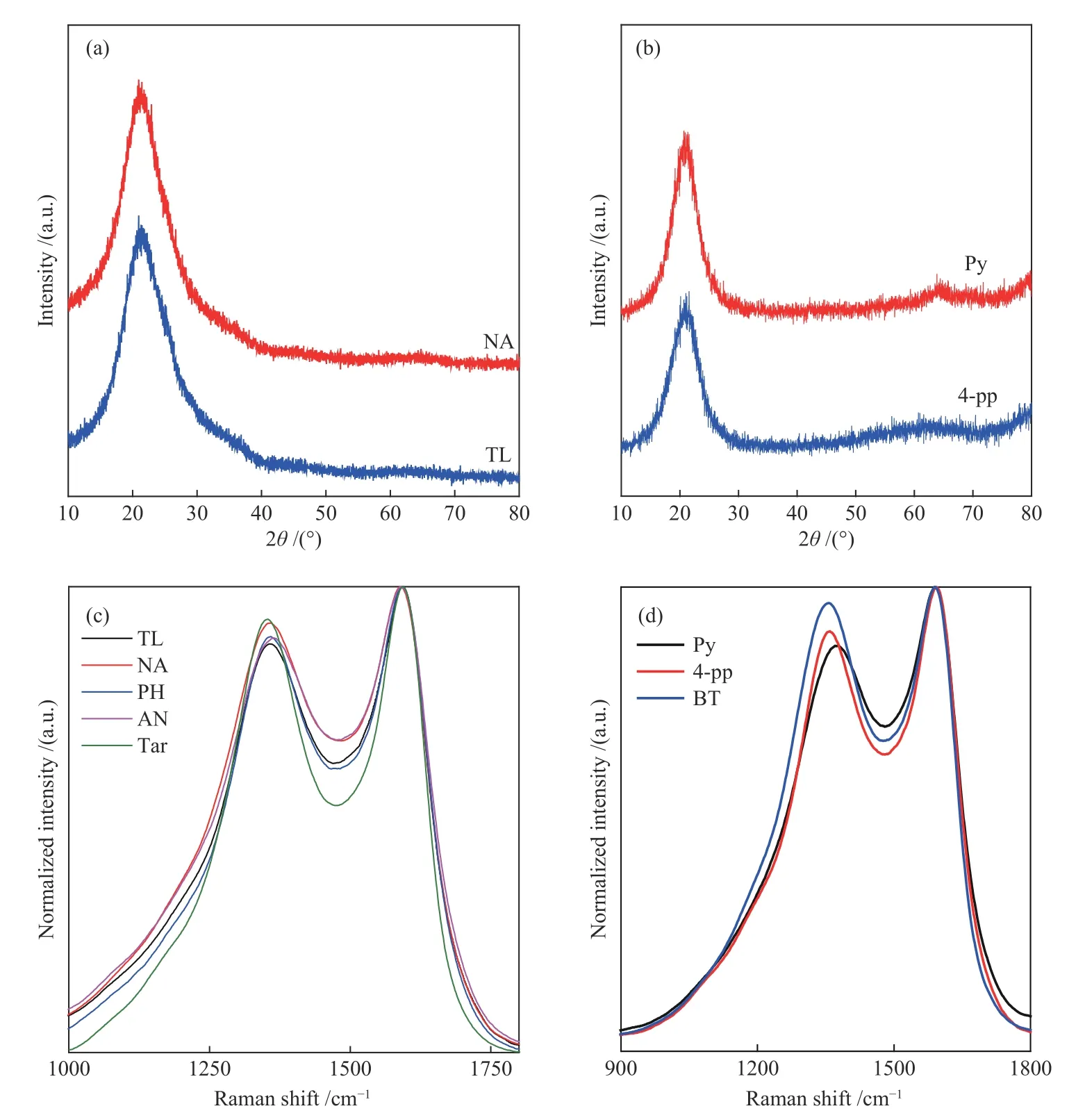
Figure 2 XRD pattern ((a),(b)) and Raman spectrum ((c),(d)) of carbon films prepared with arene/coal tar and different heteroatom compound
Figure 3 shows the TEM,HRTEM,and surface morphology of TL-based,PH-based,tar-based,and Pybased carbon film.As observed in Figure 3,TL-based,PH-based and tar-based carbon film have a layered structure with a large number of layers.There are a large number of carbon nanosheets on the Py-based carbon film, which indicates that the presence of heteroatoms affects the deposition of carbon on the carbon film.The high resolution transmission electron microscope (HRTEM) images reveal that the graphitized-like nanosheets are dispersed in TL-based,PH-based and Py-based carbon film, which could improve the conductivity of carbon films.While,graphitized-like ribbons structure can be observed in tar-based carbon film,which indicates that the mixture of various precursors increases the disordered structure of the carbon film.As can be seen from Figure 3(c),(f),(k) and (m),the surface of the TL-based carbon film is relatively flat,but there are some tiny protrusions on the surface of the PH-based,coal tar-based and Pybased carbon films.
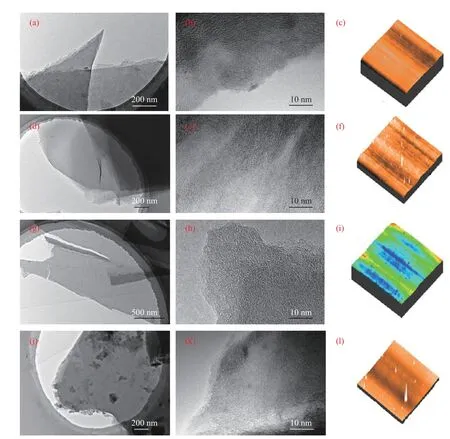
Figure 3 TEM,HRTEM,and surface morphology of carbon film prepared with arene
Figure 4 shows the film thickness of aromatic hydrocarbon-based carbon films and heteroatom compound-based carbon films.It needs to be pointed out that the thickness of the carbon film depends on the amount of precursor.For aromatic hydrocarbon-based carbon films (as shown in Figure 4(a)),the thickness of the carbon film is arranged in the following order:NAbased carbon film (208.9 nm) >TL-based carbon film(154.6 nm) >PH-based carbon film (47.5 nm) >tarbased carbon film (37.5 nm) >AN-based Carbon film(33.09 nm).This result indicates that the precursor composition has a significant effect on the thickness of the carbon films.The reason for the greatest thickness of the NA-based carbon film is that some small molecules and free radicals such as acetylene,ethylene,hydrogen, methane, etc.are generated during the pyrolysis process of naphthalene.These small molecules and free radicals promote the pyrolysis reaction of naphthalene.In order to further study the influence of the types of aromatic carbon on the surface morphology of the carbon film,the surface roughness of different aromatic hydrocarbon-based carbon films was measured,and the results are shown in Figure 4(b).It can be seen that the type of aromatic carbon source has a greater impact on the surface roughness of carbon film.NA-based carbon film has the smallest surface roughness (10.1 nm),but PH-based carbon film has the largest one (13.2 nm).
For the heteroatom compound-based carbon films,as shown in Figure 4(c),the film thickness of the Pybased carbon film is the largest at 129.3 nm;the film thickness of the BT-based carbon film is the second,at 99.44 nm;and the film thickness of the 4-pp-based carbon film is the smallest,at 32.3 nm.The boiling point of 4-pp,BT and Py is 306.5,221,115.2 °C,respectively.This indicates that the boiling point of the precursor has a great influence on the film thickness of the carbon film.As shown in Figure 4(d),the type of heteroatom compound has a greater impact on the surface roughness of carbon films.The surface roughness of 4-pp-based carbon film,Py-based carbon film and BT-based carbon film are 10.09,13.92 and 11.91 nm,respectively.This is similar to the change of the thickness of the carbon film itself,which indicates that the composition of the precursor significantly affects the surface roughness of the carbon film.
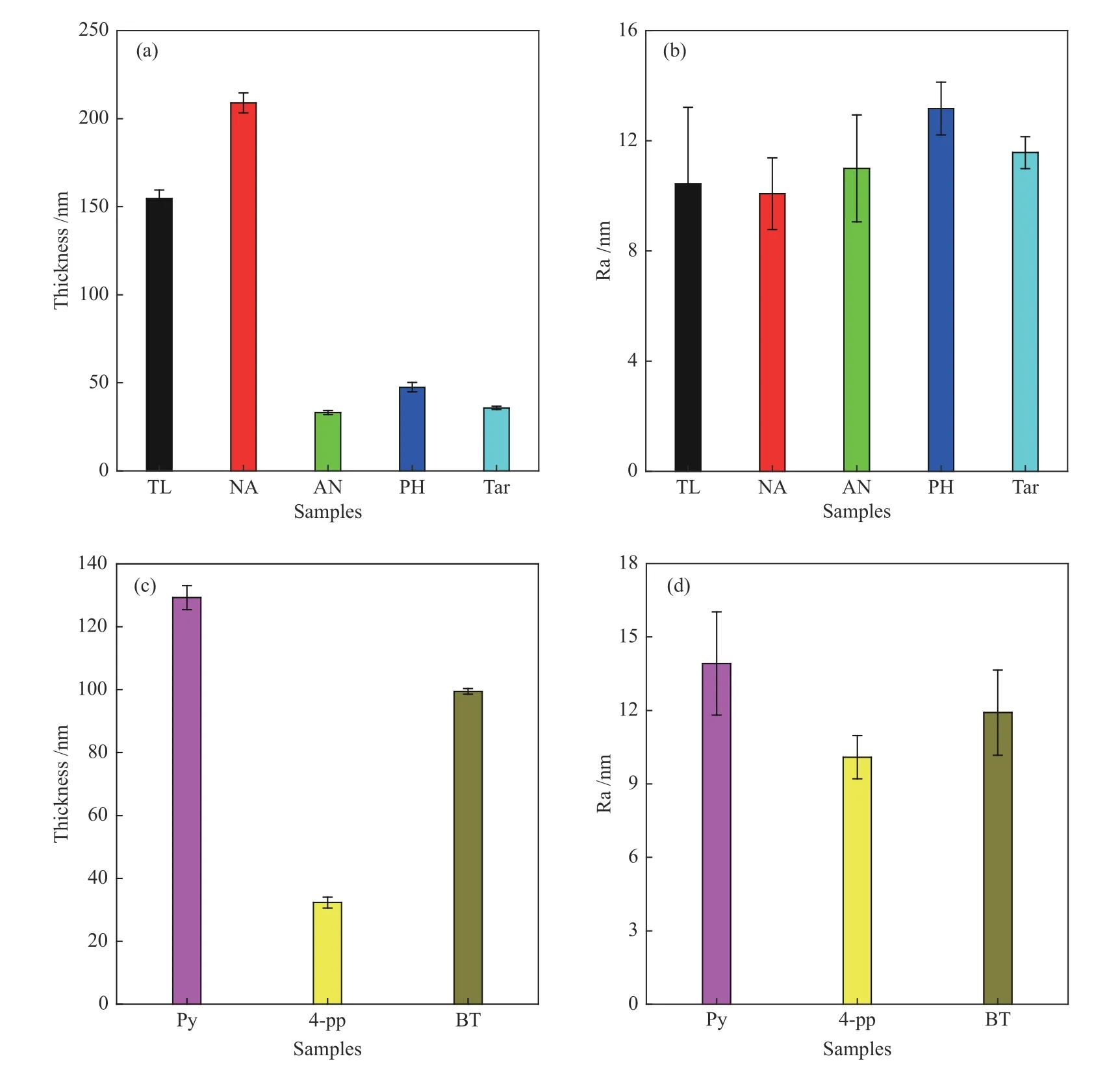
Figure 4 Film thickness ((a),(c)) and surface roughness ((b),(d)) of carbon films prepared with arene,coal tar and heteroatom compound
Figure 5 shows the carrier concentration and the mobility of as-prepared carbon films.As exhibited,the carrier concentration of PH-based carbon film and tarbased carbon film is negative, indicating that the conductivity type of the carbon film is electronic conduction.While the carrier concentration of other three groups of carbon films are positive,indicating that the conductivity types of TL-based,NA-based,and AN-based carbon film are all hole conduction.The carrier concentration of TL-based, NA-based, ANbased,and PH-based carbon film is 1.22×1022,1.79 ×1022,1.24×1022and −1.15×1022cm−3,respectively,which is 10 orders of magnitude of those of the graphene films (normally at the scale of 1011–1012cm−2)[13,14].As shown in Figure 5(b),the carrier mobility of TL-based,NA-based,AN-based,PH-based,and tar-based carbon film is 0.78,0.55,0.57,0.58 and 0.32 cm2/Vs,respectively,which is lower than that of the graphene films (> 200 cm2/Vs)[15].For the heteroatom compound-based carbon films,the carrier concentration of the 4-pp-based carbon film is positive,indicating that the conductivity type of the carbon films is hole conduction;while the carrier concentration of the Py-based and BT-based carbon films are negative,indicating that the conductivity types of these tow carbon films are both electronically conductive.The carrier concentration of Py-based,4-pp-based and BTbased carbon film are −3.77×1021,3.28×1022and−6.87×1021cm−3,respectively.This indicates that the carrier concentration of the carbon film material is related to the heteroatom species in the carbon films.It can be seen that the order of the carrier mobility of the three heteroatom compound-based carbon films is:Pybased carbon film (1.91 cm2/Vs) >BT-based carbon film (0.48 cm2/Vs) > 4-pp based carbon film(0.23 cm2/Vs).The existence of heteroatoms increases the defects of the carbon film, which hinders the migration of carriers and reduces the carrier mobility.
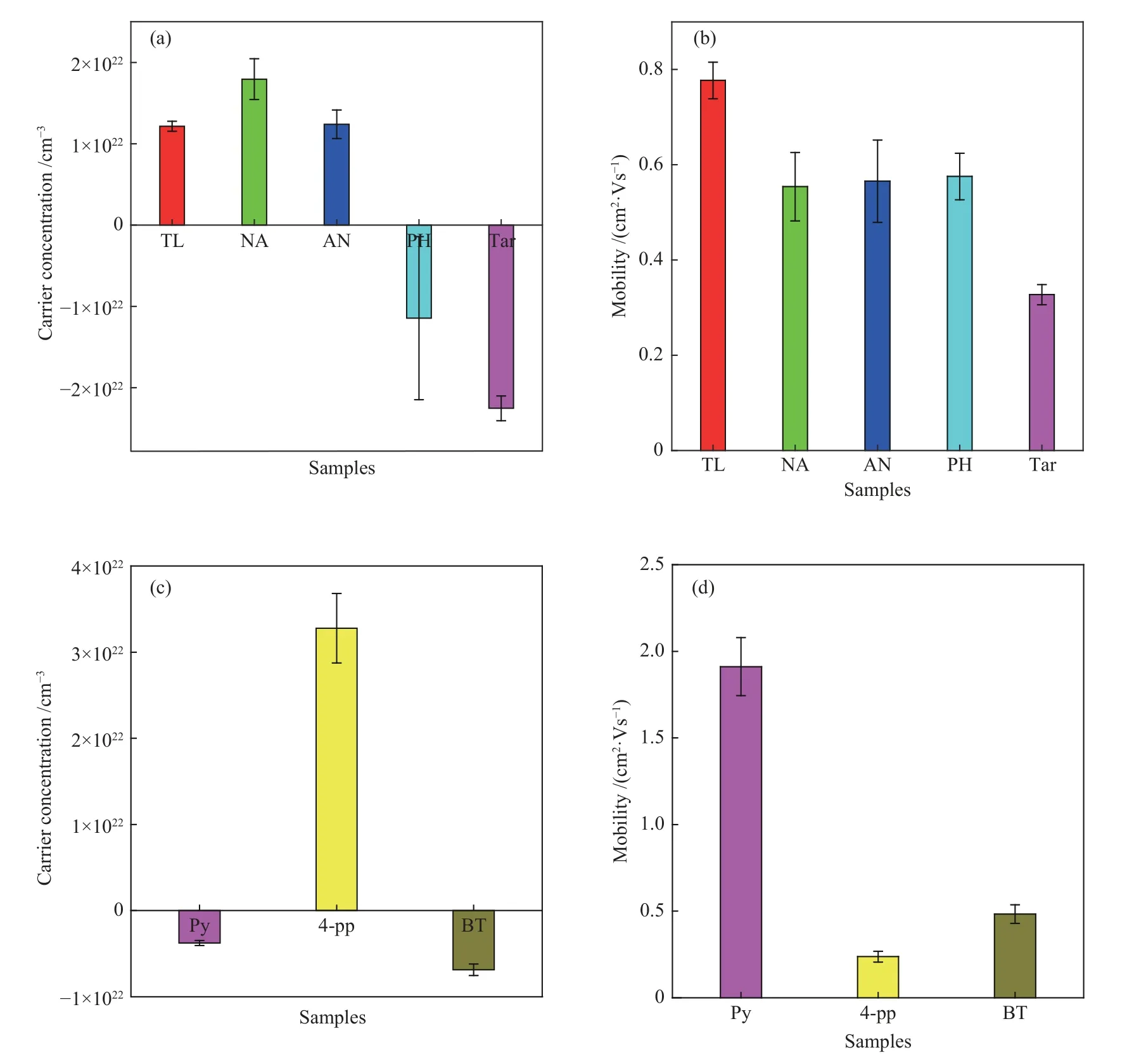
Figure 5 Carrier concentration (a),(c) and mobility (b),(d) of carbon films prepared by different arene,tar and heteroatom compound
Figure 6 shows the resistivity and sheet resistance of the as-prepared carbon films.It can be seen that the resistivity of NA-based carbon film (6.4×10−4Ω·cm)is the lowest,which may be caused by the increase ofsp2C cluster in the prepared carbon film.The sheet resistance of the aromatic hydrocarbon-based carbon films is arranged in the following order: tar-based carbon films (793.0 Ω/sq) >AN-based carbon film(298.1 Ω/sq) > PH-based carbon film (203.26 Ω/sq) >TL-based carbon film (76.19 Ω/sq) >NA-based carbon film (43.4 Ω/sq).The sheet resistance of NAbased carbon film and TL-based carbon film is much lower than that of tar,indicating that their Joule heating effect is much better than that of tar-based carbon film[12].The low sheet resistance of NA-based carbon film and TL-based carbon film mainly comes from its low resistance and large film thickness[16].
For the heteroatom compound-based carbon film,as shown in Figure 6(c) and 6(d),the resistivity of the Py-based, 4-pp-based and BT-based carbon film is 8.6×10−4,8.1×10−4and 1.9×10−3Ω·cm,respectively.The resistivity of the heteroatom compound-based carbon films is higher than that of aromatic hydrocarbon-based carbon film, indicating that the conductivity of the carbon film prepared by heteroatom compounds is worse than that of the aromatic hydrocarbon-based carbon film.The sheet resistance of the three groups of carbon film is in the following order:4-pp-based carbon film (339.6 Ω/sq) >BT-based carbon film (205.7 Ω/sq) > Py-based carbon film(100.7 Ω/sq).The Py-based film with the lowest sheet resistance is higher than that of the TL-based film.
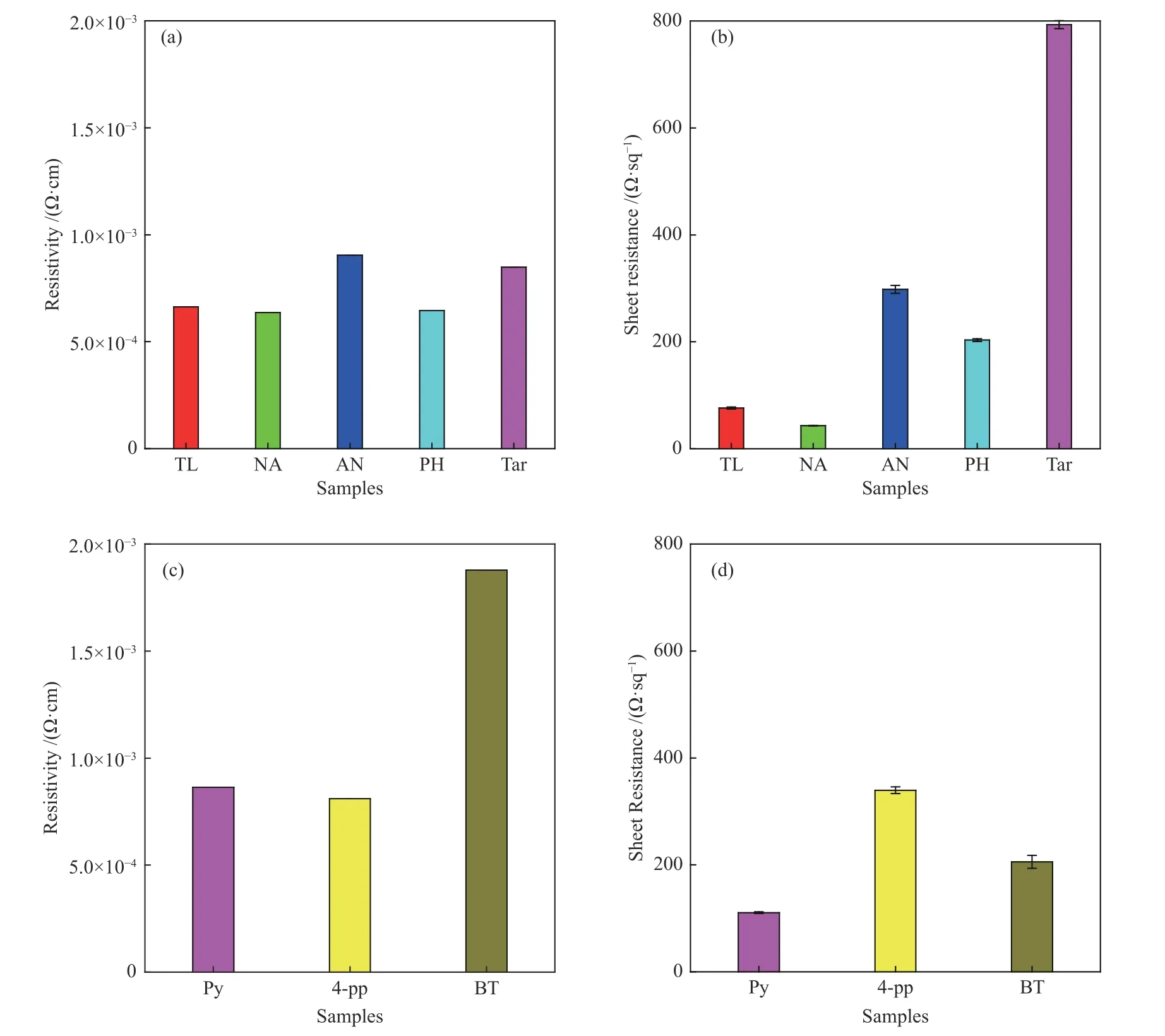
Figure 6 Resistivity (a),(c) and sheet resistance (b),(d) of carbon films prepared with arene,tar and heteroatom compound
2.2 Effect of precursor on the electrothermal properties of carbon films
The structural analysis of carbon films prepared with different precursors indicates that the structure of carbon films can be controlled by selecting the types of precursor.In order to verify the electrothermal performance of the prepared carbon film,the Joule heating performance of different carbon films under different voltages and fixed voltages was studied.As shown in Figure 7(a),under an applied DC voltage of 30 V, NA-based carbon film has the best electrothermal properties,which can be quickly heated up to 300 °C in about 30 s,and with the increase of time,the heating temperature of NA-based carbon film exceeds 300 °C and continues to increase,exceeding the measurement range of the instrument, which is superior to these anthracite-derived films on a quartz substrate (60 V, 285 °C), steam-cracker tar-derived conductive thin films (60 V,279 °C) and 1000 °C annealed reduced graphene oxide and graphene-based devices under equal bias[10,11].Under the external voltage of 30 V,the maximum heating temperature of TL-based,PH-based,AN-based,and tar-based carbon films is 262,145,120 and 108 °C,respectively.The above results clearly show that the Joule heating performance of NA,TL and Py based carbon films are significantly higher than that of the coal tar based carbon films (30 V,174 °C).[12]In order to further explore the electrothermal properties of the NA-based carbon film,the heating temperature of the NA-based carbon film under different external DC voltages was measured.As exhibited in Figure 7(b), when the external DC voltage is 10 V and 20 V,the maximum heating temperature of NA-based carbon film can reach to 91.4 and 233 °C,respectively.The above results indicate that NA-based carbon film has excellent electrothermal performance, due to its lowest sheet resistance,highersp3carbon content,and thicker films.
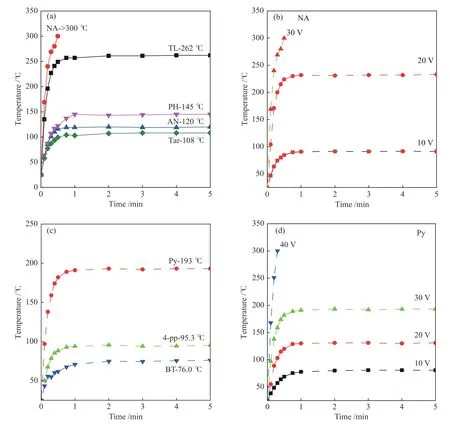
Figure 7 Electrothermal properties of arene/coal tar-based and heteroatom compound -based carbon film carbon films
Figure 7(c) shows the heating temperature of heteroatom compound-based carbon films under 30 V applied DC voltage.It can be seen that the Py-based carbon film has the best electrothermal properties.The maximum heating temperature that can be reached is 193 °C under an external DC voltage of 30 V,due to its smaller sheet resistance.The heating temperature of 4-PP-based carbon film is higher than that of BT-based carbon film.Also,the heating temperature of the Pybased carbon film under different external DC voltages was measured.As shown in Figure 7(d),in the voltage range of 10−30 V,as the external voltage increases,the maximum heating temperature of the carbon film reach to 80.9, 131 and 193 °C, respectively.When the external voltage is 40 V,the heating temperature of the carbon film exceed 300 °C in about 18 s, which exceeds the measuring range of the instrument.The above results indicate that the Py-based carbon film has good electrothermal performance, which originates from the lower defect and higher degree carbonization of the Py-based carbon film.
In order to develop a carbon film with excellent electric heating performance,it is necessary to correlate the electric heating performance of the film with its characteristics.As exhibited in Figure 8,as the film thickness increases,the sheet resistance of the carbon film gradually decreases.Therefore, the maximum temperature of the carbon film under 30 V gradually increases,which indicates that the increase of the film thickness will cause the junction resistance to decrease.It is worth noting that BT-based carbon film does not conform to the above rules, indicating that the influence of sulfur doping on the electrothermal properties of carbon films is different from that of other heteroatoms and the reason for the poor electrothermal performance of BT-based carbon film should be investigated.Then,in order to use coal tar to prepare high-performance carbon-based electric heating materials,it is better to select light components as the precursor,such as naphthalene oil or crude benzene fraction.
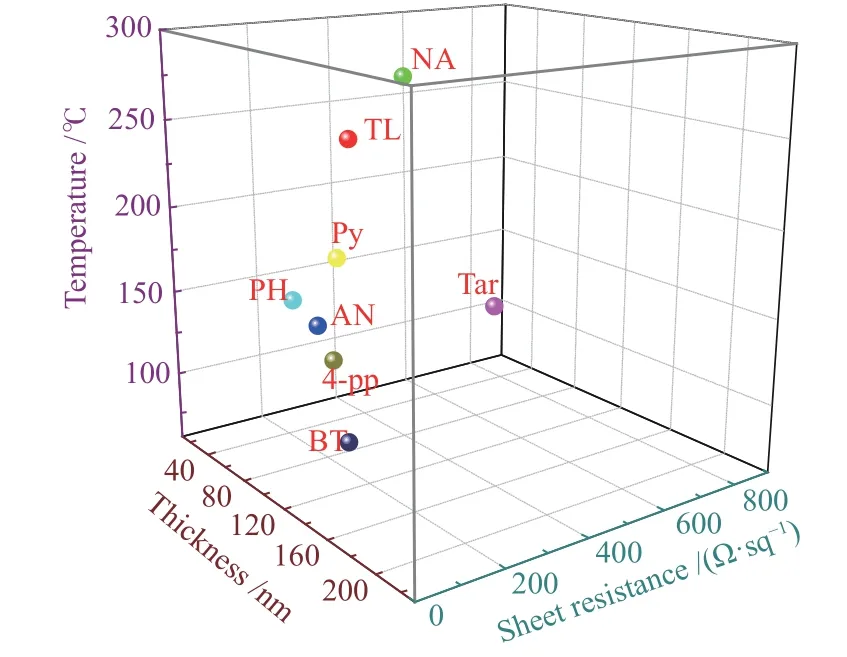
Figure 8 Relationship between sheet resistance,temperature at a voltage of 30 V,and films thickness of carbon films
3 Conclusions
In this paper,the effects of the light components(such as toluene, naphthalene, anthracene, and phenanthrene) and heteroatom compounds (such as pyridine,p-hydroxybiphenyl,and benzothiophene) in coal tar on the structure and performance of carbon film were investigated.It is found that the carrier concentration,mobility and film thickness determine the sheet resistance and electrothermal properties of the carbon film.The carrier centration of the aromatic hydrocarbon-based carbon films is higher than 1022cm−3,and the mobility is lower than 1 cm2/Vs.NAbased carbon film has the best electric heating performance.The maximum heating temperature of NA-based carbon film under 10,20 and 30 V is 91.4,233 and > 300 °C, respectively.The type of heterocyclic compound has a greater influence on the resistivity and sheet resistance of the carbon film.Electrothermal analysis shows that the electrothermal performance of the heteroatom compounds-based carbon film is lower than that of aromatic hydrocarbons-based carbon film.
Acknowledgments
The Authors are appreciative of the financial support from the Provincial Innovative Group for Processing &Clean Utilization of Coal Resource.

