Genome-wide evolution of MAPKs family and their expression in response to bacterial infection in seahorse Hippocampus erectus*
Kai WANG , Xin WANG , Qiang ZOU , Han JIANG , Rongrong ZHANG ,Yanan TIAN , Lele ZHANG , Qiang LIN
1 School of Agriculture, Ludong University, Yantai 264025, China
2 CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
3 Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou 511458, China
4 Yantai Branch of Shandong Technology Transfer Center, Chinese Academy of Sciences, Yantai 264003, China
5 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract Seahorses have evolved many unique biological traits, including a male brood pouch, the absence of caudal and pelvic f ins, and the lack of spleen and gut-associated lymphatic tissue. The mitogenactivated protein kinases (MAPKs) are known to be involved in various important biological processes including growth, diff erentiation, immunity, and stress responses. Therefore, we hypothesized that the adaptive evolution and expression of the MAPK gene family in seahorse may diff er from those of other teleost species. We identif ied positive selection sites in the erk2, erk5, jnk1, and p38α MAPK genes of the lined seahorse Hippocampus erectus and tiger-tailed seahorse Hippocampus comes. A novel expression prof ile of MAPK cascade genes was found in seahorse larvae during the f irst day after birth based on the RNA-seq data of H. erectus, which ref lected vital signs of immune response to its parental immune system.The expression patterns of the four positively selected MAPK genes were analyzed following the bacterial challenge of Vibrio fortis, revealing their upregulation pattern in brood pouch and other immune tissues.This study enriched our knowledge of the evolution of the H. erectus MAPK subfamilies, and could help better understanding the functional role of MAPKs in teleosts.
Keyword: mitogen-activated protein kinases (MAPKs); Hippocampus erectus; genomic structure; positive selection; immune regulation
1 INTRODUCTION
The mitogen-activated protein kinases (MAPKs)are an evolutionarily conserved family of serine/threonine protein kinases, involved in the regulation of a variety of cellular processes including proliferation, diff erentiation, and apoptosis (Cargnello and Roux, 2011; Plotnikov et al., 2011; Arthur and Ley, 2013). Previous literatures have demonstrated that all eukaryotic cells possess multiple MAPK pathways to recognize and respond to extracellular stimuli, which correspondingly regulate various cellular activities including metabolism, motility,proliferation, and diff erentiation (Krens et al., 2006).The three major MAPK subfamilies, extracellular signal regulated kinase (erk), c-Jun NH2-terminal kinase (jnk), and the p38, were characterized as typical MAPKs in multicellular organisms(Sopontammarak et al., 2005). The MAPKs can phosphorylate their downstream substrates as transcription factors when they are activated by phosphorylation upon threonine and tyrosine residues with in a conserved Thr-Xaa-Tyr motif (Raman et al.,2007).
Environmental stresses and extracellular stimuli including hormones, growth factors, cytokine,transforming growth factor (TGF)-related agents that through Ser-Thr kinase receptors can activate MAPK signaling pathways (Kyriakis and Avruch, 2012).Once activated, the signal will transduced via the“core signaling module” consisting of a three-tier phosphorylation cascade driven sequentially by MAP kinase kinase kinases (MKKKs), MAP kinase kinases(MKKs) and MAP kinases (MAPKs) (Kyriakis and Avruch, 2012; Sun et al., 2016). At present, 14 mammalian MAPKs have been characterized into seven groups. Among these groups, the extracellular signal-regulated kinase 1 and 2 (erk1/2), the c-Jun N-terminal kinases 1-3 (jnk1-3), the p38s (p38α,β,γ,andδ), anderk5are called the conventional MAPKs,while the other MAPKs (Nemo-like kinase (nlk),erk3/4, anderk7/8) sharing distinct mode of activation are classif ied as atypical MAPKs (Coulombe and Meloche, 2007; Cargnello and Roux, 2011; Plotnikov et al., 2011). Unlike the intensive concern in mammalian species, information about evolutionary origins, patterns and functions of the conventional MAPKs in teleost are limited.
Majority attention has been drawn to revealing how MAPKs functions in innate immunity, stress responses, development, and cellular functions in teleost species. It has been testif ied that JNKs participate in pattern recognition receptors (PRRs)induced inf lammation and transduction of osmosensory signals (Kültz and Avila, 2001; Marshall et al., 2005; Ding et al., 2018), as well as promote cell regeneration (He et al., 2016). ERKs have fundamental roles in modulating cellular growth, mitosis,metabolism, and individual development (Codina et al., 2008; Krens et al., 2008; Fuentes et al., 2011;Sánchez-Gurmaches et al., 2013), and they have been found responding to PRRs induced immune challenges inEpinipheluscoioides,Larimichthyscrocea, andSalmosalar(Iliev et al., 2013; Jia et al., 2015; Ding et al., 2018), as well as virus infection inS.salar(Olavarria et al., 2015). Moreover, ERKs can also respond to stresses, such as osmotic pressure,environmental neurotoxic metal, and thermal variation (Kültz and Avila, 2001; Leal et al., 2006;Keller et al., 2008; Jia et al., 2015). In aspect of immunologic functions, p38s can be activated by Lipopolysaccharide (LPS) and cytosine-phosphateguanosine (CpG) (Iliev et al., 2013; Zhu et al., 2014;Olavarria et al., 2015). Activation induced by toxins,thermal, starvation, and osmotic pressure in many teleost f ishes conf irmed a functional role of p38 pathway in stress responses (Marshall et al., 2005;Leal et al., 2006; Urushibara et al., 2009; Feidantsis et al., 2012; Antonopoulou et al., 2013; Li et al., 2016;Marshall et al., 2017). Additionally, p38 involves in synchronous embryonic cleavage and myocardial regeneration inDaniorerio(Jopling et al., 2012). The members of MAPKs vary a lot in diff erent teleost species, as well as the functional performance of an identical member. Therefore, our understanding of how MAPKs evolve and function in teleost is very necessary.
Seahorse is characterized by sedentary behavior with low swimming capacities, small home ranges,which evolves a unique morphology includes an armored body, a highly derived head shape, a prehensile tail, and the absence of caudal and pelvic f ins (Lin et al., 2016; Luo et al., 2016). Its specialized life-history traits, including male-pregnancy with a brood pouch, low fecundity and high site f idelity,have attracted considerable attention in adaptive evolution and marine conservation (Foster and Vincent, 2004; Qin et al., 2017). The unique body morphology and specialized life history traits has made them good f lagship species for threats and solutions in marine conservation (Zhang et al., 2017;Wang et al., 2019). In addition, the lack of spleen and gut-associated lymphatic tissue (GALT) may has resulted in a partial but natural “immunodef iciency”of seahorse (Matsunaga and Rahman, 1998; Galtier et al., 2009; Luo et al., 2016). Considering the vital functions of the MAPK gene family in immune response, we hypothesized that the adaptive evolution and expression characteristics of the MAPK gene family in the seahorse may have diff erent characteristics from other teleost f ishes. The draft genomes of the lined seahorseHippocampuserectusprovide a valuable information for molecularevolutionary inference (Lin et al., 2017). It is possible for us to conduct systematic genomic analysis of MAPKs, as well as its correlations to the other knownteleosts. The present study aimed to determine the conventional member, gene structure, evolutionary history, and expression pattern of MAPKs in the lined seahorseH.erectus. The results can enrich our knowledge of the evolution history of MAPKs, and will facilitate better understanding of the functional role of MAPKs in teleosts.
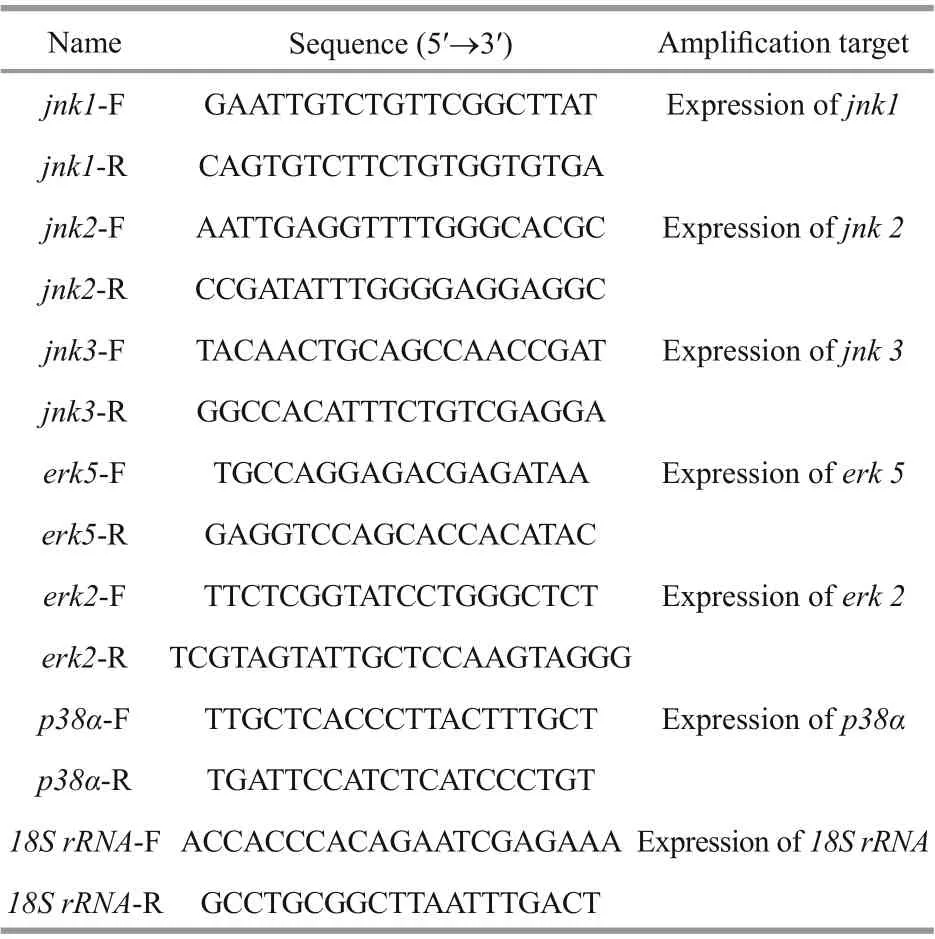
Table 1 Primers used in this study
2 MATERIAL AND METHOD
2.1 Experimental animals and sample collection
Hippocampuserectuswere cultured in the aquaculture system of seahorse center in Ludong University, and all breeding methods were carried out in accordance with relevant guidelines and regulations of the Animal Care and Use Committee of the Ludong University. Lined seahorses were cultured in recirculating seawater tanks (50 cm×40 cm×30 cm)with aerated water (salinity: 31.5±0.5, temperature:25.5±0.5 °C, and pH: 8.2±0.1). The seahorses were fed with frozenMysisspp. three times a day (08꞉00,12꞉00, and 16꞉00), as characterized by Luo et al.(2016). Thirty-six adult seahorses (body height:12.7±0.8 cm, wet bodyweight: 13.1±0.8 g) were used in theVibriofortischallenge experiment. These seahorses were divided into three groups (12 inds./group) collected, and each group was reared in three re-circulating seawater tanks (4 inds./tank).
The f ishes of three groups were intraperitoneally injected with 20-μL low-concentration bacterial suspension (1.0×107colony forming units/mL;Infected 1), 20-μL high-concentration bacterial suspension (1.0×108colony forming units/mL;Infected 2), and 20-μL phosphate buff er saline (PBS)(control group), respectively. All seahorse individuals were anesthetized with 0.035% Tricaine Methanesulfonate (MS-222) (Sigma-Aldrich, Castle Hill, NSW, Australia) and dissected rapidly at 24-h post-infection. The tissues were frozen in liquid nitrogen and stored at -80 °C until RNA extraction.We conf irm that all experimental protocols were approved by the Animal Care and Use Committee of the Ludong University.
2.2 RNA isolation, cDNA synthesis, and real-time quantitative PCR analysis
Total RNA was extracted using TRIzol reagent(TaKaRa, Dalian, China) according to the manufacturer’s instructions. The f irst-strand cDNA was synthesized using an oligo (dT) primer with the RNA Eraser Reverse Transcription Kit (TaKaRa,Japan). Specif ic PCR primers were designed to amplify these cDNA fragments by using Primer Premier 5.0 (Table 1). The real-time quantitative PCR(qPCR) was performed on Roche Light Cycler 480 using SYBR Premix Ex Taq™ (TaKaRa, Japan), and the PCR conditions were as follows: 40 cycles at 94 °C for 20 s, 52 °C for 20 s, and 72 °C for 15 s. Seahorse 18S rRNA was amplif ied to conf irm the expression level of the housekeeping gene. Relative gene expression was determined using the 2-ΔΔCtcomparative quantif ication method (Livak and Schmittgen, 2001).
2.3 RNA-seq data analysis
RNA-seq data ofH.erectuswas downloaded from the NCBI Sequence Read Archive under accession number of SRA392578 (Lin et al., 2016). HTSeq v0.6.1 was used to count the numbers of reads mapped to each gene. Then, the expected number of Reads Per Kilobase of transcript sequence per Millions base pairs sequenced (RPKM) of each gene was calculated based on the length of the gene and read counts mapped to each gene.
2.4 Gene identif ication and sequence analysis
The whole genome sequence databases of the lined seahorse (H.erectus) were searched using the available teleosts’ MAPK protein sequences,including those fromDaniorerio,Poeciliaformosa,Oryziaslatipes,Larimichthyscrocea,Oreochromisniloticus,Xiphophorusmaculatus, andCynoglossussemilaevis. TBLASTN algorithm was used to obtain the initial pool of MAPK family genes using a cut-offE-value of 10-5. All MAPK genes obtained fromH.erectusgenome were further conf irmed against the published transcriptome sequences ofH.erectususing the BLASTN program (Lin et al., 2017).The exon/intron organization for MAPK genes were drawn by comparing the cDNA sequences with their corresponding genomic DNA sequences using the Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn). The protein domains were predicted by Simple Modular Architecture Research Tool (SMART) (http://smart.emblheidelberg.de).
2.5 Phylogenetic analyses
All MAPK protein sequences obtained fromH.erectus,D.rerio,P.formosa,O.latipes,L.crocea,O.niloticus,X.maculatus, andC.semilaeviswere aligned using MAFFT version 7 (Katoh and Standley,2013). The best amino acid substitution model was chosen under the Akaike Information Criterion (AIC)as calculated in ProtTest (Abascal et al., 2005).Maximum-likelihood (ML) analysis was implemented in PhyML 3.0 with 1 000 bootstrap replicates(Guindon et al., 2010). The f inal ML tree was visualized using FigTree v1.4.2.
2.6 Selection analyses
To assess the rates of non-synonymous (Ka) and synonymous (Ks) substitutions for all MAPK codon sites and branches of the phylogeny of MAPKs, the branch-site model (BSM) and branch model (BM)were tested using the CODEML program of PAML v4.8 package (Yang, 2007). The BM was employed under two assumptions: one-ratio model and freeratio model. For BSM, comparison between BSM A and the corresponding null model were constructed,and the codon sites under positive selection (Ka/Ks>1)in the foreground branches was detected.
The likelihood ratio test (LRT) was constructed to verify that the best models f itted the data, and Bayes Empirical Bayes (BEB) approach was applied to identify amino acid codon sites under positive selection based on a posterior probability threshold of 0.95. TheKa/Ksratios of the main branch of the phylogeny of MAPKs were labeled and the positive selected sites were displayed in a graphic view of sequence alignment using BioEdit v7.1.3.0.
2.7 Statistical analyses
Statistical analyses were conducted using the programs SPSS 19.0 (IBM, US). The gene expression in diff erent seahorse treatment groups were analyzed using the one-way analysis of variance (ANOVA).
3 RESULT
3.1 Gene identif ication and structure analysis of MAPK genes of H. erectus
Seven MAPK genes, includingerk1(also known asmapk3),erk2(mapk1),erk5(mapk7),jnk1(mapk8),jnk2(mapk9),jnk3(mapk10), andp38α(mapk14),were identif ied from theH.erectusgenome, and the predicted sequences were further conf irmed against the published transcriptome data ofH.erectus. The open reading frames oferk1,erk2,erk5,jnk1,jnk2,jnk3, andp38αwere 1 185, 1 857, 3 156, 1 695, 1 368,1 536, and 1 137 bp, respectively, and encoded 394,618, 1 051, 564, 455, 511, and 378 amino acids(Fig.1). In addition, the genomic DNA sequences of each MAPK genes were obtained from theH.erectusgenome based on the genome annotation f ile (gff 3 format).
The exon-intron junctions were determined by comparing the cDNA sequences with their corresponding genomic DNA sequences. We found thaterk2andjnk3were organized into 13 exons and 12 introns, andjnk1,jnk2,p38αwere composed of 12 exons. Moreover, theerk1anderk5were only composed of 8 and 6 exons, respectively. Some introns are extremely long (>4 kb), including the fourth and f ifth introns inerk2, the eleventh intron inp38αandjnk2, and the f irst intron injnk3. The quantity of exons exhibited little diff erence among theerk2,jnk3,p38α,jnk1, andjnk2(13, 13, 12, 12, and 12,respectively) (Fig.1).
3.2 Phylogenetic analysis of MAPK genes of H.erectus
In this study, we constructed a phylogenetic tree of the 56 MAPK genes from 8 teleost species (Fig.2).The ML tree shows that the MAPK genes formed three well-def ined clades, corresponding toerk,jnk,andp38αMAPK subfamilies. As we predicted, each gene formed its own clade, which provided a solid phylogenetic evidence that was necessary to conf irm the accuracy of identif ication of theH.erectusMAPKs. Our results show thaterk1anderk2formed a sister group, and then clustered witherk5. The phylogeny suggested a sister relationship betweenjnk1andjnk3, and then clustered withjnk2. Thep38αwas identif ied as the sister group of thejnksubfamily.Diff erentH.erectusMAPK genes had a diff erent clustering pattern when compared in gene homology with other teleosts.H.erectuswas clustered withC.semilaevisin the clades oferk1anderk5, while in the phylogeny ofjnk1andjnk3,H.erectuswas clustered withO.latipesandD.rerio.
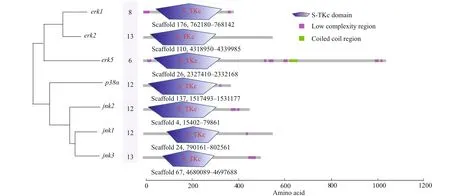
Fig.1 The gene structures of MAPK genes in H. erectus genome
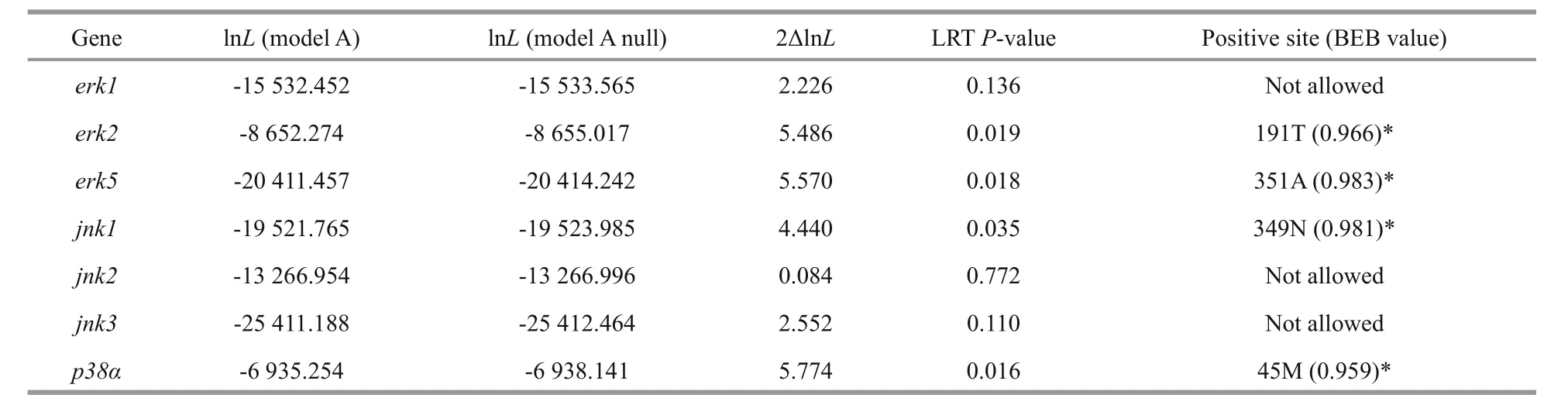
Table 2 Likelihood values and parameter estimates of positive selection sites by site-specif ic modeling for the H. erectus and H. comes MAPK family genes
Selective pressure of diff erent MAPK subfamilies after gene duplication were detected. TheKa/Ksvalues were calculated across all branches using the branch model of CODEML. The results suggest that there was a substantial diff erence in selective pressure among the MAPK subfamilies. Signatures of positive selection inerk5(Ka/Ks=3.231),jnk1(Ka/Ks=1.468),p38α(Ka/Ks=1.036) were revealed in the phylogeny,which may suggested that adaptive changes in these genes (Fig.2). TheKa/Ksvalues estimated inerk1,erk2,jnk2, andjnk3were less than 1 (0.536, 0.965,0.278, and 0.201, respectively).
3.3 Positive selection analysis of MAPK genes of H. erectus
The branch-site model in CODEML was used to reveal whetherHippocampusMAPK genes were subjected to positive selection. We set theH.erectusandH.comesas a foreground branch, the specif ic codons identif ied by the BEB approach with a posterior probability of 95% are listed in the Table 2.We found that four positive selection sites among four MAPK genes of seahorse that showed BEB values >0.95, includingerk2,erk5,jnk1, andp38α,which may have a plasticity in function (Fig.3, Table 2). The 191T inerk2and the 351A inerk5were identif ied as positive selection sites with a BEB posterior probability of 96.6% and 98.3%,respectively. Positively selected sites located in the amino acid positions 349 and 45 were detected in thejnk1andp38αwith a posterior probability of 98.1%and 95.9%.
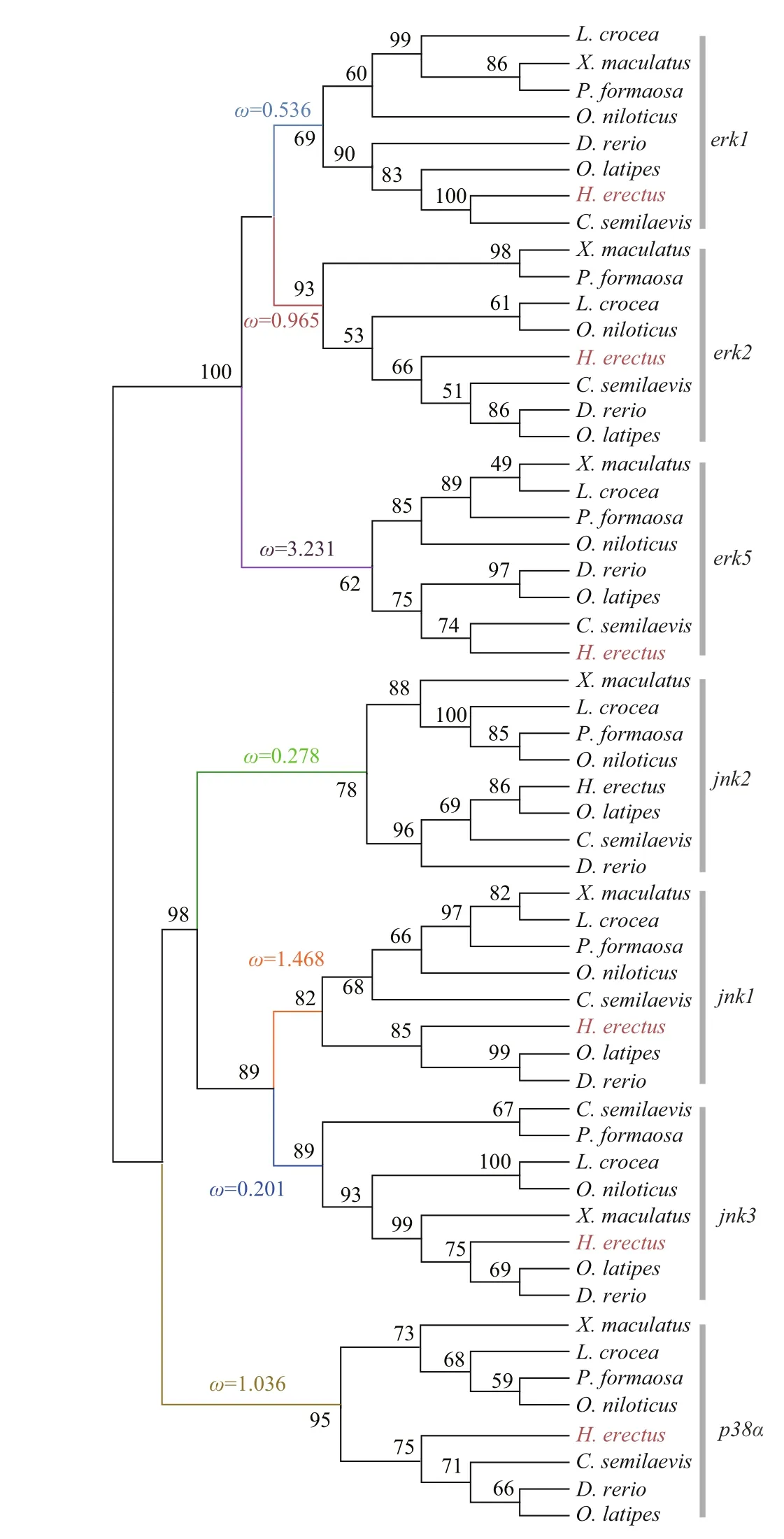
Fig.2 Phylogenetic tree and selection pressure of MAPK genes from the lined seahorse ( H. erectus) and other f ish
3.4 Expression prof iles of genes involved in the MAPK cascade signal transduction pathway
In total, 34 genes involved in the MAPK cascade signal transduction pathway were identif ied from the RNA-seq data, and most genes have shown diff erentexpression trends between diff erent tissues and development stages (Fig.4). A stage-specif ic gene expression prof ile was revealed from the comparative transcriptome analysis, and low expression level of many genes were detected in the testis and brood pouch at the age of 5 months, includingraf(raf proto oncogene serine/threonine protein kinase),mlk2(mixed lineage kinase 2),mlk3,mekk4(mitogenactivated protein kinase kinase kinase 4),tao1(serine/threonine-protein kinase tao1),tao2,mkk2(mitogenactivated protein kinase kinase 2),mkk7,erk1,msk2(ribosomal protein s6 kinase alpha-4), etc. In the f irst 30 days after birth, a novel expression prof ile of MAPK cascade genes was found during the f irst day after birth. Compared with other develop stages, the expression level of many genes were lowest in the 1-day-old larvae, includingtao1,tao2,ask1(apoptosis signal-regulating kinase 1),mkk1,mkk4,mkk5,mkk6,p38α,jnk3,rsk2(ribosomal s6 kinase 2),rsk3,msk1,mk2, andmk3. However, the genes includingraf,mos,tak1,mkk2,erk1,p38β,jnk2, andmnk1showed highest expression level in the 1-day-old larvae (Fig.4).
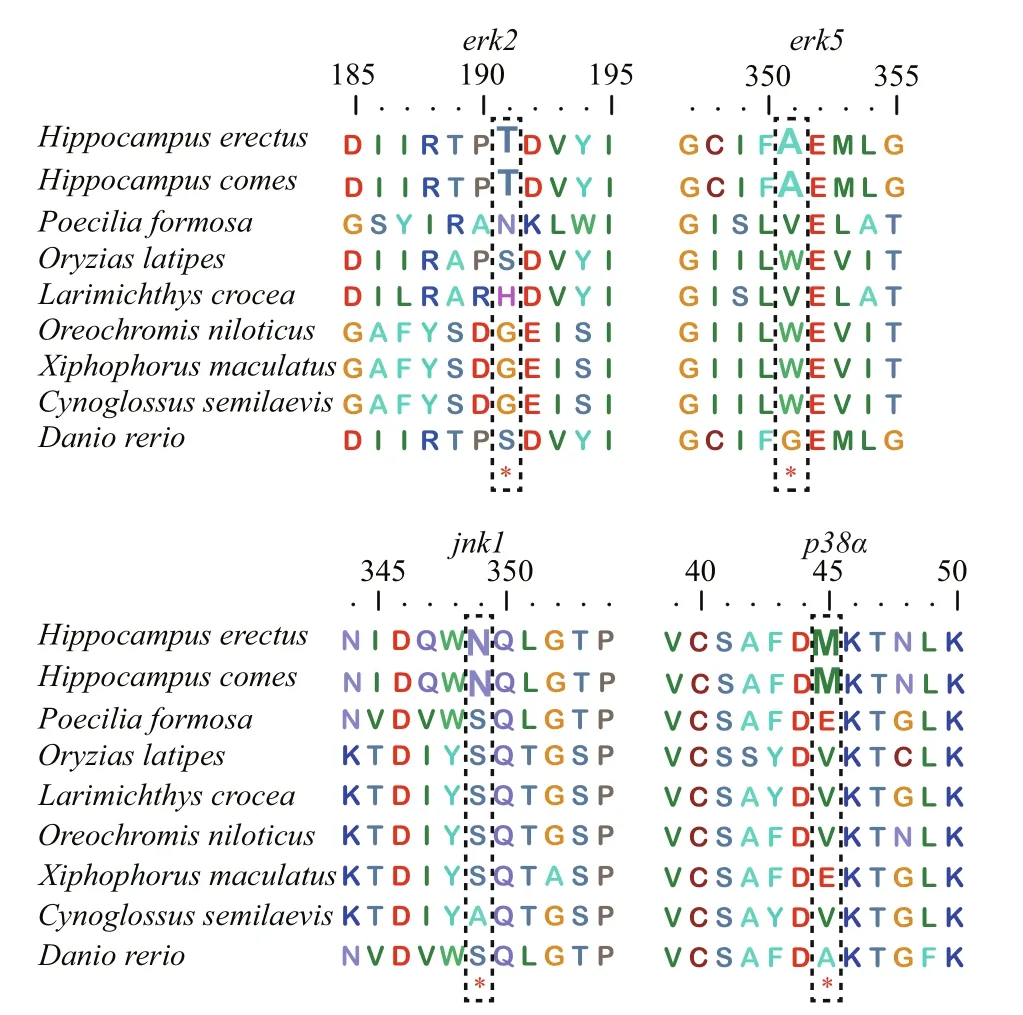
Fig.3 Positive selection sites detected in MAPK family genes
3.5 Expression prof iles of MAPK genes in seahorses after challenge with V. fortis
The expressions levels of the four positively selected MAPK genes together withjnk2,jnk3were analyzed in nine tissues of theH.erectus(Fig.5). The result show the expression of the mRNA oferk2andjnk2gene was highest in intestine, the mRNA oferk5,jnk1, andp38αwere all most abundantly expressed in the ovary, and the gene expression ofjnk3was highest in gill.
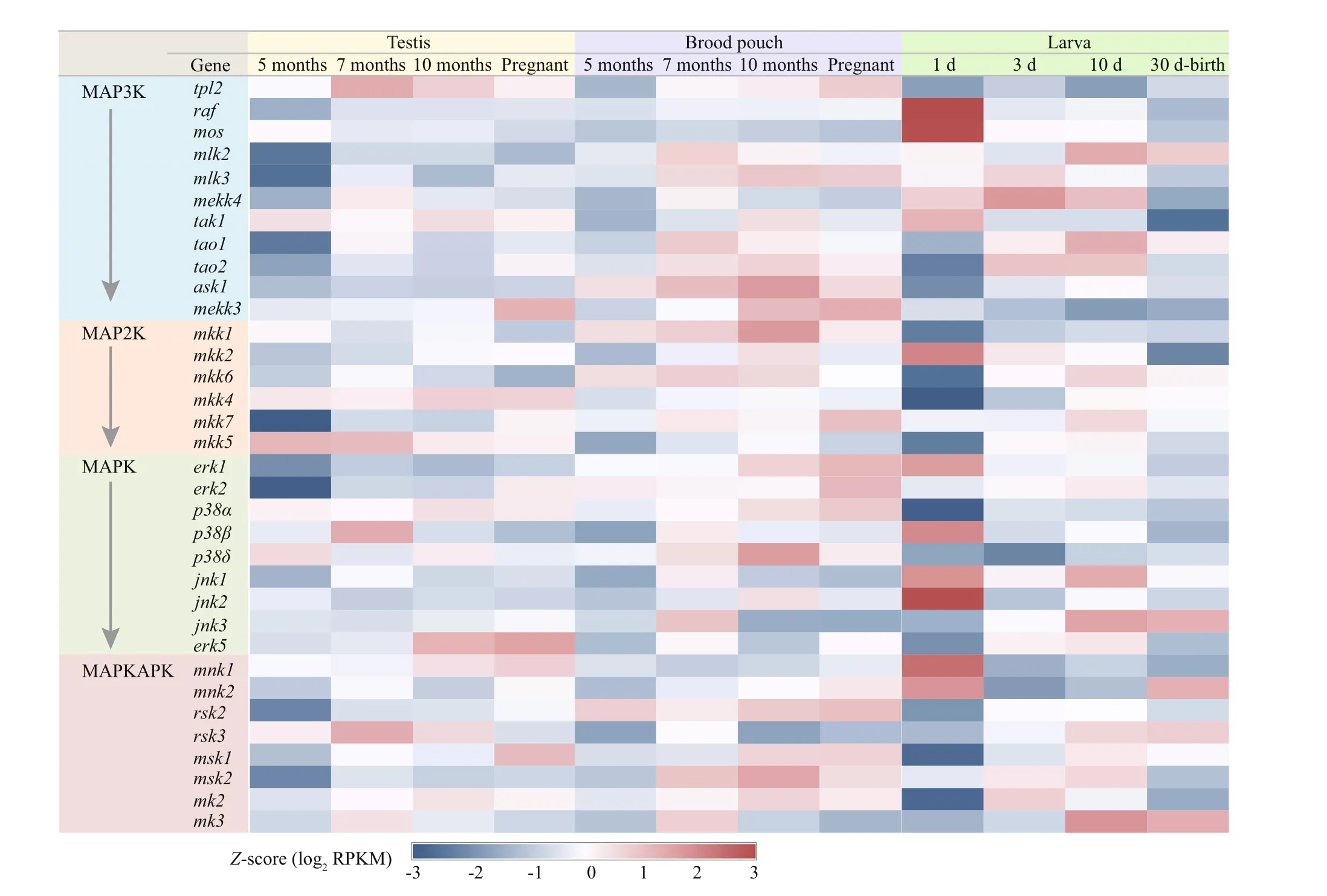
Fig.4 Transcriptomics data analysis of the lined seahorse
Challenge of the lined seahorse with theV.fortisresulted in a signif icant up-regulation of theerk2,erk5,jnk1,jnk2,jnk3, andp38αgenes in diff erent tissues (Fig.6). The mRNA expression level oferk2,erk5, andjnk3in the liver were signif icantly increased after injection with the pathogenic bacteriaV.fortis.In the gill, the transcript oferk2,erk5,jnk1,jnk2,jnk3, andp38αwere signif icantly increased after experimental challenge. However, the relative level of expression of the four genes tested in ovary and testis does not change signif icantly post-infection,except for thejnk1. The mRNA expression level oferk2,jnk1,jnk2, andp38αwere signif icantly increased after challenge in the intestine. Moreover, in the brood pouch, the mRNA expression level oferk2,erk5, andjnk1were signif icantly increased after challenge, and expression of these three genes does not change signif icantly following the injection of low and high concentrations ofV.fortis(Fig.6).
4 DISCUSSION
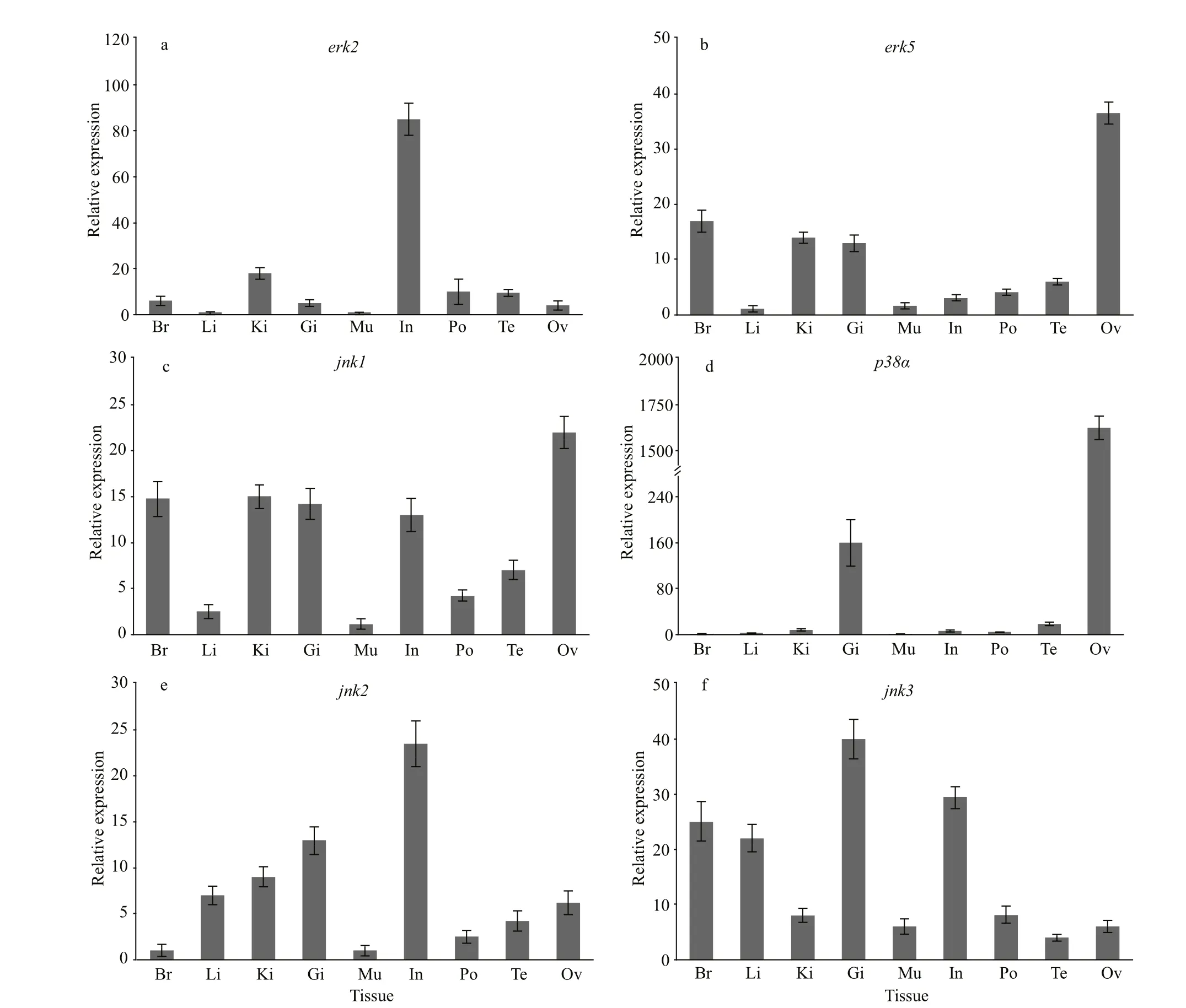
Fig.5 The expression levels of MAPK family genes in diff erent tissues
Previous genome-wide studies of the seahorse have indicated a substantial loss of conserved noncoding elements, which were indicated that some peculiar physiological mechanism in metabolism and regulation is developed in seahorse (Lin et al., 2016).MAPK genes are important transcription factors that convert extracellular stimuli into a wide range of fundamental cellular responses. Although the genomic structures ofH.erectusMAPK genes are varied, the evolutionarily conserved serine/threonine protein kinase catalytic (S-TKc) domain was found in all MAPK proteins, which were similar to those found in other species (Jia et al., 2015). The gene structure of MAPK genes suggested the high conservation in gene splicing during the evolutionary history. The specif ic occurrence of intron losses in theerk1anderk7subfamilies might be associated with adaptive evolution of the teleosts by enhancing the gene expression level of both MAPK genes (Kawaguchi et al., 2010; Li et al., 2011). The evolution of MAPK gene family is highly conserved throughout the eukaryotic kingdoms (Cook et al., 1997; Hamel et al.,2012). However, the detailed evolutionary history of the vertebrate MAPK family is largely unclear (Li et al., 2011). Gene duplications of the MAPK family genes were found to be ubiquitous in the evolutionary history, and the whole-genome duplication (WGD) is believed to be a really important source of evolutionary changes. Additionally, the redundant genes derived from WGD are considered to be acquire new or altered functions (Sémon and Wolfe, 2007; Inoue et al., 2015). The evolutionary history of teleost MAPK family needs to be conf irmed by a systematic phylogenetic analysis. We found that these teleosts were conventional in MAPK family gene members,and the MAPK family had been formed through multiple duplications at least prior to the diversif ication of teleost f ishes (Li et al., 2011). The deep divergence of MAPK genes may have benef ited from the extra event of whole-genome duplication in teleost f ish,which is proposed to have provided suffi cient genetic material for the adaptive evolution (Toloza-Villalobos et al., 2015).
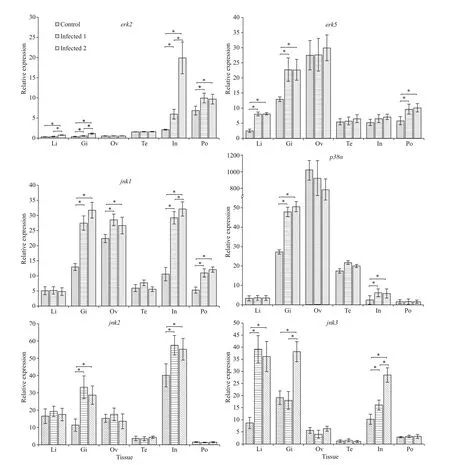
Fig.6 Relative expression of the MAPK genes in various tissues of the healthy lined seahorses and V. fortis challenged lined seahorses
Whether the natural selection has driven the evolution of the teleost MAPK family remains unknown, and it will be interesting to f ind compelling evidence of positive selections acting of MAPK genes inH.erectusandH.comes. The MAPK family genes have been considered important in the adaptive evolution of the vertebrates (Li et al., 2011). The selection results based on the branch model indicating that purifying selection dominated the evolution of these genes with strong functional constraints.Previous study suggested that MAPK genes have high level of selective constraints are under strong functional constraints, although there is an expansion of MAPK gene family (Wu et al., 2010; Li et al.,2011). However, diff erentH.erectusMAPK genes have been under diff erent selective pressures. The positive selection oferk2,erk5,jnk1, andp38αobserved in the present study could help us to better understand the evolutionary history of seahorses and its adaptation to the complex and changeable off shore environment. The specif ic gene expression pattern and the positive selection detected in the corresponding gene ofH.erectusmay ref lected a plasticity in gene function, which will facilitated the radiation of the group.The innovation of “male pregnancy” in seahorses and pipef ishes, together with novel expression patterns of key immune and pregnancy genes in their reproductive tissues, were considered to be an important research system for studying vertebrate immune system (Roth et al., 2020). A problem for pregnancy to evolve is the rejection of the embryo that is recognized as foreign tissue by the vertebrate’s immune system (La Rocca et al., 2014; Roth et al.,2020). In the present study, a novel expression prof ile of many MAPK cascade genes was found in the seahorse larvae during the f irst day after birth, which may ref lects a signs of immune response to its parental immune system. The brood pouch, where the entire embryonic development takes place, has shown signif icant transcriptional changes in response to pathogen infection as reported by Roth et al. (2020).However, as reproductive organs, the ovaries, and testes did not respond suffi ciently to external infections,which may indicates that the brood pouch is an important immune barrier in seahorse. In addition, the results suggest that liver and gill, the important immune organs in teleost f ish, may play an important role in immune protection. The seahorse has a siphonlike snout and abandoned predatory life, which may result a less injury and infection in its gastrointestinal tract (Luo et al., 2016). Previous studies have conf irmed that the seahorse lacks a spleen and GALT(Galtier et al., 2009; Litman et al., 2010; Luo et al.,2016), and we found that the intestinal has a signif icant upregulation response to bacterial infection, which may be attributed to the intestinal immune compensation to “immunodef iciency” of the seahorse.Because the seahorses are well known for its breeding strategy and novel immunological characters, the adaptive change in their MAPK genes with vital immune functions may provide clues for the studies of breeding adaptation in seahorses.
5 CONCLUSION
Seven MAPK subfamilies includingerk1,erk2,erk5,jnk1,jnk2,jnk3, andp38αwere successfully identif ied from the lined seahorseHippocampuserectus. We characterized their gene structure and protein domains, and found intron losses in theerk1anderk7subfamilies. Phylogeny of the MAPK family genes suggested multiple duplication events prior to the diversif ication of teleosts were occurred. Selective pressure of diff erent MAPK subfamilies indicated the adaptive changes inerk5,jnk1, andp38α, and positive selection sites were found inerk2,erk5,jnk1, andp38α. The expression pattern ofjnk2,jnk3, and four positively selected MAPK genes were analyzed in nine tissues of theH.erectus, among whicherk5,jnk1, andp38αwere most abundantly expressed in the ovary. A novel expression prof ile of MAPK cascade genes was found in the seahorse larvae during the f irst day after birth, which ref lected vital signs of immune response to its parental immune system. The expression patterns of these MAPK genes were analyzed following the bacterial challenge ofV.fortis,revealing their upregulation pattern in brood pouch and other immune tissues. This study will enrich our knowledge of the evolution of theH.erectusMAPK subfamilies, and facilitate better understanding of the functional role of MAPKs in teleosts.
6 DATA AVAILABILITY STATEMENT
Data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2021年6期
Journal of Oceanology and Limnology2021年6期
- Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
