Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
Changyou CHEN , Pingping WANG ,**, Long-Fei WU , Tao SONG ,4
1 Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China
2 France-China Joint Laboratory for Evolution and Development of Magnetotactic Multicellular Organisms, Chinese Academy of Sciences, Beijing 100029, China
3 Aix Marseille University, CNRS, LCB, Marseille F-13402, France
4 University of the Chinese Academy of Sciences, Beijing 100049, China
Abstract Magnetotactic bacteria are capable of biosynthesizing magnetic nanoparticles, also called magnetosomes, and swimming along magnetic f ield lines. The abilities endow the whole cells of magnetotactic bacteria with such applications as targeted therapy and manipulation of microrobots. We have shown that the intact marine magnetotactic bacteria MO-1 kill effi ciently antibiotic-resistant pathogen Staphylococcus aureus in vivo, but the biocompatibility of this marine bacterium is unknown. In this study,the strain MO-1 was chosen to analyze its biocompatibility and potential for biomedicine applications.Results showed that MO-1 cells could be guided at 37 °C under an external magnetic f ield and swim in the blood plasma and urine. They could keep active locomotivity within 40 min in the plasma and urine,although their velocity slowed down. When incubated with human cells, magnetotactic bacteria MO-1 had no obvious eff ects on cellular viability at low dose, while the cell toxicity increased with the augmentation of the quantity of the MO-1 cells added. In the in-vivo experiments, the median lethal dose of magnetotactic bacteria MO-1 in rats was determined to be 7.9×10 10 bacteria/kg. These results provided the foundation for the biocompatibility and safety evaluations of magnetotactic bacteria MO-1 and suggested that they could be basically used in clinical targeted therapy.
Keyword: magnetotactic bacteria; motility; cell interaction; median lethal dose
1 INTRODUCTION
Magnetotactic bacteria (MTB) are a group of phylogenetically diverse and Gram-negative microorganisms with the ability of synthesizing magnetite (Fe3O4) or greigite (Fe3S4) nanoparticles in a genetically controlled manner (Blakemore, 1982;Bazylinski and Frankel, 2004; Simmons et al., 2006;Faivre and Schüler, 2008). These nanoparticles, also called magnetosomes, are arranged in a chain-like manner, which helps magnetotactic bacteria to swim along magnetic lines (Lefèvre and Wu, 2013).Magnetotactic bacteria also have the f lagella that enable them to swim autonomously and effi ciently(Zhang and Wu, 2020). Based on diff erent types of swimming behavior, magnetotactic bacteria can be divided into two main categories: one is polar strain that swims persistently in one direction along the magnetic f ield, and the other one is axial strain that swims in either direction along magnetic f ield lines(Bazylinski and Frankel, 2004). The features of magnetosomes and autonomous movements along the magnetic f ield endow them with rapid responsibility to external magnetic f ield.
As a biosynthetic magnetic nanoparticle,magnetosome is coated by a biomembrane, which could be easily decorated for in vitro uses, such as DNA extraction and immunoassays (Matsunaga et al.,2004). Magnetosomes could also be used in vivo as a contrast agent (Hu et al., 2010; Heinke et al., 2017),drug carriers (Sun et al., 2007; Alphandéry et al.,2017), and a tumor photo-/magneto-thermal transmitter (Alphandéry et al., 2011, 2012; Chen et al., 2016a). However, they are not capable of autonomous swimming and do not target the deep area in the body even when an external magnetic f ield is applied, thereby greatly limiting their applications.
Applications of the whole cell of magnetotactic bacteria have recently attracted considerable attention.The whole magnetotactic bacteria could not only be guided to target the deep area but also perform as energy converters, which are conducive to microrobot construction and targeted therapy (Fdez-Gubieda et al.,2020). Magnetotactic bacteria are suitable actuators of bacterial microrobot due to their autonomous swimming ability.Magnetosopirrillumgryphiswalense(MSR-1), an axial strain, was constructed as a guidable delivery vehicle for cargo transport and release(Alsaiari et al., 2016). It was also integrated with ciprofloxacin-loaded microtubes to build controllable microrobots, and the robots could target an infectious biofilm under the guidance of an external magnetic f ield and then release the antibiotics (Stanton et al.,2017). A polar magnetotactic bacterium may be more suitable as an actuator than the above axial strain because it swims persistently in one direction along the magnetic f ield. A polar marine magnetotactic coccus,designated strain MC-1 is one of the best actuators for building microrobots. MC-1 bacteria pushed a microsphere and swam along the magnetic f ield, thus showing a great potential in manipulated movement under large weight loading (Martel et al., 2006).Further, MC-1 cells were used to transport drug-loaded nanoliposomes to target the tumor in the blood under the control of magnetic f ield (Taherkhani et al., 2014;Felfoul et al., 2016). Another polar magnetotactic bacterium, magnetotactic ovoid strain MO-1, is also a highly suitable candidate for constructing microrobots.Magnetotactic ovoid strain MO-1 was isolated from the Mediterranean Sea, and it is a kind of chemoautotrophic bacterium (Lefèvre et al., 2009).MO-1-derived microrobots were constructed by attaching microbeads to the cell surface, and the microrobots could swim in the microf luidic channel under the control of magnetic f ield (Ma et al., 2012).MO-1 cells have also been fabricated as a separating microrobot to target separate pathogens and then kill them using diff erent external magnetic f ields (Chen et al., 2014, 2017b). By using magnetosomes in the cell body, intact magnetotactic bacteria could produce positive magnetic resonance imaging contrast for visualizing mouse tumor xenografts, thereby providing a potential tool for improved cancer imaging visualization in preclinical and translational studies(Benoit et al., 2009). The whole cell of magnetotactic bacteria could also transmit the magnetic f ield energy into the thermal energy through the intracellular nanoparticles. When applied in the alternating magnetic f ield, they produced heat and then strongly aff ected cancer cell proliferation on clinical conditions (Gandia et al., 2019). Magnetotactic bacterium-based magnetic hyperthermia was also used to target and kill pathogenic cells, thus could potentially treat infectious diseases(Chen et al., 2016b). Aside from thermal energy,magnetotactic bacteria could transmit the magnetic f ield energy to the mechanical force, which could inhibit the viability of pathogen (Chen et al., 2017a).
Although the whole magnetotactic bacteria show many potentials in the construction of microrobots and targeted therapy, their biocompatibility and biosecurity should be considered seriously. On the one hand, when magnetotactic bacteria enter into the body f luid, such as blood and urine, for drug delivery and targeted treatment, their status and swimming ability require suffi cient evaluation. When incubated in the blood, the terminal velocity ofMagnetococcusmarinusstrain MC-1 decayed for a duration of 40 min (Martel et al., 2009). On the other hand,magnetotactic bacteria, as foreign substances, are certainly attacked by the immune system in the human body, and the toxicity should be assessed. As for magneto-ovoid strain MO-1, which has been used for the construction of bacterial microrobots and targeted therapy, the biocompatible studies in the blood and urine are still unknown until now, and they need to be carried out.In the present study, magneto-ovoid strain MO-1 was used to investigate its biocompatibility for in vivo applications. The swimming ability of MO-1 bacteria in the blood and urine was f irst assessed. The interaction of bacteria and human cells was examined to show the biocompatibility and the cellular safe dose. Finally, the median lethal dose (LD50) of magnetotactic bacteria in rats was determined for in vivo biomedical utilization.
2 MATERIAL AND METHOD
2.1 Material
Chemical regents containing NaCl, MgCl2, Na2SO4,CaCl2, KCl, NH4Cl, NaHCO3, K2HPO4, and NaNO3were purchased from BBI Co., Ltd. Succinic acid,L-cysteine, tryntone, and yeast extract were bought from AMRESCO Commercial Finance, LLC. MTT was purchased from Sigma-Aldrich. The mineral solution and vitamin solution for magnetotactic ovoid strain MO-1 were prepared in accordance with previous reports (Frankel et al., 1997). Ferric quinate was also prepared. Artif icial urine was purchased from Qingdao Jisskang Biotechnology Co., Ltd. (China),and its pH was adjusted to 7.5 by using NaOH solution.
2.2 Animal
Adult male Sprague-Dawley (SD) rats weighing 180-200 g were used in the LD50experiments and purchased from Beijing HFK Bio-Technology Co.,Ltd. (China). Blood was acquired after the rats were anesthetized, and then the plasma was obtained by centrifuging the rat blood.
2.3 Bacterial culture and transmission electron microscopy (TEM)
The magnetotactic ovoid strain MO-1 was grown at room temperature in EMS2 medium as previously reported (Lefèvre et al., 2009). The EMS2 medium was f irst added with (per liter of deionized water; pH 6.8) 19.45-g NaCl, 5.9-g MgCl2, 3.24-g Na2SO4, 1.8-g CaCl2, 0.55-g KCl, 0.2-g NH4Cl, 1.26-g NaHCO3,0.6-g L-cysteine, 3-g HEPES, and 0.35-g agarose.The medium was then autoclaved at 120 °C for 20 min. After the medium was cooled to approximately 45 °C, the following solutions were added (per liter)to previously sterile-f iltered stock solutions in the following order: 0.5-mL vitamin solution, 0.5-mL ferric quinate (0.4 mmol/L), 3-mL sodium thiosulfate(40% w/v), 4-mL dipotassium hydrogen phosphate(1.86% w/v), and 5-mL modif ied Wolfe’s mineral solution. Ultrathin copper grids were immersed in the bacterial suspensions to observe the MO-1 cell morphology, and then the grids were dried in a clean bench after retrieval for observation using a transmission electron microscope (Hitachi H-7650B;Hitachi, Japan).
2.4 Speed analysis of magnetotactic bacteria in plasma and urine
Speed analysis of magnetotactic bacteria MO-1 in the plasma and urine was carried out in a microf luidic chip. The microf luidic channel contains four corners,and its plane geometry was shown in the previous report (Ma et al., 2012). The width and depth of the channel were 200 μm and 20 μm, respectively. The rat plasma or artif icial urine was pushed into a microf luidic channel. After one of the micropools in the chip was blocked, the MO-1 solution was injected into the other micropool. A magnetic f ield was applied to guide the MO-1 cells in the microf luidic channel.The movement of MO-1 swarm in the plasma or artif icial urine was observed under 40×magnif ication in the dark-f ield mode. The swimming behavior was recorded using a Canon 500D camera that recorded 1 920×1 080 pixel images at 20 frames per second.The experiments were conducted at 37 °C to mimic the in vivo biomedical applications of human physiological temperature and repeated thrice. The temperature controlling instrument of Live Cell Imaging System was used to keep the microf luidic chip with MO-1 cell samples at 37 °C. All the blood and urine solutions were at human physiological pH.The average swimming velocities were calculated using the MTrackJ plugin for ImageJ (Murat et al.,2015; Zhang et al., 2017).
2.5 Cell culture and MTT assay
Human immortalized epidermal cell HaCaT (No.3111C0001CCC000373) and human cervical carcinoma cell Hela (No. 3111C0001CCC000011;China Infrastructure of Cell Line Resources, Beijing,China) were maintained in Dulbecco’s modif ied Eagle’s medium (DMEM, Gibco/Life Technologies,Grand Island, NY, USA) supplemented with 10%fetal bovine serum (Australian origin, Hyclone Laboratories, Logan, UT, USA), 100-IU/mL penicillin(Sigma-Aldrich, St. Louis, MO, USA), and 100-μg/mL streptomycin (Sigma-Aldrich) at 37 °C in 5%CO2. The HaCaT and Hela cells were seeded at 10 000 cells/well in 100-μL DMEM in a 96-well plate and incubated for 24 h. The medium was then replaced with the magnetotactic bacteria MO-1 working solution for the succeeding experiments. The magnetotactic bacteria MO-1 working solution (per well in 100-μL DMEM) contains 0 (control sample),2×105, 4×105, 8×105, 1.6×106, 3.3×106, 6.5×106,1.3×107, 2.5×107, and 5×107bacteria.

Fig.1 Magnetotactic ovoid strain MO-1
After incubation was performed for 24 h, the viability of the HaCaT and Hela cells was analyzed by MTT method. In brief, the cell medium was replaced with 100-μL DMEM without phenol red(Gibco) containing MTT agent and incubated at 37 °C in the dark for 4 h. The medium was discarded, and 100 μL of dimethyl sulfoxide (Sigma-Aldrich) was added to the well. After shaking for 15 min, the cell plates were centrifuged at 3 000 r/min for 5 min, and the supernatant was transferred to a new 96-well plate. The absorbance was measured at 570 nm with a reference at 630 nm by using a microplate absorbance reader (168-1130 model, Bio-Rad Laboratories,Hercules, CA, USA). The relative cell viability (%)relative to control was calculated using the following equation: (Asample/Acontrol)×100% (whereAis the absorbance). The experiments were repeated thrice.
2.6 LD 50 of magnetotactic bacteria in rats
The magnetotactic ovoid strain MO-1 in exponential growth was collected using a magnetic block and suspended in phosphate buff er solution(PBS, pH7.0). Then, 16 SD rats were randomly assigned to four groups (n=4), and 5×108, 5×109,5×1010, and 5×1011MO-1 cells in 3-mL PBS were injected into four groups of rats intravenously. The animals in each group were observed for 1 week.Afterwards, Behrens-Kärber method was used to calculate the LD50and 95% conf idence interval (CI)(Zhang et al., 2015):
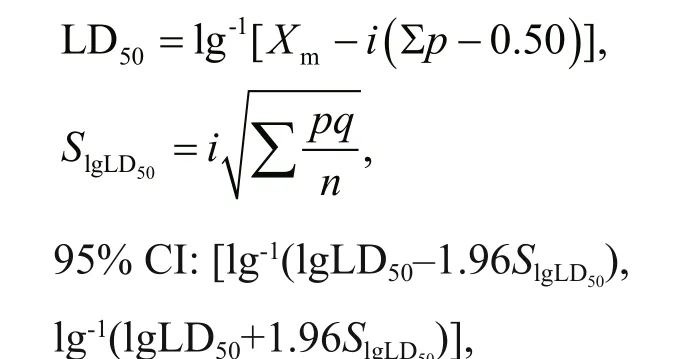
whereXmis the logarithm of the maximum dose;iis the diff erence in the logarithm of adjacent concentrations;pandqare the lethal success and failure rates in each group, respectively; andnis the number of rats in each group.
3 RESULT AND DISCUSSION
3.1 Characterization of magnetotactic bacteria MO-1
Magneto-ovoid strain MO-1 is a marine species affi liated with zeta proteobacterial strains, and it grew at aerobic-anaerobic interfaces in the culture f lasks.The TEM results showed that magnetotactic bacteria MO-1 owned two f lagellar bundles on the long hemisphere of the cell (Fig.1a, black arrows). They have an autonomous swimming ability toward North Pole in a magnetic f ield. TEM images showed that they also synthesized bio-membrane-coated magnetosomes with an average number of 11.1±2.94(Fig.1b), which assisted them to feel and move along the magnetic f ields. The morphologies of most MO-1 magnetosome particles have an elongated cubooctahedral shape, with the length of 77.06±11.1 nm,width of 71.54±10.59 nm respectively (n=149). The features of fast autonomous movement and magnetotaxis may lead them to reach the deep site inside the organism body for drug delivery and targeted therapy.
3.2 Speed of magnetotactic bacteria MO-1 in rat plasma and artif icial urine
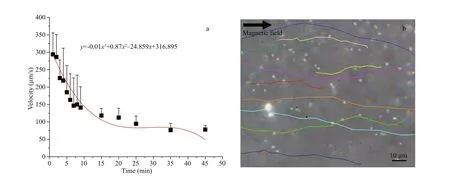
Fig.2 Speed of magnetotactic bacteria MO-1 in rat plasma under external magnetic f ield
Movement ability is an important characteristic for MO-1 cells to perform a task. Generally, in vivo application scenarios in biomedical areas are always accompanied with the body liquid environment, such as blood and urine. Here, the magnetotactic velocity of magnetotactic bacteria MO-1 in the blood plasma and artif icial urine was assessed at the human physiological temperature of 37 °C to mimic the in vivo biological application scenarios. The rat plasma was acquired, and the MO-1 cells and the plasma were injected into the microf luidic chip. At 37 °C,when a magnetic f ield with a strength of 2.2 mT was applied, MO-1 cells could swim along the magnetic f ield in the blood plasma liquid, the fresh MO-1 cells swam at the beginning speed of about 300 μm/s, while the swimming speed decreased as the time pasted(Fig.2a & b). In the f irst 4 min, the MO-1 cells swam at the velocity of above 200 μm/s and then travelled at more than 100 μm/s in the following 20 min. In the next time, the MO-1 cells continued to swim at a lower speed below 100 μm/s. During the entire process, the MO-1 cells were capable of responding to the external magnetic f ield guidance at 37 °C(Fig.2b). The experimental results showed that magnetotactic bacteria MO-1 could survive and keep their swimming motility in the plasma at the human physiological temperature for at least 45 min.
When artif icial urine was used as another model of body f luid, the state of magnetotactic bacteria MO-1 at the human physiological temperature (37 °C) was similar to that in the blood plasma (Fig.3a). The MO-1 cells could be navigated by an external magnetic f ield and swim in the urine at a speed of above 200 μm/s during the f irst 6 min. The bacteria over time continued swimming at the speed of more than 130 μm/s for 20 min. Finally, their speed further decreased in the next time but still moved in the urine at a lower speed below 100 μm/s (Fig.3a & b). The swimming experimental results conf irmed that the MO-1 cells could survive and swim for at least 40 min in the urine at physiological temperature.
Targeted therapy or drug delivery in the human body mediated by magnetotactic bacteria probably need the ability of these bacteria to swim autonomously in the body f luid. The results of this study demonstrated that magnetotactic bacteria MO-1 are capable of surviving and swimming in the blood and urine.According to the average speed and surviving time,the distance that magnetotactic bacteria MO-1 swam in the blood for 45 min or in the urine for 40 min at physiological temperature could even reach up to approximately 40 cm. Considering the size of human organ and the principle of proximal intervention with external carriers, the magnetotactic bacteria MO-1 swimming distance is enough to satisfy the requirements of drug delivery and targeted therapy.The blood results are also similar to those when other magnetotactic bacteria of MC-1 were used (Martel et al., 2009). Other factors, such as blood f low velocity and vascular branch, were not considered in the present study. However, it was reported that magnetotaxis enabled MTB to overcome counterdirectional f low and migrate eff ectively through porous micromodels (Rismani Yazdi et al., 2018a, b).As to the scene that the velocity of MO-1 cells actually decreased as the incubation time increased, the main reason is possibly the osmotic pressure induced by diff erent salt concentrations. For example, the NaCl concentrations in the blood and urine are 8.5 and 4.2 g/L respectively, much lower than those(19.45 g/L) needed by MO-1 to grow. Consistently, it has also been implied that the velocity of the bacteria could also be controlled by varying the concentration of Na+in the medium (Lefèvre et al., 2009). Besides,a magnetic f ield with a higher strength could slow down the speed of magnetotactic bacteria (Yang et al.,2012). It is also suggested from the survival and movement abilities that magnetotactic bacteria do not proliferate spontaneously inside an animal nor cause severe infection and pathogenicity. Thus, their safety for the in vivo application is much better than that of other pathogenic bacteria.
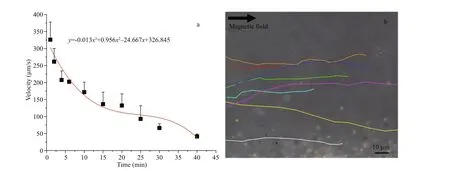
Fig.3 Speed of magnetotactic bacteria MO-1 in artif icial urine under external magnetic f ield
3.3 Interaction of magnetotactic bacteria MO-1 with human HaCaT and Hela cells
Although the infection and pathogenicity of magnetotactic bacteria as a therapeutic tool may be negligible according to the analysis, the dose eff ect at the cellular level and the in vivo toxicity of magnetotactic bacteria MO-1 remained to be studied.In the present study, the biocompatibility and biological safety dose of magnetotactic bacteria MO-1 at the cellular level were examined via MTT assay. HaCaT and Hela cells (1×104cells) were treated with magnetotactic bacteria MO-1 at diff erent bacterial cell concentrations ranging from 0 to 5×107bacteria/well in 100-μL DMEM in a 96-well plate for 24 h. As shown in Fig.4a, the incubation of MO-1 cells at the concentration of less than 3.3×106bacteria/well had no eff ect on the viability of the HaCaT cells. The cellular viability decreased by approximately 40% at the concentration of 6.5×106bacteria/well and continued to decrease as the number of MO-1 cells was increased to 5×107bacteria/well (Fig.4a). As for Hela cells, coculture of MO-1 cells did not induce cell toxicity at the bacterial concentration of less than 1.6×106bacteria/well (Fig.4b). However, when the MO-1 concentration was at 3.3×106bacteria/well, the viability of Hela cells decreased to about 80% and continued to decrease with the increased concentration of magnetotactic bacteria MO-1. A potential reason for the increased cytotoxicity may be that increased magnetotactic bacteria touched with cells and then were phagocytosed, leading to increases in oxidative stress and reactive oxygen species inside the cell(Wang et al., 2014, 2017).
Fig.4 The cell viability of HaCaT (a) and Hela (b) cells after interaction with magnetotactic bacteria MO-1
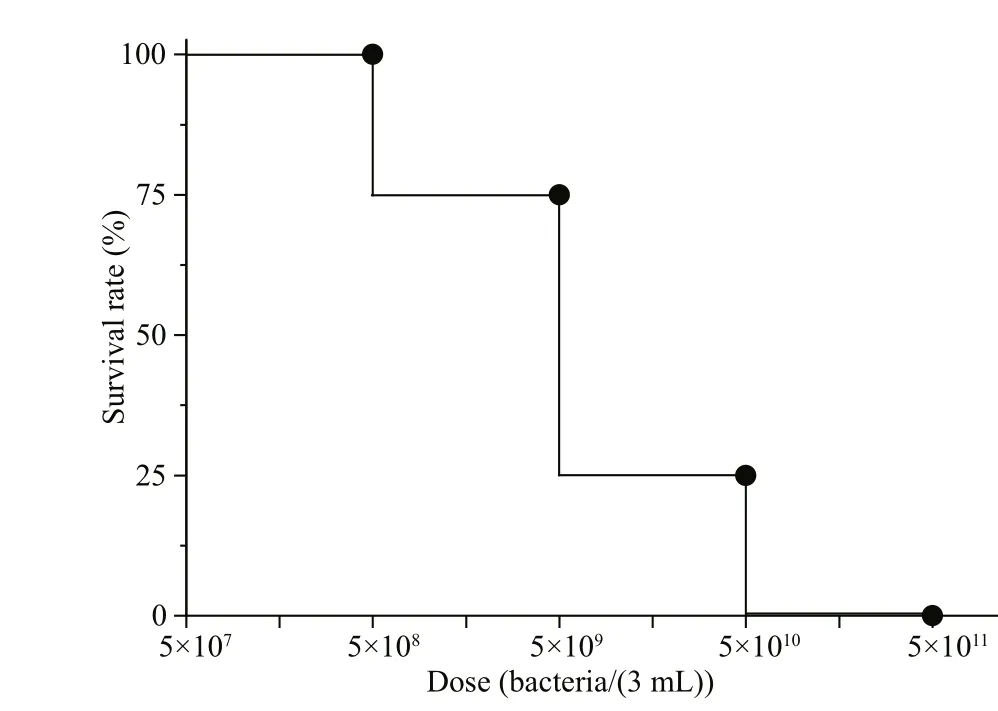
Fig.5 Determination of LD50 of magnetotactic bacteria in rats
Previous reports showed that 6.5×106bacteria ofMagnetococcusmarinusstrain MC-1 incubated with 1×105murine J774A.1 and NIH/3T3 cells in a 96-well plate induced a huge decrease in cellular viability to approximately 30% (Taherkhani et al., 2014). The decrease in cellular viability caused by magnetotactic bacteria MO-1 in the present study was much smaller than the results of strain MC-1. The diff erence may be attributed to the diff erent cell lines and added bacterial species. The method of coating nanoliposome on the surface of MC-1 bacteria was proposed to reduce the toxicity of magnetotactic bacteria (Taherkhani et al.,2014).
3.4 Determination of LD 50 of magnetotactic bacteria in rats
From an application perspective, the eff ect of magnetotactic bacteria on the whole human body must be considered. Diff erent doses of magnetotactic bacteria MO-1 were injected into rats in each test group to acquire the LD50, and the rats were observed persistently for 1 week. As shown in Fig.5, the survival rate decreased as the doses of magnetotactic bacteria MO-1 were increased. The experimental animals did not die in the groups of 5×108bacteria/(3 mL), one died in the 5×109bacteria/(3 mL) group,three died in the 5×1010bacteria/(3 mL) group, and all animals died in the 5×1011bacteria/(3 mL) group.Behrens-Kärber method was used to calculate the LD50of the rats (0.2 kg) at 7.9×1010bacteria/kg, with a 95% CI of 1.99×1010-3.155×1011bacteria/kg.According to a guidance (U.S. Food and Drug Administration, 2005), the dose per unit body weight of an adult (60 kg) is equivalent to 0.16 of that of rats(0.2 kg). Therefore, the LD50of an adult human(60 kg) was calculated to be 1.264×1010MO-1 bacteria/kg.
With the abilities of magnetic nanoparticle synthesis and autonomous swimming, magnetotactic bacteria have been used as contrast agents and in vivo drug delivery. As a contrast agent,MagnetospirillummagneticumAMB-1 was intravenously injected into mice with 1×109cells (Benoit et al., 2009). The number of magnetotactic bacteria MC-1 intravenously injected into rats for drug delivery was reported to be 1×108cells (Felfoul et al., 2016). Thus, the actual quantity of magnetotactic bacteria for in vivo uses was much less than its LD50(7.9×1010MO-1 bacteria/kg in rats) obtained in the present study. Of course,diff erent species of magnetotactic bacteria may have diff erent LD50values, thereby needing further investigations. Regardless of the species of magnetotactic bacteria, after entering the body from the blood vessels or urethra, these bacteria may interact with the initially encountered organ or tissue and then interact with diff erent kinds of cells,especially immune cells. Whether magnetotactic bacteria induced an intense immune response or not depends mainly on the immunogenicity and quantity of the bacteria. In any case, as a kind of Gram-negative bacteria, the endotoxins of magnetotactic bacteria MO-1 should be paid with suffi cient attention when used in biomedical applications (Alphandéry, 2020).On one hand, agents such as poly-L-lysine could be modif ied to reduce or eradicate the endotoxins of magnetotactic bacteria (Hamdous et al., 2017;Mandawala et al., 2017). On the other hand, rats could actually overcome some endotoxins of MO-1 cells according to the study. Nevertheless, the endotoxins of magnetotactic bacteria / magnetosomes released from bacterial cells into the animal body should be considered seriously. Taken together, based on the LD50obtained in the present study, the dose of MO-1 cells may be safe and it could satisfy the requirement of clinical tumor therapy.
4 CONCLUSION
The biocompatibility of magnetotactic bacteria was determined. The MO-1 cells were able to swim along magnetic f ield lines in blood and urine and survived 37 °C for approximately 40 min. When HaCaT and Hela cells were incubated with magnetotactic bacteria MO-1, their cellular eff ect was dependent on the dose below 3.3×107bacteria/mL,which was well tolerated by human cells. In the animal experiments, an LD50of 7.9×1010bacteria/kg in rats was determined. These data provided the key to understand the biocompatibility and safety of magnetotactic bacteria to a certain extent and suggested that magnetotactic ovoid strain MO-1 could be used in targeted therapy. However, the distribution of whole bacteria in the body should be further studied.
5 DATA AVAILABILITY STATEMENT
The data supporting the f indings of this study are shown in this article. Otherwise, please contact the corresponding author on reasonable request.
6 ACKNOWLEDGMENT
We thank Dr. Haitao CHEN for the assistance in analyzing the swimming speed of magnetotactic bacteria MO-1 in plasma and urine by using the MTrackJ plugin for ImageJ.
 Journal of Oceanology and Limnology2021年6期
Journal of Oceanology and Limnology2021年6期
- Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Observations on a magnetotactic bacteria-grazing ciliate in sediment from the intertidal zone of Huiquan Bay, China*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
- Three-dimensional mesoscale eddy identif ication and tracking algorithm based on pressure anomalies
