Seasonal and regional diff erences in long-term changes in large mesozooplankton (>505 μm) biomass and abundance in a semi-enclosed subtropical bay*
Ping DU , Zhibing JIANG , Yuanli ZHU , Yibo LIAO ,3, Quanzhen CHEN ,Jiangning ZENG , Lu SHOU ,**
1 Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources (MNR),Hangzhou 310012, China
2 Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3 Key Laboratory of Ocean Space Resource Management Technology, MNR, Hangzhou 310012, China
Abstract Obvious spatiotemporal heterogeneity is a distinct characteristic of ecosystems in subtropical bays. To aid targeted management and ecological restoration in long and narrow semi-enclosed subtropical bays, we analyzed seasonal and regional diff erences in long-term changes (1980-2019) in the biomass and abundance of large mesozooplankton (LMZ; >505 μm) in Xiangshan Bay, Zhejiang, China. We found spatiotemporal heterogeneity in the historical changes of LMZ. Signif icant negative trends in LMZ biomass were found in the inner and middle bay during the warm season (summer and autumn), when the nutrient concentration (especially dissolved inorganic nitrogen) and temperature increased simultaneously. Nutrient changes in Xiangshan Bay began in the late 1980s or early 1990s, coinciding with large-scale f ish cage development. A rapid decline in LMZ biomass occurred after 2005 when power plants commenced operation,accelerating the warming trend. Therefore, the joint stress of eutrophication and warming likely precipitated the decline in LMZ biomass. Conversely, a signif icant increase in LMZ biomass was found in the outer bay in spring. This trend was consistent with the trend of LMZ biomass near the Changjiang (Yangtze)River estuary, which indicates that the pelagic ecosystem in the outer bay was aff ected by water from the Changjiang River estuary during spring. Based on our results, ecosystem management and restoration in semi-enclosed subtropical bays should focus on internal waters, which have a poor capacity for water exchange. For Xiangshan Bay, the changes in the Changjiang River estuary ecosystem during the cold season (winter and spring) should also be considered.
Keyword: large mesozooplankton; long-term changes; spatiotemporal heterogeneity; Xiangshan Bay
1 INTRODUCTION
Zooplankton plays a pivotal role in marine food webs by transferring carbon f ixed by phytoplankton and microzooplankton to higher trophic levels(Hughes, 2000). Variations in zooplankton are mainly related to hydro-environmental changes; moreover,zooplankton responses can amplify subtle environmental variations that are diffi cult to detect through the assessment of single physical variables(Fernández-Urruzola et al., 2014). Understanding the relationship between the environment and the zooplankton community, based on long-term observations, is essential to precisely grasp the inf luence of environmental changes on marine ecosystems.
Long-term studies in many areas have shown that global zooplankton are undergoing diff erent changes(Uye, 1994; Kideys et al., 2000; Piontkovski and Castellani, 2009; Glibert, 2010; García-Comas et al.,2011; Steinberg et al., 2012; Valencia et al., 2016;Wang et al., 2016a). The major environmental changes in subtropical bay systems include anthropogenic increases in nutrient inputs and temperature. However,the responses of zooplankton communities have diff ered owing to diff erences in plankton composition.In Tokyo Bay during the 1980s, for example, larger copepods that dominanted in the 1950s were replaced by small copepods (Oithonanana), and the abundances of Scyphomedusae and Ctenophora increased (Uye, 1994). In the Sacramento-San Joaquin River Delta, the dominant copepod species changed fromEurytemoraaffi nistoLimnoithonatetraspina(Glibert, 2010). In the neritic northwestern Black Sea,gelatinous zooplankton (Noctilucascintillansand ctenophores) increased considerably, accompanied by decreases in the abundances of crustacean and fodder zooplankton (Kideys et al., 2000). In the San Francisco estuary, the abundance ratio of copepods to cladocerans changed (Lehman et al., 2010). In the Changjiang (Yangtze) River estuary, an increase in zooplankton biomass and abundance and a shift in zooplankton community structure have occurred over the past 30 years (Wang et al., 2016a).
Xiangshan Bay (XSB) is a typical anthropogenically aff ected system suff ering from increased eutrophication and warming. XSB was once excellent for f ish spawning and breeding because of its abundant nutrients, rich food organisms, and relatively stable environment (Wang et al., 2017). However, over the last three decades, XSB has been aff ected by largescale human activities, such as the expansion of aquaculture (especially f ish cages) and coastal industries since the 1990s, as well as the operation of power plants since 2005 (Du et al., 2017). Human activities aggravated the eutrophication and warming to diff erent degrees (Ye et al., 2017b; Jiang et al.,2019a). Over the past 35 years, primary productivity and phytoplankton biomass have increased, especially the proportion of micro-chlorophylla(Chla) (20-200 μm) (Jiang et al., 2019a). Conversely, the abundance and biomass of large mesozooplankton(LMZ; >505 μm) have decreased (Du, 2018;Supplementary Fig.S1a), although changes in the LMZ dominant species have not been obvious throughout the bay (Luo et al., 2018). Meanwhile,f ishery resources, including f ish, crabs, shrimp, and cephalopods, have decreased sharply (Editorial Committee of the Bay Chorography in China (ECBCC),1992; Tang et al., 2012; Supplementary Fig.S1b).
Signif icant spatiotemporal heterogeneity is a distinct characteristic of subtropical bays. To better manage and restore these areas, it is critical to identify the spatiotemporal heterogeneity in ecosystem changes and key drivers in diff erent areas. XSB is a long and narrow semi-enclosed bay with signif icant spatial and seasonal heterogeneity in terms of environmental parameters and the plankton community (Du et al., 2015, 2019; Jiang et al., 2019b).The bay has traditionally been divided into three regions: the BI(inner bay), BM(middle bay), and BO(outer bay) (Ye et al., 2017a; Fig.1). The water in the BIis mainly aff ected by runoff near the bottom of the bay, while the water in the BOis greatly aff ected by the water mass originating from the East China Sea(ECBCC, 1992). The salinity from BIto BOranges from 17 to 27 (Du et al., 2020). Four distinct seasons(spring, summer, autumn, and winter) occur throughout the year in XSB. The water temperature from winter to summer ranges from 9.0 to 28.7 °C(Du et al., 2020). The BOof XSB is subject to the southward-f lowing, turbid, inshore Changjiang Diluted Water during the cold/dry season (spring and winter) and the northward-f lowing, relatively clear,off shore Taiwan Warm Current during the warm/wet season (summer and autumn) (ECBCC, 1992).According to existing records, algal blooms in XSB occur mainly during the cold season and in the inner section (Jiang et al., 2019b). Although XSB adjoins the Changjiang River estuary (Fig.1), LMZ abundance and biomass in XSB were found to decrease, in contrast to the trend in zooplankton changes near the Changjiang River estuary (Wang et al., 2016a).Therefore, we hypothesize that regional and seasonal diff erences exist in the historical changes of LMZ abundance and biomass in XSB.
In this study, we aimed to examine whether there are regional and seasonal diff erences in the long-term changes in LMZ abundance and biomass in XSB, and determine whether changes in LMZ are associated with increases in nutrients and temperature. This study provides a research idea and results to support regionally and seasonally diff erentiated management and ecological restoration in long and narrow semienclosed subtropical bays. Meanwhile, these f indings will be valuable for studies on pelagic ecosystem changes in regions synchronously aff ected by warming and eutrophication.

Fig.1 Map of Xiangshan Bay, China, and the partition diagram
2 MATERIAL AND METHOD
2.1 Study area
XSB is a long and narrow bay (approximately 60-km long and 3-8-km wide) in China (29.40°N-29.75°N, 121.40°E-122.05°E) (Fig.1). The bay covers a tidal f lat area of 198 km2and a water area of 365 km2, and the water depths range from 5 to 20 m.The bay was traditionally divided into three regions based on topography, hydrological features, and human activities (Ye et al., 2017a; Fig.1). Ninety percent of the water exchange takes approximately 80, 60, and 15 days in the inner (BI), middle (BM), and outer (BO) sections, respectively (Dong and Su, 1999)(Fig.1). Accordingly, the BOis aff ected by the water mass originating from the highly saline East China Sea, while the BIis more aff ected by freshwater inputs. The salinity from BIto BOranges from 17 to 27 (Du et al., 2020). Freshwater inputs from 95 rivers around the bay, most of which are in the BIand BM,result in low salinity along the inner regions of the bay (ECBCC, 1992). Additionally, XSB is surrounded by dense population areas, and human activities(coastal power plants, aquaculture, and terrestrial inputs) are mostly concentrated in the BIand BM(Nobre et al., 2010). Combined with the inf luence of poor seawater exchange, pollutants easily accumulate in the BIand BM(Fan and Jin, 1989; Ye et al., 2017a).The Ninghai (4×600 MW+2×1 000 MW) and Wushashan (4×600 MW) Power Plants are located in the BIand BM, respectively, with large thermal discharge volumes of 87.5 and 53 m3/s, respectively.The areas that experience a 1 °C increase around the Ninghai and Wushashan Power Plants can approach 20 and 60 km2, respectively (Huang and Ye, 2014).Additionally, strong wind- and tide-induced vertical mixing and sediment resuspension result in increased turbidity in the BO, while high clarity and water column stability are observed in the BI. Under the inf luence of the East Asian monsoon, there are four distinct seasons in XSB. The average water temperatures are approximately 15, 27, 20, and 10 °C in the spring, summer, autumn, and winter,respectively. During the cold season (spring and winter), the southward monsoon brings turbid Changjiang Diluted Water to the BO, whereas during the warm season (summer and autumn), the northward monsoon brings the Taiwan Warm Current that is relatively warmer and clearer to the BO(Gao et al.,1990). Furthermore, precipitation is signif icantlyhigher from June to September than during other months, resulting in decreased salinity in the warm season (Jiang et al., 2019b). Thus, there are spatial and seasonal gradients and variations in temperature,salinity, nutrients, and turbidity, which result from the combined inf luence of topography, hydrological features, human activities, and monsoons.

Table 1 List of historical data on zooplankton and nutrients in Xiangshan Bay
2.2 Data source
The China National Oceanic Census began in 1959, while environmental surveys in gulfs began in the early 1980s. Mesozooplankton are zooplankton ranging in size from 200 to 20 000 μm. All early zooplankton samples in China were collected using a plankton net with a mesh size of 505 μm, in accordance with the Marine Investigation Criterion, and have been designated “large mesozooplankton (LMZ)”.Therefore, in this study, we analyzed the long-term changes in the abundance and biomass of these LMZ,which range in size from 505 to 20 000 μm.
The zooplankton and nutrient data in this study were derived from the literatures and our own studies(Table 1). Samples were collected monthly in 1980/1981 and quarterly in other years; thus, in 1980/1981, the seasonal biomass and abundance values are three-month averages, whereas in other years, the values are those from the quarterly surveys.The historical data for each sampling region contained a certain number of stations (Table 1). The zooplankton data generated from the 2000 to 2019 samples were all collected and analyzed by the Second Institute of Oceanography, Ministry of Natural Resources (SIO, MNR), China. Zooplankton samples were collected using a plankton net(diameter: 80 cm, mesh size: 505 μm, length:140 cm) via vertical tows from 2 m above the bottom depth to the surface. All samples collected were stored in 5% formalin in 1-L plastic bottles. The volume of f iltered water was measured using a digital f low meter. In the laboratory, mesozooplankton samples were f iltered through a silk sieve with a mesh size of 160 mm and then weighed with a 0.1-mg electronic balance after picking out of sundries.Taxonomic identif ication and enumeration was carried out using a stereoscope (Zeiss SteREO Discovery.V8) and a microscope (Leica DM2500).Adult mesozooplankton, crustacean larvae, and other larvae were identif ied to the species, family, and class levels, respectively. Zooplankton biomass was determined as the ratio of the wet weight of zooplankton to the f iltered water volume, and zooplankton abundance was the ratio of the number of individuals to the f iltered water volume. The nutrient data generated from the 2006 to 2019samples were collected and analyzed by SIO, MNR.The nutrients included dissolved inorganic nitrogen(DIN=nitrate (NO3-N)+nitrite (NO2-N)+ammonium(NH4-N)) and dissolved inorganic phosphate(DIP=phosphate (PO4-P)) concentrations, which showed clear historical changes. To analyze NO3-N,NO2-N, NH4-N, and PO4-P, 1-L water samples were f iltered through 0.45-μm pore size mixed cellulose ester f ilters, and the nutrients were measured using colorimetric methods according to GB 17378.4-1998/2007.
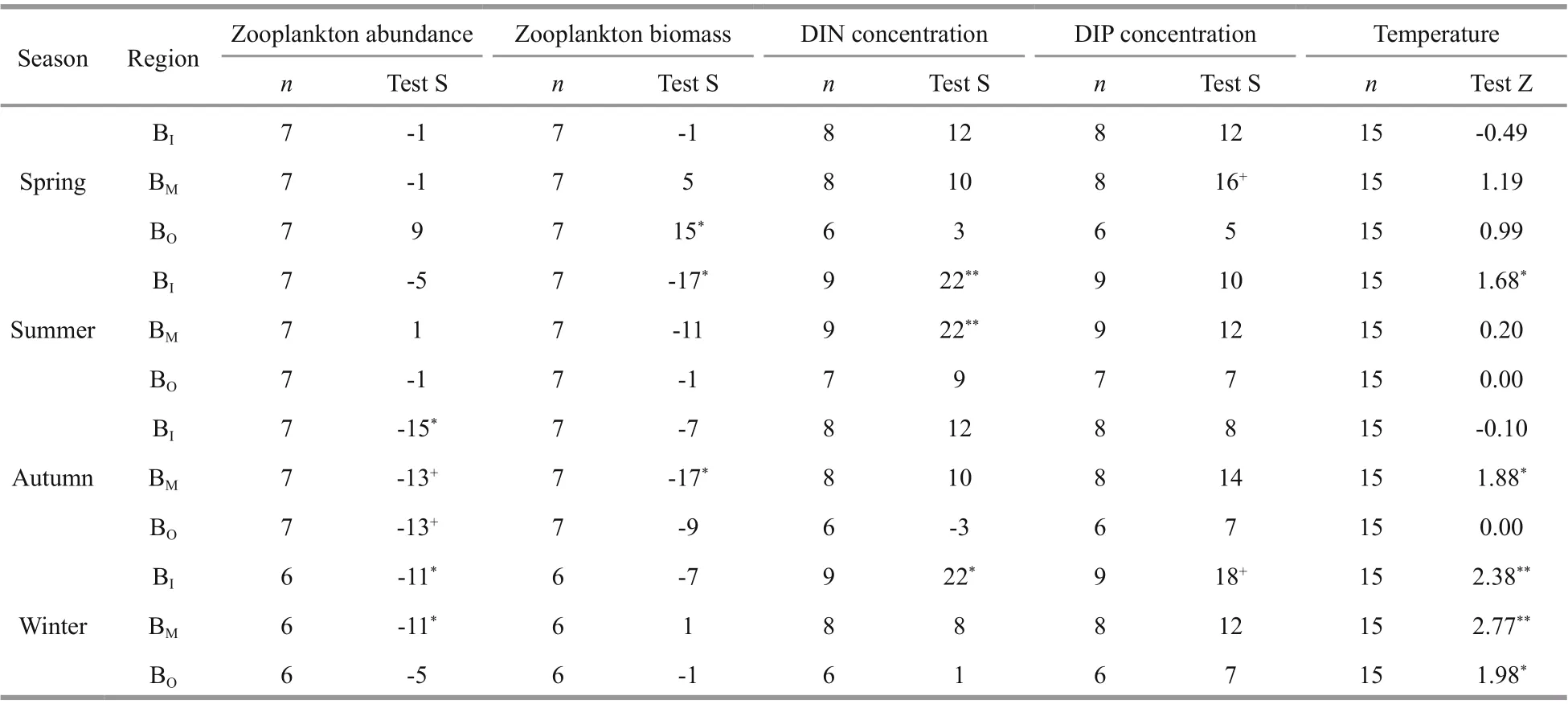
Table 2 Results of the Mann-Kendall test for monotonic trends in the abundance and biomass of large mesozooplankton,concentrations of DIN and DIP, and temperature among three regions over four seasons
Although these historical data were not collected from the same stations or at the same time, they covered specif ic regions and seasons. Therefore, the error between past and present data collected from diff erent stations is likely minimal. Furthermore, to reduce the error caused by monthly diff erences, the most zooplankton data representing winter, spring,summer, and autumn were collected in January, April,July, and October, respectively (only the samples from autumn 2010 and spring 2019 were collected in mid-November and mid-May, respectively).
To determine the warming trend in XSB, and considering that power plants began operating in 2005, the monthly mean sea surface temperature(SST) from 2003 to 2017 was derived from the Moderate-resolution Imaging Spectroradiometer(MODIS) Aqua Level 3 Product-Monthly SST4 (4-μ nighttime measurements with 4-km resolution;https://oceancolor.gsfc.nasa.gov/l3/).
2.3 Data analysis
The Mann-Kendall test in the “Kendall” package in R v.3.5.1 software (The R Development Core Team, 2020) was used to detect monotonic increases in zooplankton abundance and biomass, as well as DIN and DIP concentrations and SST. Sen’s nonparametric method was used to estimate the true slope of an existing trend (Salmi et al., 2002). Changes were considered signif icant atP<0.05. Test S was exported to indicate monotonic trend when the number of yearsn≤10, while Test Z was exported to indicate monotonic trend whenn>10.
3 RESULT
3.1 Regional and seasonal diff erences in zooplankton changes
We found regional and seasonal diff erences in the variation trends of LMZ abundance and biomass(Figs.2-3; Table 2). Trends of decreasing abundance and biomass were observed in most of the seasons and regions, while increasing abundance and biomass trends were observed in BOduring spring. The rapid decline in abundance and biomass occurred after 2005.
Sharp abundance declines were observed in winter and autumn. The approximately 7-fold decline in the BIduring autumn (from 204 ind./m3in the 1980s to 28 ind./m3in 2018), 14-fold decline in the BIduring winter (from 170 ind./m3in the 1980s to 12 ind./m3in 2019) and 7-fold decline in the BMduring winter(from 75 ind./m3in the 1980s to 11 ind./m3in 2019)were signif icant (P<0.05) (Fig.2; Table 2).
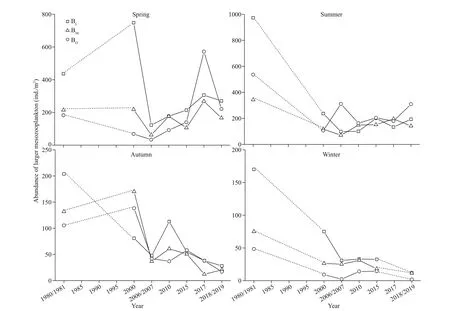
Fig.2 Historical changes in large mesozooplankton abundance in diff erent seasons and regions
Sharp biomass declines were observed in the warm season (summer and autumn). The biomass-declining trend was increasingly clear from the BOto BIduring summer, whereas the increasing trend was increasingly clear from the BIto BOduring spring. The approximately 10-fold decline in the BIduring summer (from 187 mg/m3in the 1980s to 16 mg/m3in 2018), 5-fold decline in the BMduring autumn (from 48 mg/m3in the 1980s to 10 mg/m3in 2018) and 2-fold increase in the BOduring spring (from 148 mg/m3in the 1980s to 278 mg/m3in 2019) were signif icant (P<0.05) (Fig.3; Table 2).
The seasonal changes in LMZ abundance and biomass were as follows. In spring, both the biomass and abundance in the BOincreased, and biomass increased signif icantly (Figs.2-3; Table 2).Calanussinicuswas the dominant species in the BO, and its relative abundance remained at almost 40% to 50% in the 1980s and 2019. In summer, the biomasses of LMZ in the BIand BMdeclined greatly (Fig.3; Table 2), while the abundances declined slightly or increased(Fig.2; Table 2), which indicates that the mean individual biomass (ratio of biomass to abundance,B/A) declined. The changes in dominant species during summer were not obvious, and the dominant species in the BIwere stillAcartiapacif icaand Brachyura larvae. However, the relative abundance ofA.pacif icadeclined from approximately 50% in the 1980s to 15% in 2017 and 2019, whereas that of Brachyura larvae increased from 30% to 40%.Meanwhile, the relative abundance ofOithonanana(a small copepod) reached 10% in 2017. In autumn,both the biomasses and abundances of LMZ in the three regions of XSB declined greatly. In winter, the decreasing trend of abundance was more obvious than that of biomass, due to the higher biomass in 2006, which was caused by the presence of some species (Diastylistricincta, Amphipoda spp.,C.sinicus) with individuals larger thanCentropagesdorsispinatus.

Fig.3 Historical changes in large mesozooplankton biomass in diff erent seasons and regions
3.2 Regional and seasonal diff erences in changes in nutrients and temperature
The average annual concentrations of DIN and DIP increased in almost all seasons and regions from 1981/1982 to 2019, by approximately three times for DIN and more than two times for DIP. The increases in DIN and DIP both began in the late 1980s or early 1990s. The increases in the BIand BMwere greater than those in the BOin each season. Signif icant increases in DIN were observed in the BIand BMduring summer and in the BIduring winter (P<0.05).No signif icant increases in DIP were observed(P>0.05) (Figs.4-5; Table 2).
For the years 2003 to 2017, signif icant increases in SST were detected in the BI, BM, and BOduring winter,by 2.27, 2.68, and 1.60 °C, respectively, and at rates of 1.52, 1.79, and 1.07 °C/decade, respectively. Signif icant increases were also found in the BIduring summer, by 0.33 °C at a rate of 0.22 °C/decade, and in the BMduring autumn, by 0.68 °C at a rate of 0.45 °C/decade,according to the Mann-Kendall test and Sen’s slope estimation (Supplementary Fig.S2). Moreover, SST in the BMincreased during all four seasons. The SST in the BOincreased during the cold season but did not change during the warm season. SST in the BIdecreased slightly in the spring and autumn.
3.3 Coupled changes in zooplankton and environmental factors
During the warm season, the LMZ biomass declined signif icantly in the regions where the temperature increased signif icantly, while the nutrient concentration (especially DIN) also increased greatly in these regions, namely, in the BIduring summer and in the BMduring autumn (Figs.2-5; Table 2). During winter, signif icant declining trends in LMZ abundance were observed in the region where the DIN concentration and temperature increased signif icantly simultaneously.

Fig.4 Historical changes in dissolved inorganic nitrogen (DIN) concentrations in diff erent seasons and regions
4 DISCUSSION
In accordance with our hypothesis, our study results indicate that regional and seasonal diff erences exist in the historical changes of LMZ (>505 μm)abundance and biomass in XSB. The regions with declining LMZ biomass trends during the warm season were accompanied by simultaneous increases in nutrients and temperature. The increases in LMZ abundance and biomass in the BOduring spring appeared to be aff ected by water from the Changjiang River estuary outside the bay.
4.1 Simultaneous increases in nutrient concentration and temperature depressed LMZ biomass in the B I and B M during the warm seasons
In the context of global warming, increases in temperature and eutrophication are widespread in many bays and along coasts during periods of rapid economic development; however, the responses of pelagic ecosystems are not exactly the same (Ning et al., 2010; Kimmel et al., 2012; Jiang et al., 2014;Wang et al., 2016a). Eutrophication and warming generally enhance phytoplankton biomass, with increases in the proportions of either small-cell species (Uye, 1994; Glibert, 2010; Hao et al., 2016)or large-cell species (Jiang et al., 2019a) in diff erent areas because of latitudinal diff erences, as well as changes in the nutrient proportions and nitrogen forms among the sea areas. However, the responses of zooplankton and f ish to eutrophication and increased phytoplankton are more complex and may be determined by trophic interactions in the middle of the food web (Micheli, 1999; Shurin et al., 2002;Stibor et al., 2004).
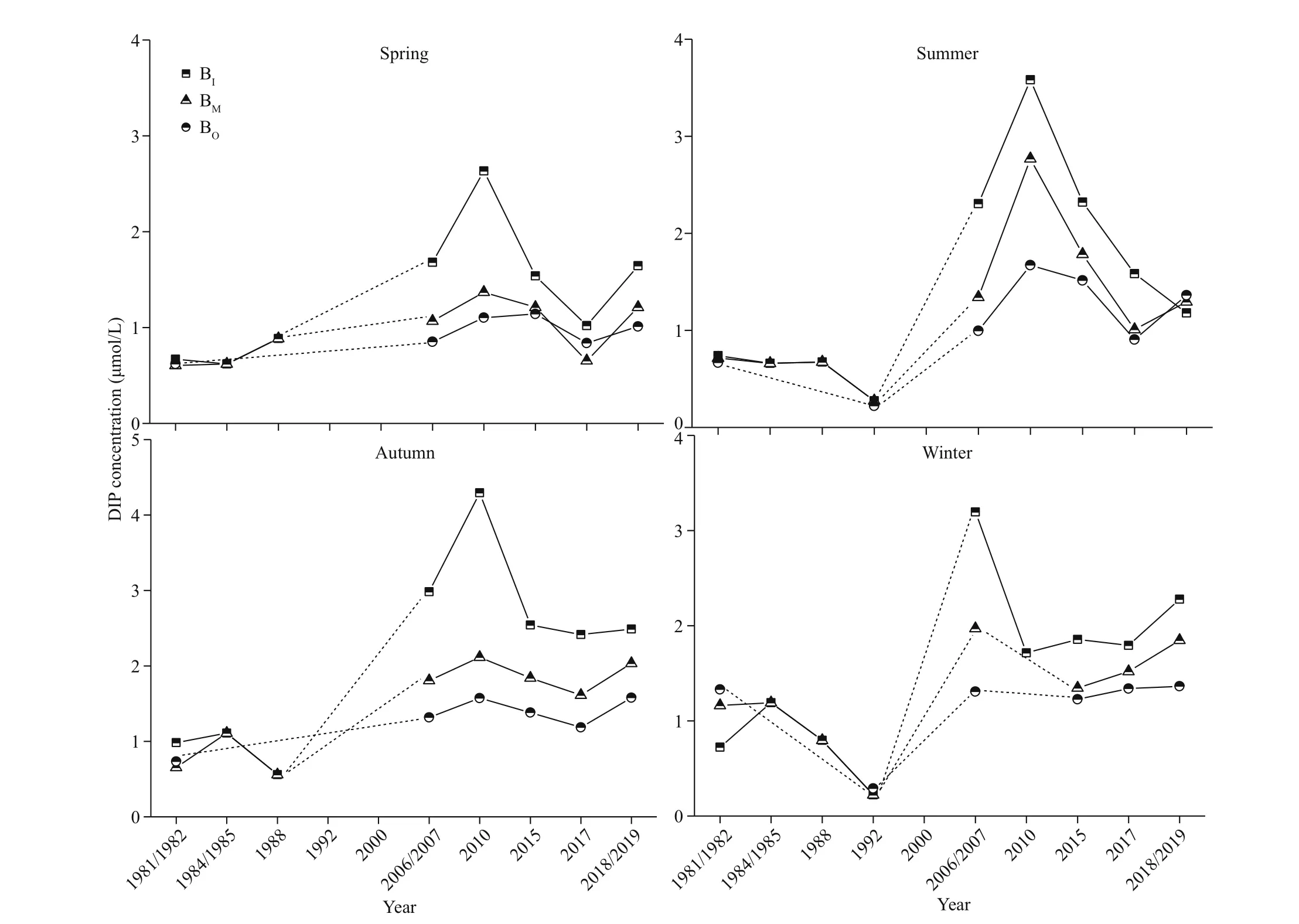
Fig.5 Historical changes in dissolved inorganic phosphate (DIP) concentrations in diff erent seasons and regions
After examining several parameters in XSB that have been recorded since the early 1980s, we found that the increases in water temperature, and DIN, DIP,and Chl-aconcentrations, and the decrease in total f isheries resources may co-vary with the decline in LMZ biomass (Supplementary Fig.S1). Nutrient concentrations started changing in the late 1980s or early 1990s (Figs.4-5), coinciding with large-scale f ish cage development in XSB. The increased phytoplankton biomass in XSB corresponded to the nutrient changes. Beginning in 2005, the thermal discharge by power plants in the BIand BMof XSB accelerated the warming trend because of the long water-residence time. The rapid decline in LMZ biomass that occurred after 2005 coincided with the time at which the power plants commenced operation.Therefore, the decline in LMZ biomass was likely related to the joint stress of increased temperature and nutrients.
In subtropical systems, increasing water temperature often promotes an increase in mesozooplankton (>200 μm) abundance (Salvador and Bersano, 2017), but an inverse relationship generally exists between zooplankton body size and temperature (Daufresne et al., 2009). The high temperatures and eutrophic conditions promoted an increase in the abundance of small-bodied copepods,such asOithonanana,Paracalanusparvus, and copepod nauplii, which are frequently observed in coastal ecosystems (Park and Marshall, 2000; Lam-Hoai et al., 2006; Salvador and Bersano, 2017). A recent study on mesozooplankton (>200 μm) in XSB showed that the number of small mesozooplankton(200-505 μm; e.g.,O.nana,Limnoithonatetraspina,Brachyura larvae, copepod nauplii) was higher in summer and in the BIunder higher temperatures and nutrient concentrations (Du et al., 2020). In this study,the decreasing trends in the biomasses of LMZ(>505 μm) were more obvious than the decreases in abundance in the BIduring summer and in the BMduring autumn (Figs.2-3; Table 2), which indicates that the B/A may decline. At the same time, changes in the relative abundances of dominant species were observed in the BIduring summer. The abundance ofA.pacif ica(a medium-sized copepod) declined in the summer, probably because of a temperature increase near the power plant outlet up to 33 °C that exceeded its thermal tolerance (Du et al., 2017). Instead, the relative abundances of Brachyura larvae andO.nana(a small-sized copepod) increased. Therefore, we propose that the decline in the biomass of LMZ in XSB during summer was mainly because of zooplankton miniaturization caused by increases in nutrients and, especially, temperature. We speculate that the decrease in LMZ abundance in the BIand BMduring winter may be caused by the miniaturization ofCentropagesdorsispinatus, which remained the main dominant species in the 1980s and 2019. Unfortunately,there were no historical data on the abundance of small mesozooplankton (200-505 μm).
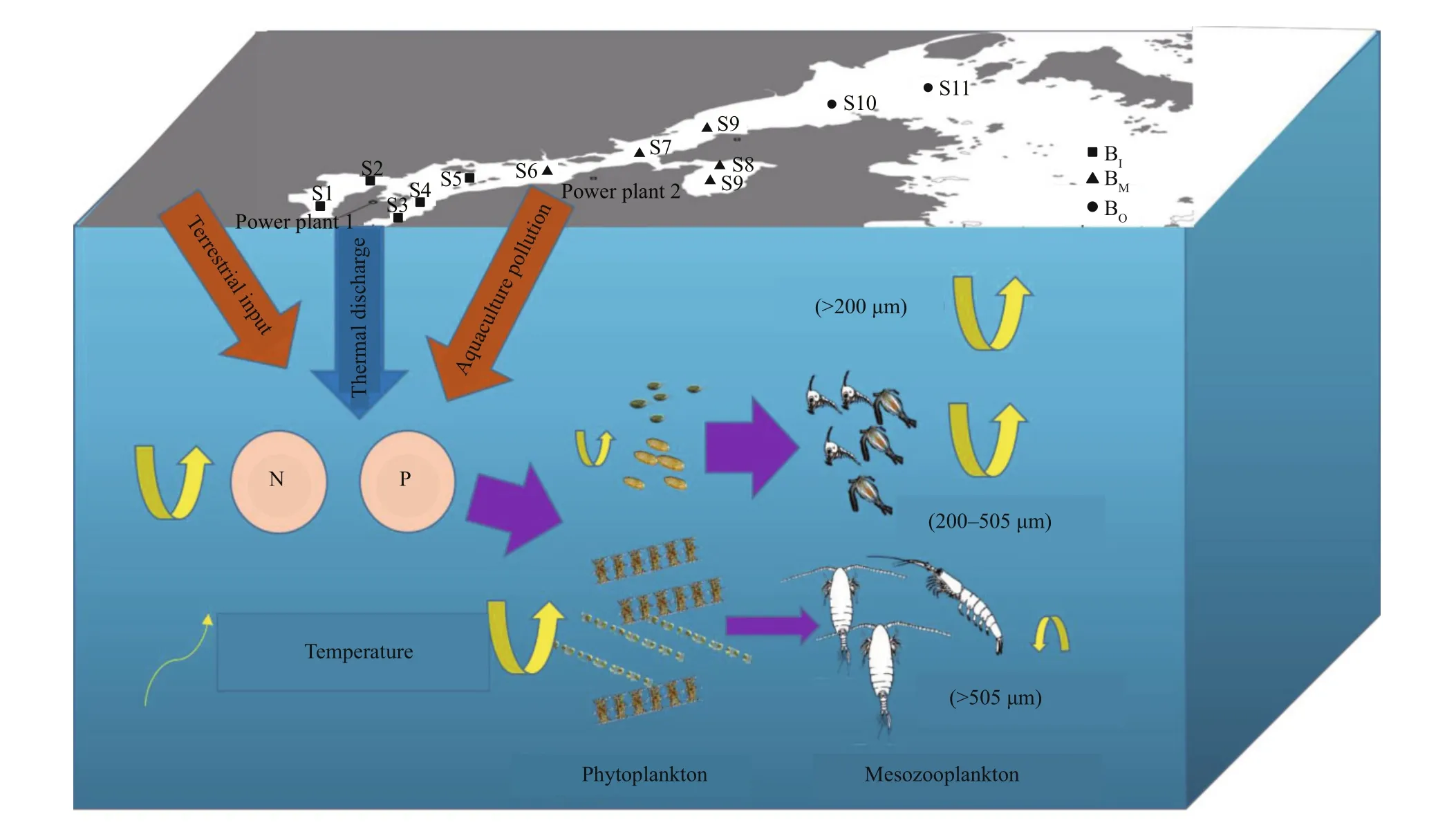
Fig.6 Schematic diagram of changes in the pelagic ecosystem of Xiangshan Bay, China
Given the trends of temperature, nutrients, Chla,and f isheries resources in the time series (Figs.2-5 and Supplementary Fig.S1), we propose the following scenario to explain the long-term changes in the pelagic ecosystem in the BIand BMof XSB since the 1980s (Fig.6). Eutrophication and warming increased food resource availability (increased phytoplankton biomass) for zooplankton (Jiang et al., 2019a) but caused zooplankton to decrease in size. Eutrophic waters are often characterized by larger phytoplankton species, and anthropogenic nutrient enrichment increases cell size at mid-high latitudes (Cloern,2018). Diatoms have always been dominant in XSB(relative abundance >80%); furthermore, some largecelled dinof lagellate species, which were not dominant in 2000 or 2006/2007, have become dominant since 2015 (Jiang et al., 2019a). The zooplankton miniaturization caused the large phytoplankton to lose eff ective feeding control; therefore, the micro-Chl-a(20-200 μm) contribution increased sharply and formed chronic blooms, especially in the BI(Jiang et al., 2019a). The mismatch between the phytoplankton supply and food demand of zooplankton prevented the energy from transferring to higher trophic organisms; thus, the transition from a high-energy food chain to a low-energy one caused the f isheries resources to decline in combination with overf ishing (ECBCC, 1992; Parsons and Lalli, 2002;Tang et al., 2012; Supplementary Fig.S1).
4.2 The pelagic ecosystem in the B O during spring was mainly aff ected by Changjiang River estuary water
Under the inf luence of the East Asian monsoon, the BOof XSB is subjected to southward-f lowing Changjiang Diluted Water during spring and winter(ECBCC, 1992; Zeng et al., 2017).Calanussinicusis the dominant LMZ species in Changjiang Diluted Water during spring, as well as the most important dominant species in the BOof XSB during spring. The biomasses of LMZ near the Changjiang River estuary during spring in 1999, 2001, and 2011 (674.28,542.18, and 415.6 mg/m3, respectively) were approximately two times higher than those in the late 1950s and 1980s (244.59 and 277.80 mg/m3) (Wang et al., 2004, 2016b), which is consistent with the trend of LMZ biomasses in the BOof XSB.
The increases in the total LMZ biomass in the spring of 1999 and 2001 were mainly caused by an increase in the number of the dominant speciesC.sinicus(Wang et al., 2004). A study on long-term zooplankton variations based on annual averages from 1982 to 2011 near the Changjiang River estuary found a slight, nonsignif icant upward trend in zooplankton abundance and biomass (Wang et al.,2016a). However, the abundance of most crustacean zooplankton ( Euphausiacea, Mysidacea, and Copepoda) decreased while gelatinous zooplankton(medusae and tunicates) and Polychaeta increased (Li et al., 2010; Wang et al., 2016a). Studies on the longterm variations in zooplankton in Tokyo Bay, the San Francisco Estuary, shallow regions of the northwestern Black Sea, and Chesapeake Bay, which suff ered from eutrophication, all revealed that crustacean zooplankton and fodder zooplankton decreased in abundance and size (Uye, 1994; Kideys et al., 2000;Glibert, 2010; Kimmel et al., 2012); however, once carnivorous gelatinous zooplankton (medusae and ctenophores) increased or invaded, the total zooplankton biomass and abundance increased(Kideys et al., 2000). These changes are not benef icial for f ishery resources (Parsons and Lalli, 2002).
In recent decades, the East China Sea shelf has experienced major environmental and ecological changes—including eutrophication, red tides,hypoxia, and warming—especially near the Changjiang River estuary (Li et al., 2010; Ning et al.,2011; Jiang et al., 2014; Kong et al., 2016). Medusae comprise the third largest group of zooplankton in the East China Sea (Xu, 2006). Medusa outbreaks have occurred in the northern East China Sea in successive years since the 1990s, especially after 2003 (Dong et al., 2010). High medusae abundance mostly occurred in the northern East China Sea outside the Changjiang Diluted Water during spring, while low abundance occurred in coastal waters, because the salinity ranges for most medusae species in the East China Sea are narrow, with the optimal salinity being 32-34 (Xu,2009), while the salinity ranges in XSB were 17-27.In surveys conducted over the last 15 years,scyphozoans, cubozoans, and their larvae have not been found in XSB. Only some hydromedusae (Eirenesp.,Ectopleurasp.,Aglaurahemistoma,Liriopetetraphylla), siphonophores (Diphyeschamissonis,Muggiaeaatlantica), ctenophores (Pleurobrachiaglobose), and Chaetognatha (Zonosagittabedoti,Flaccisagittaenf lata) often appeared in XSB,especially in the BO, from May to October, when the BOwas mainly aff ected by the northward-f lowing Taiwan Warm Current. Therefore, this bay is unlikely to be invaded by large medusae (scyphozoans or cubozoans) in the short term (Xu, 2009; Luo et al.,2012; Wang et al., 2012; Zuo et al., 2016).
5 CONCLUSION
We found regional and seasonal diff erences in the historical changes in the abundance and biomass of LMZ in XSB. The sharp abundance declines in the BIand BMduring winter and autumn resulted in a decline in the annual mean abundance of LMZ throughout the bay. The sharp biomass declines in the BIand BMduring the warm season (summer and autumn)resulted in the decline in the annual mean biomass throughout the bay. The signif icant decline in LMZ biomass in the BIand BMduring the warm season was coupled with simultaneous increases in nutrients and temperature. The mismatch between the phytoplankton supply and food demand of zooplankton prevented energy from being transferred to higher trophic levels.Thus the decline in the energy transfer effi ciency of the food chain, combined with overf ishing, likely resulted in the decrease in f isheries resources. The increase in the abundance and biomass of LMZ in the BOduring spring appeared to be aff ected by the Changjiang River estuary water. Fortunately, XSB is unlikely to be invaded by large medusae. We recommend that the ecosystem management and ecological restoration in subtropical semi-enclosed bays be focused on internal waters, which have a poor water exchange capacity. Regrettably, it is diffi cult to determine a quantitative relationship between zooplankton and environmental factors because of the lack of synchronous time-series data, as well as the complex interactions in the ecosystem. In addition,zooplankton changes may also be caused by other,more important, unknown factors, because we measured only a few parameters. Thus, continuous plankton monitoring in XSB should be performed in the future. Meanwhile, the trophic transfer effi ciency of food chains in subtropical bays, including microbial loops, warrants further study.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are included in this published article and its supplementary information f iles.
7 ACKNOWLEDGMENT
We are grateful to Xiaoya LIU, Jingjing ZHANG,and Xiangyu SUN in the SIO, MNR, China for their cooperation with the nutrient analysis. We are also grateful to the anonymous reviewers for improving the quality of the present manuscript.
 Journal of Oceanology and Limnology2021年6期
Journal of Oceanology and Limnology2021年6期
- Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
