Distinct root system acclimation patterns of seagrass Zostera japonica in sediments of diff erent trophic status: a research by X-ray computed tomography*
Xiaoyue SONG , Yi ZHOU , Jiangning ZENG , , Lu SHOU , Xiaomei ZHANG ,Shidong YUE , Wei GAO , Weihua FENG , Zhifu WANG , Ping DU
1 Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, China
2 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
3 College of Tropical Crops, Hainan University, Haikou 570228, China
4 Key Laboratory of Engineering Oceanography, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, China
Abstract Conspecif ic seagrass living in diff ering environments may develop diff erent root system acclimation patterns. We applied X-ray computed tomography (CT) for imaging and quantifying roots systems of Zostera japonica collected from typical oligotrophic and eutrophic sediments in two coastal sites of northern China, and determined sediment physicochemical properties that might inf luence root system morphology, density, and distribution. The trophic status of sediments had little inf luence on the Z. japonica root length, and diameters of root and rhizome. However, Z. japonica in oligotrophic sediment developed the root system with longer rhizome node, deeper rhizome distribution, and larger allocation to below-ground tissues in order to acquire more nutrients and relieve the N def iciency. And the lower root and rhizome densities of Z. japonica in eutrophic sediment were mainly caused by fewer shoots and shorter longevity, which was resulted from the more serious sulf ide inhibition. Our results systematically revealed the eff ect of sediment trophic status on the phenotypic plasticity, quantity, and distribution of Z. japonica root system, and demonstrated the feasibly of X-ray CT in seagrass root system research.
Keyword: Zostera japonica; root system; acclimation pattern; sediment; trophic status; X-ray computed tomography
1 INTRODUCTION
Seagrass are angiosperms unique in the marine environment, and seagrass meadows provide important habitats for other marine organisms.Around 70 seagrass species have adapted to neritic environments around the world (Short et al., 2007),including coastal rocky, sandy, and muddy substrates,and estuaries, bays, lagoons, mangrove forests, and coral reefs. Seagrass root and rhizome systems contribute to this adaptability, since they anchor against water f low and enable the exploitation of sediment-associated nutrients (Stapel et al., 1996;Rubio et al., 2007). Their relatively simple branching may also minimize the distance for oxygen transport from shoots to roots (Kiswara et al., 2009). Meanwhile,sulf ide, and especially dissolved H2S, is highly toxic for seagrass (Pedersen and Kristensen, 2015; Martin et al., 2019). Seagrass can aerate their below-ground tissues and lose oxygen from roots to prevent sulf ide intrusion from the surrounding sediment (Connell et al., 1999; Koren et al., 2015; Martin et al., 2019).Oxygen dynamics of seagrass roots alter sediment redox conditions and inf luence benthic biogeochemical processes (Martin et al., 2019), causing diff erences between bacterial communities associated with the roots and those associated with bulk sediments of seagrass meadows (Isaksen and Finster, 1996; Jensen et al., 2007; Ettinger et al., 2017). In addition, iron plaque formation on roots and rhizomes has been observed in seagrass and other aquatic macrophytes with implication for sulf ide intrusion (Povidisa et al.,2009).
There are distinct acclimation strategies among root systems of diff erent seagrass species. For instance, in coastal zones of northern China,Zosteramarinainhabits sediment andPhyllospadixiwatensisinhabits rocky areas.P.iwatensisdevelops a more complex root system with denser root hairs and inf lated root ends, facilitating its attachment to a rocky substrate(Li et al., 2019). Root architecture diff ers markedly between seagrass species, where relatively long-lived and slow-growing species are characterized by short internodes, fewer unbranched roots per node, and dense root hair; meanwhile, faster-growing species have more numerous roots per node (Kiswara et al.,2009). Belowground biomass of diff erent seagrass species in the same meadow show considerable vertical stratif ication within sediments, where larger species tend to extend deeper into the sediments than smaller ones (Duarte et al., 1998). This likely reduces potential interspecif ic competition for sediment resources, especially in the uppermost layers (Duarte et al., 1998). Some species show unique root system adaptations, such asPosidoniaoceanicaadults commonly lacking root hairs and regularly forming f lourishing mycorrhizae through a specif ic association with a single pleosporalean fungus (Borovec and Vohník, 2018; Vohník et al., 2019).
Seagrass have the capacity to acclimate their morphological, physiological, and mechanical traits to their local conditions, which is regarded as its phenotypic plasticity (Jensen and Bell, 2001;Bercovich et al., 2019). Seagrass leaves can acclimate to low light conditions though a variety of physiological and morphological mechanisms, like enlarging chloroplast density and specif ic leaf area, to enhance photosynthetic capacity (McDonald et al.,2016; Beca-Carretero et al., 2019). Conspecif ic seagrass in distinct environments will also develop diff erent root system acclimations patterns. Seagrass could achieve advantages in acquiring nutrients, by root architecture and root plasticity, in contrasting sediment types that diff er in nutrient availability, such as carbonaceous nutrient-poor sediments as well as muddy and nutrient-rich sediments (Erftemeijer and Middelburg, 1993; Kamp-Nielsen et al., 2002). The root systems of two temperate seagrass,PosidoniaaustralisandPosidoniasinuosa, display architectural and morphological plasticity with season, and to a lesser extent, nutrient addition (Hovey et al., 2012).Similarly, root lengths and nitrogen-obtaining patterns of seagrass in an off shore atoll diff ered from the same species in continental habitats in South China Sea(Jiang et al., 2019).
Zosterajaponicais widespread along coastal areas of the North Pacif ic Ocean and its meadows support a variety of marine ecosystem functions (Miki, 1933;Abe et al., 2009; Zhang et al., 2019), for example, theZ.japonicameadow in the Huanghe (Yellow) River estuary is one of the largest seagrass meadows in China and a key habitat for many marine organisms in the National Nature Reserve of Huanghe River estuary (Zhang et al., 2019). Despite its broad distribution and signif icant ecological role, there has been no specif ic research intoZ.japonicaroot systems. Multiple factors aff ect seagrass root systems,including hydrodynamics, illumination, nutrition, and substrate physicochemical characteristics. Previous investigations of typicalZ.japonicameadows in China indicated clear diff erences in the ratio of aboveground biomass to below-ground biomass in diff erent areas of distribution (Zhang et al., 2015, 2019). A better understanding of its root system acclimation to diff erent substrates will be useful for the conservation and restoration ofZ.japonicameadows and relevant seagrass ecosystem.
Previous investigations of seagrass root systems were carried out by rinsing attached sediment and using water to spread the roots for visual observation and analysis (Kiswara et al., 2009; Hovey et al., 2011;Jiang et al., 2019). Most of the time, root systems are treated simply as below-ground biomass, which results in the loss of in situ root system information.Several researchers have applied minirhizotron tubes to visualize and quantify the root standing crop,production, mortality, and lifespan ofZ.marinaroots(Johnson et al., 2016) and optical nanoparticle-basedsensors to visualize oxygen dynamics around rhizomes and roots (Koren et al., 2015; Martin et al.,2019). In recent decades, X-ray computed tomography(CT) has been used for imaging and quantifying 3D root systems of various plants (Tracy et al., 2010;Koebernick et al., 2014; Blaser et al., 2018), but this has not yet been applied to seagrass root systems.
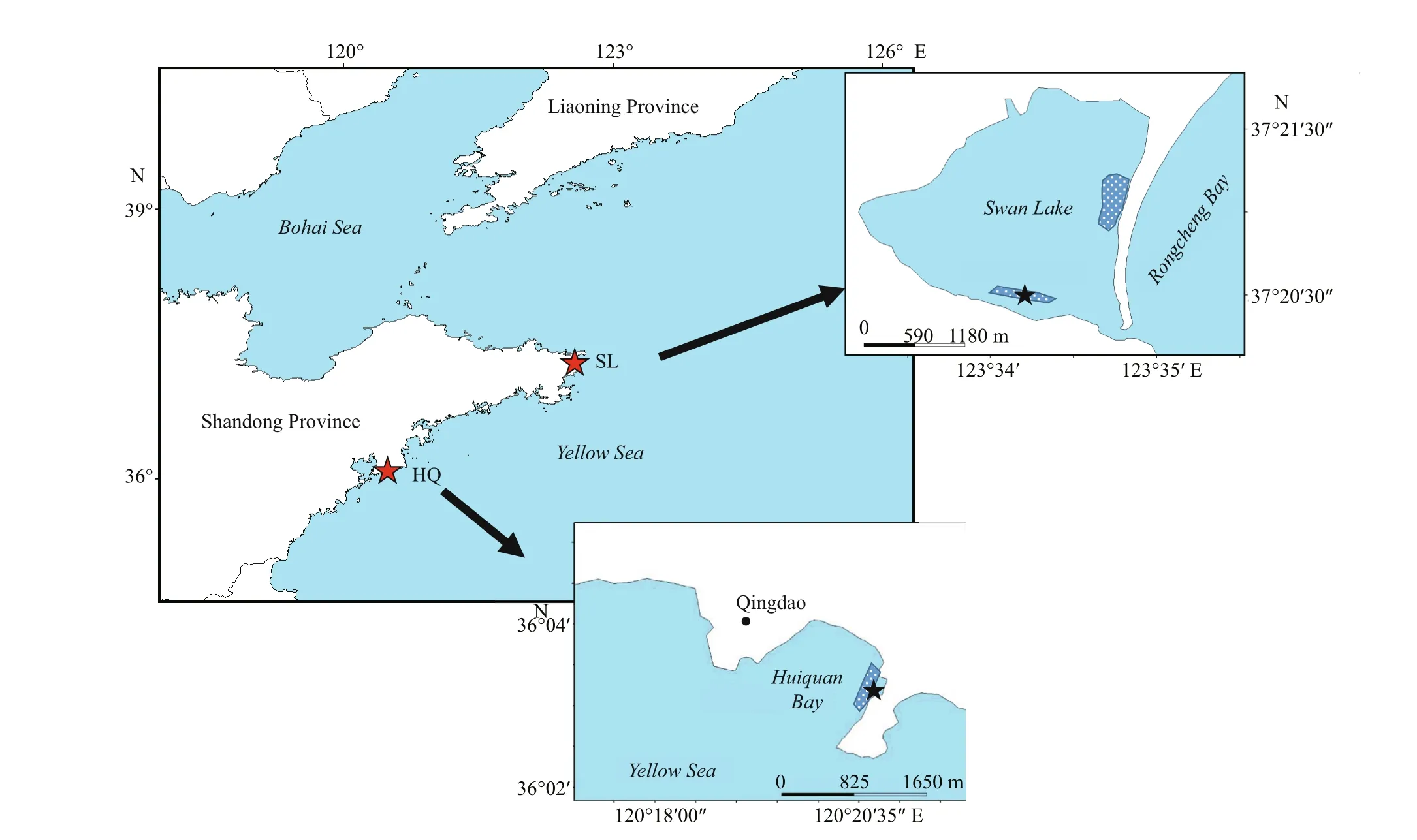
Fig.1 The locations of two Zostera japonica meadows and sampling sites of diff erent sediment trophic status, Swan Lake(SL) (37°21′24″N; 122°35′21″E) and Huiquan Bay (HQ) (36°3′34″N; 120°21′5″E) in Shandong Peninsula of China
In this study, we hypothesis thatZ.japonicaroot system in sediments of diff erent trophic status developed diff erent acclimation patterns, and the nutrient condition and sulf ide content diff erence therein were the primary reasons causing that. We collectedZ.japonicaroot and sediment samples from typical oligotrophic and eutrophic sediments and used X-ray CT imaging to observe the diff erences in root systems between these environments, then compared these with sediment physicochemical properties, with the aim of describing the mechanisms by whichZ.japonicaroot system acclimated to common sediment types of distinct trophic states.
2 MATERIAL AND METHOD
2.1 Site description and sampling
Our samples were collected fromZ.japonicameadows in a lagoon named Swan Lake in Weihai(37°21′24″N; 122°35′21″E) and Huiquan Bay in Qingdao (36°3′34″N; 120°21′5″E), Shandong Province, China (Fig.1). Persistent meadows ofZ.japonicaexist in both locations. Swan Lake has eutrophic sediment due to sewage input and relative closed lagoon topography (Zhang et al., 2014, 2015),the sampling site was located near the south bank where the inf luence of sewage was heavier than other area. Whereas sediment in Huiquan Bay is comparatively oligotrophic (Zhang et al., 2020).
Sampling of both locations was carried out during the summer of 2018 at 25-32 °C air temperature. We collected three samples 10-m apart in each location at low tide, with each sample consisting of a sediment core 11.5 cm in diameter and 15-cm deep. Cores were f ixed in polyvinyl chloride tubes and transported to a laboratory at 4 °C. Shoot density, total biomass,percentage of below-ground biomass, root length, and rhizome node length were obtained from a simultaneous investigation that sampled 10 quadrats(20 cm×20 cm) in each site, as described in Zhang et al. (2015, 2019). All shoots in each quadrat were placed in plastic bags and transported to the laboratory in darkness at low temperature.
2.2 X-ray computed tomography
The above-ground tissues of seagrass were removed and collected. Sediment cores were scanned using an industrial X-ray CT scanner(phoenix v|tome|x m, General Electric Company,U.S.A.) at 180 kV and 210 μA (40 W). A total of 1 800 equiangular projections were acquired through 360° with an exposure time of 2 000 ms,resulting in a scan time of 60 min for each core.The surfaces of cores were automatically recognized basing on the enormous diff erence between sediment and other objects, like air and polyvinyl chloride tubes. The acquired images were reconstructed using a f iltered back-projection algorithm in VGSTUDIO MAX software (Volume Graphics Company, Germany). The achieved resolution of 3D tomogram was 80 μm.CT images were processed using the “Rootine”method (Gao et al., 2019a, b). In brief, reconstructed 16-bit images were converted to 8-bit to reduce the image size in Fiji/ImageJ (National Institutes of Health, U.S.A). The converted images were f iltered according to non-local means. “Tubeness” f ilters were then applied at multiple scales to group tubular rhizomes and roots according to diameter. “3D Hysteresis thresholding” was used to binarize the greyscale “Tubeness” images. “Size opening” was then used to remove isolated segmentation noise. The length of rhizomes and roots were quantif ied voxel by voxel (90 μm×90 μm×90 μm) using “Skeletonize(2D/3D)” and “Analyze Skeleton” plugins in Fiji/ImageJ. Then the relative densities were calculated,and the root-per-rhizome length was acquired through the division of total root length by total rhizome length. The volume of rhizomes and roots was calculated by counting the voxel numbers of objects,and the relative densities were calculated. The quotients of the total volumes and lengths of rhizomes and roots were equal to their average cross-sectional areas; if they were assumed to be round, the approximate diameters of rhizomes and roots could be calculated. It is worth noting that the obvious upright thicker lines in superf icial layer, like those in HQ-1 and HQ-3, were sheathes of shoots, and were not calculated as rhizomes.
2.3 Analysis of nutrients of leaves and physicochemical properties of sediments
Zosterajaponicaleaves samples from 5 quadrats per site were lyophilized at -50 °C for 60 h, and carbon (C) and nitrogen (N) contents were measured using a PE2400 Series II elemental analyzer.Phosphorus (P) content was determined by using the phosphomolybdenum blue method modif ied for particulate total P determination (Zhou et al., 2003).And then the relative C/N and N/P were calculated.
Since X-ray CT results indicated that the root systems of all six samples were in the top 10 cm,sediment physicochemical analysis was also carried out in this range. The top 10 cm of sediment in each core sample was sliced and evenly divided into f ive subsamples (0-2-cm, 2-4-cm, 4-6-cm, 6-8-cm, and 8-10-cm depth). Grain size distribution was analyzed using a Laser Particle Counter (LS13320, Beckman,USA).Fresh sediment subsamples were lyophilized at-50 °C for 60 h and the water contents were determined by calculating their weight loss percentage. The lyophilized samples were sieved through 150-μm mesh prior to organic matter analysis. For total organic carbon (TOC) analysis, a sample was acidif ied with excessive 1-mol/L HCl to remove carbonates,and organic carbon content was determined using an elemental analyzer (PE2400 Series II, PerkinElmer,Norwalk, CT, USA). Total nitrogen (TN) contents were determined using the same elemental analyzer.Total phosphorus (TP) and acid volatile sulf ide (AVS)contents were determined using phosphomolybdenum blue (Zhou et al., 2003) and methylene blue spectrophotometric methods (Bradley and Stolt,2006), respectively.
2.4 Statistical analysis
Shoot density, total biomass, percentage of belowground biomass, root length, rhizome node length,root-per-rhizome length, densities and diameters of total roots and rhizomes, and leaf nutrient elements composition ofZ.japonicain Huiquan Bay and Swan Lake were compared using at-test in SPSS v. 20(IBM, U.S.A). Three-way mixed-eff ect ANOVA including site, depth, and random eff ect core was adopted to analyze the density diff erences of root length, root volume, rhizome length, and rhizome volume ofZ.japonica, and TOC, TN, TP, and AVS of sediments, respectively. Based on the results of above analysis, two-way mixed-eff ect ANOVA including depth and random eff ect core was adopted to analyze rhizome length, and rhizome volume densities in each site. Diff erences of those statistical analysis were considered signif icant atP< 0.05.

Table 1 Shoot, rhizome, and root characteristics of Zostera japonica
3 RESULT
3.1 Shoot, rhizome, and root characteristics of Z. japonica
The s hoot, rhizome, and root characteristics ofZ.japonicain Huiquan Bay and Swan Lake were compared and presented in Table 1 & Supplementary Table S2. Based on collections ofZ.japonicafrom quadrats, shoot density, and total biomass were about 2.5-fold greater in Huiquan Bay than Swan Lake(shoot density:t(18)=2.965,P< 0.05; total biomass:t(18)=4.064,P<0.05). Meanwhile, the percentage of below-ground biomass ofZ.japonicain Huiquan Bay was signif icantly higher than that in Swan Lake by the 1.3-fold (t(18)=3.044,P<0.05). There was no statistical diff erence between the root lengths ofZ.japonicain two sites (t(18)=-0.323,P>0.05).However, the rhizome node length ofZ.japonicain Huiquan Bay was signif icantly longer than that in Swan Lake (t(18)=2.712,P<0.05). Based on CT scans of cores, root and rhizome densities were about 4.3-and 3.7-fold higher in Huiquan Bay than Swan Lake(root length density:t(4)=4.198,P<0.05; root volume density:t(4)=4.004,P<0.05; rhizome length density:t(4)=2.926,P<0.05; rhizome volume density:t(4)=2.847,P<0.05). The root-per-rhizome length ofZ.japonicain Huiquan Bay was not statistically diff erent from that in Swan Lake (t(4)=-0.146 4,P> 0.05). Moreover, there were no signif icant diff erences between the diameters of both root and rhizome ofZ.japonicain two research sites (root diameter:t(4)=-0.696 4,P>0.05; rhizome diameter:t(4)=-0.455 6,P>0.05).

Table 2 Nutrient elements compostion of Zostera japonica leaves
3.2 Nutrient elements composition of Z. japonica leaves
The C, N, and P contents and the C/N, N/P ofZ.japonicaleaves in Huiquan Bay and Swan Lake were presented in Table 2. There were no signif icant diff erences between the C and P contents ofZ.japonicaleaves in two sites (C:t(8)=-0.483 7,P>0.05; P:t(8)=0.062,P>0.05). However, the N content ofZ.japonicaleaves in Huiquan Bay was lower than that in Swan Lake (t(8)=4.639,P<0.01).Furthermore, the C/N ofZ.japonicaleaves in Huiquan Bay was signif icantly higher than that from Swan Lake (t(8)=7.313 7,P<0.01); however, the N/P ofZ.japonicaleaves in Huiquan Bay was signif icantly lower (t(8)=-2.794,P<0.05).
3.3 Root system images and distribution
The 3D X-ray CT data were projected to show the vertical distribution of roots and rhizomes in two dimensions (Fig.2). The roots were showed in thinner lines of yellow color, and rhizomes were showed in thicker lines of purple color. Generally, below-ground materials were denser at Huiquan Bay than Swan Lake and also distributed deeper in the sediment(Fig.3; Table 3).
In Swan Lake, maximum root length density occurred in the top layer at 3.42±1.28 mm/cm3(Fig.3a& Supplementary Table S2). Conversely, in Huiquan Bay, maximum root density occurred in the 4-6-cm layer at 11.45±1.61 mm/cm3. In Swan Lake, the main proportion of root length (85.92%) was located in the upper layers of 0-6 cm, while in Huiquan Bay, themain proportion (77.27%) was observed in the 6-10-cm layers (Fig.3a). According to corresponding result of the three-way ANOVA for mixed eff ects including core (Table 3), signif icant diff erence existed between the root length densities ofZ.japonicain Huiquan Bay and Swan Lake, however, they were not aff ected by depth (site:F(1,19)=16.461,P<0.001; depth:F(4,19)=0.920,P>0.05). Root volume density followed a similar statistical pattern to root length density (Fig.3b) (site:F(1,19)=16.690,P<0.001;depth:F(4,19)=0.934,P>0.05), given that root diameters were similar between sites (Table 1).

Table 3 Results of three-way mixed-eff ect ANOVA of Zostera japonica root and rhizome densities in Huiquan Bay (HQ) and Swan Lake (SL)
There was no statistical diff erence between the diameters of rhizomes in two sampling sites (Table 1). The maximum rhizome length density ofZ.japonicain Huiquan Bay was 3.67±0.24 mm/cm3at 2-4 cm, compared to 1.61±0.58 mm/cm3at 0-2 cm in Swan Lake. In Huiquan Bay, the rhizome at the 0-6 cm accounted for 87.47% of the total rhizome length, however, the rhizome at 0-4 cm accounted for 86.38% of the total rhizome length in Swan Lake(Fig.3c). According to corresponding result of the three-way ANOVA for mixed eff ects including core(Table 3), there was a signif icant diff erence between the rhizome length densities ofZ.japonicain Huiquan Bay and Swan Lake, and which was also signif icantly aff ected by depth eff ect (site:F(1,19)=8.988,P<0.01;depth:F(4,19)=3.479,P<0.05). On the basis of the two-way ANOVA and multiple comparison for mixed eff ects including core, in Huiquan Bay, although there was no statistical diff erence among that rhizome length densities of diff erent depths (F(4,8)=2.238,P> 0.05), the rhizome volume densities in layers of 2-4 cm was signif icantly higher than in 8-10-cm layers (Fig.3d) (F(4,8)=4.278,P<0.05); meanwhile,in Swan Lake, the rhizome length and volume densities in 0-4-cm layers were both higher than those in 6-10-cm layers (Fig.3c & d) (rhizome length density:F(4,8)=6.845,P<0.05; rhizome volume density:F(4,8)=3.900,P<0.05).
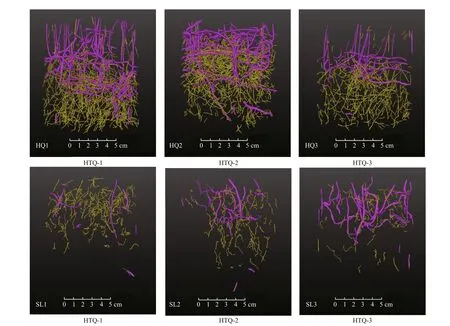
Fig.2 Zostera japonica root systems visualized as 2D vertical projection of the 3D data extracted from X-ray CT images,which were collected from Huiquan Bay (HQ) and Swan Lake (SL), respectively
3.4 Physicochemical properties of sediments
3.4.1 Grain size distribution
There was no signif icant diff erence between Huiquan Bay and Swan Lake in sediment type. The sediments of top 4 cm in two sites both mainly consisted of the sand and gravel component (Huiquan Bay: 90.28%±9.73%; Swan Lake: 69.39%±21.79%),and the main components of sediments in 4-10 cm were silt (Huiquan Bay: 43.90%±18.26%; Swan Lake: 44.87%±11.47%) and sand and gravel (Huiquan Bay: 51.71%±24.86%; Swan Lake: 34.57%±12.65%).
3.4.2 Total organic carbon and total nitrogen
The quantif ication and analysis of the chemical properties were presented in Fig.4, Table 4, &Supplementary Table S1. Swan Lake sediment had accumulated more organic matters. TOC and TN contents at Swan Lake were signif icantly higher than at Huiquan Bay (TOC:F(1,19)=464.296,P< 0.001;TN:F(1,19)=389.057,P<0.001). For the same layer,TOC content of sediment at Swan Lake was about 6.2-fold greater than at Huiquan Bay, and TN content was 12.1-fold greater. There were no signif icant eff ects of depth on the TOC and TN contents of all samples (TOC:F(4,19)=2.522,P> 0.05; TN:F(4,19)=1.323,P> 0.05). As the great disparity in organic matter and nitrogen contents, the sediments in Swan Lake and Huiquan Bay were regarded as eutrophic and oligotrophic, respectively.
3.4.3 Total phosphorus and acid volatile sulfate
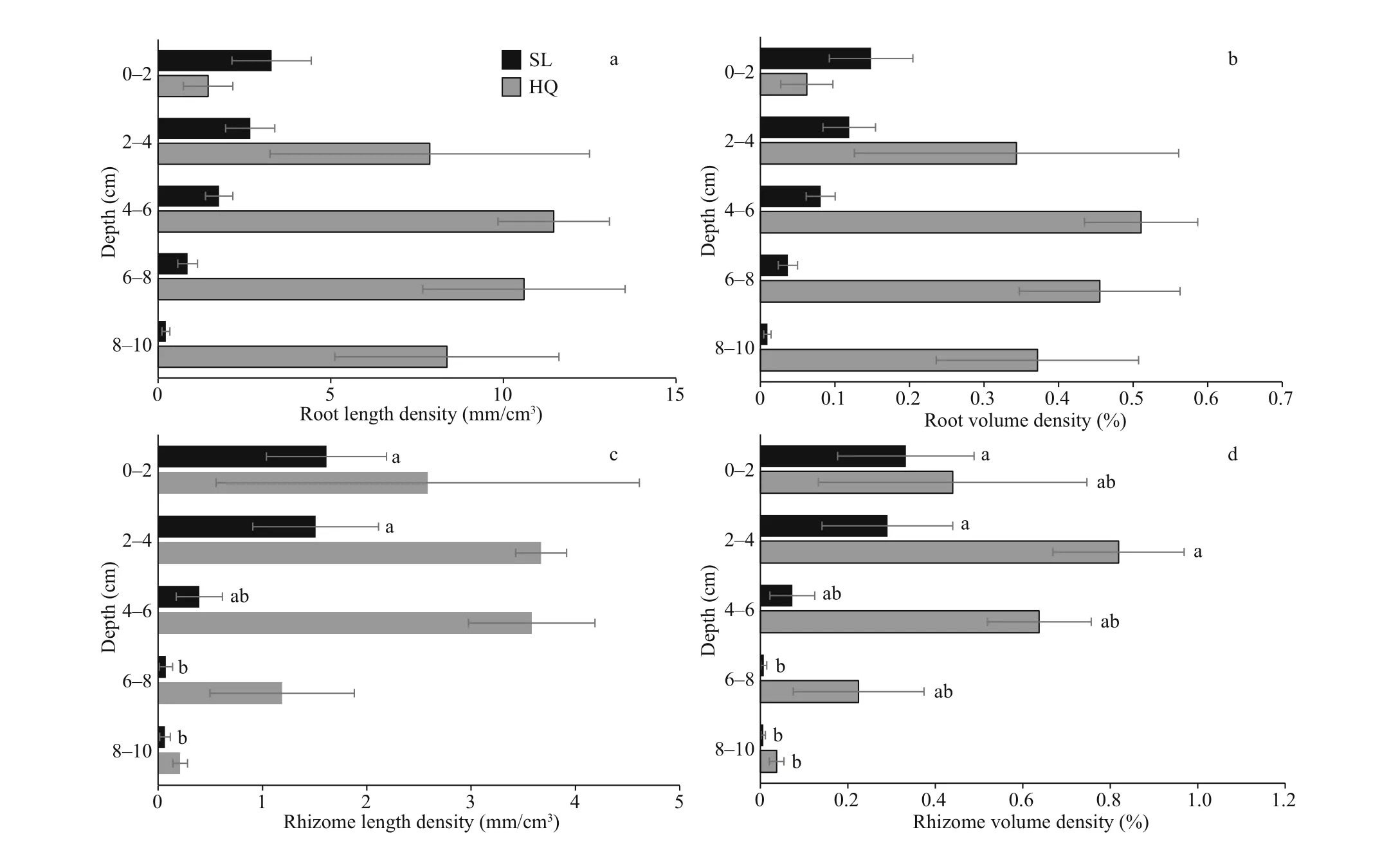
Fig.3 Root (a, b) and rhizome (c, d) densities of Zostera japonica from Huiquan Bay (HQ) and Swan Lake (SL) in diff erent layers

Fig.4 Total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), and acid volatile sulf ide (AVS) contents in sediments of Zostera japonica core samples from Huiquan Bay (HQ) and Swan Lake (SL)
TP content at Swan Lake was signif icantly lower than at Huiquan Bay (F(1,19)=241.975,P<0.001),and 2.6-fold greater at Huiquan Bay in corresponding layers. Depth has signif icant eff ect on TP in three-way ANOVA (F(4,19)=4.315,P< 0.05).
In Huiquan Bay, the minimum and maximum average AVS contents respectively appeared at 0-2 cm and 8-10 cm (Fig.4). The maximum AVS content in Swan Lake appeared at 2-4 cm, and the minimum was in 8-10 cm (Fig.4). The AVS in Swan Lake was signif icantly higher than that in Huiquan Bay (F(1,19)=15.964,P< 0.01), and no signif icant eff ect of depth on AVS was observed according to the two-way ANOVA (F(4,19)=0.92,P< 0.05).
4 DISCUSSION
By the use of X-ray CT technology for the rhizomes and roots ofZ.japonicain oligotrophic and eutrophicsediments, we found the signif icant diff erences in root system morphology, density, and distribution.Through the analysis combing chemical properties of sediments and nutrients of seagrass leaves, the reasons contributing to the diff erence of root and rhizome acclimation patterns in sediments of distinct trophic status were addressed. In addition, the advantages and disadvantages of the application of X-ray CT technology in seagrass research are discussed.

Table 4 Three-way mixed-eff ect ANOVA of chemical properties of sediments in Zostera japonica meadows in Huiquan Bay (HQ) and Swan Lake (SL)
4.1 Similarities and diff erences of Z. japonica root system acclimation characteristics
Here, we compared the morphology, density, and distribution characteristics of roots and rhizomes ofZ.japonicain oligotrophic and eutrophic sediments.The trophic status of sediments had little inf luence on the root length, root and rhizome diameters ofZ.japonica, which was demonstrated by Table 1. This result was consistent with the study of Kiswara et al.(2009) on root systems of six tropical seagrass species in Indonesian waters, which suggested that diff erences in sediment nutrient availability had little eff ect on root architecture parameters, such as length and diameter of per root order. However, the similarity in root lengths from sediments with diff erent trophic status was inconsistent with several researches suggesting that seagrass in oligotrophic environment tended to own longer root length (Cabaço et al., 2009;Jiang et al., 2019). The longer root of seagrass is benef icial to obtain adequate nutrients, and rhizomes with a wider diameter allow the storage of carbohydrates and nitrogenous compounds (Vermaat,2009). In our research,Z.japonicahad not adopted these traits to acclimate the oligotrophic sediment.The comparison of our and previous researches suggested that under diff erence of sediment tropic status, the seagrass phenotype plasticity in root system diff ers among seagrass species.
However,Z.japonicain oligotrophic sediment owned longer rhizome node (Table 1). This result was consistent with the previous study onZ.noltii(Cabaço et al., 2009). There is obvious positive correlation between the node length and growing speed of seagrass (Kiswara et al., 2009; Vermaat, 2009). Thus,the longerZ.japonicanode length showed its relative rapid expanding rate of rhizomes in oligotrophic sediment. In addition,Z.japonicarhizome preferred to constrict its range to upper sediment layer, and its rhizome vertical distribution in oligotrophic sediment was wider. Although the mixed-eff ect ANOVA indicated the inf luence of depth on rhizome volume densities in both oligotrophic and eutrophic sediments,depth only had signif icant inf luence on the rhizome length density in eutrophic sediment of Swan Lake(Table 3). In addition, the length density was the preferable indicator for the root system distribution characteristics (Li et al., 2014; Gao et al., 2019a).Thus, theZ.japonicarhizome in oligotrophic sediment distributed relatively deeper than that in eutrophic sediment.
The trophic status of sediments inf luenced the rhizome and root densities ofZ.japonica, those in oligotrophic sediment were obviously higher(Table 1). The three-way ANOVA indicated that site,which was featured with distinct sediment trophic status, was the dominant eff ect on rhizome and root densities ofZ.japonica(Table 3). This is consistent with previous studies showing that seagrass species in low-nutrient habitats exhibited more extensive root systems to enhance their potential for acquiring nutrients from the sediments (Perez et al., 1994;Touchette and Burkholder, 2000; Hovey et al., 2011).However, theZ.japonicaroot system of higher density in oligotrophic sediment mainly ref lected its corresponding higher shoot density. The signif icant diff erence of below-ground biomass percentages(Table 1) contributed to explain the distinct root system densities in two sites, but that of seagrass in oligotrophic sediment was merely 1.32-fold than eutrophic sediment. The root and rhizome length densities of Huiquan Bay were about 4.3- and 3.2-fold than those of Swan Lake in respective, thus shoot density should be the primary eff ect leading to the distinct root system densities. Meanwhile, the rootper-rhizome lengths without signif icant diff erence in two sites (Table 1) meant the below-ground parts of seagrass in either site had not allocated more to their root development, and that helped to support the signif icance of shoot density for the diff erence of root system density. Besides the shoot density, the root system longevity diff erence between twoZ.japonicameadows was the other signif icant factor leading to their distinct densities. According to our previous research on these two seagrass meadows, about 6%shoots in the Swan Lake were recruited by overwintering rhizomes, but that percentage in Huiquan Bay reached 66% (Zhang et al., 2020).
4.2 Reasons causing diff erent Z. japonica root system acclimation characteristics
In this study,Z.japonicain oligotrophic sediment endured N def iciency, and the sulf ide, as a phytotoxin,threated the living ofZ.japonicain eutrophic sediment.Seagrass derive N and P from sediment pore water(especially ammonium) and the water column (most nitrate), and the ratios of carbon, nitrogen and phosphorous (C꞉N꞉P) of seagrass leaves tend towards 550꞉30꞉1, which was called “seagrass Redf ield ratios”(Atkinson and Smith, 1983). Duarte (1990) suggested that when N and P contents of seagrass leaves are lower than 1.8% and 0.2%, respectively, the plants are strongly nutrient limited, but not vice versa. In our study, the P contents ofZ.japonicaleaves in both sites were higher than 0.2% (Table 2), and the N/P of leaves from both sites were obviously lower than 30, thus it did not appear to be restricted by P. And the leaf N content and C/N in Swan Lake suggested thatZ.japonicain the eutrophic sediment hadn’t suff ered N def iciency, but corresponding values in Huiquan Bay demonstrated thatZ.japonicain the oligotrophic sediment was under N def iciency. The sulf ide of higher concentration resulted in more serious intrusion toZ.japonicain eutrophic sediment. Sulf ide intrusion in seagrass is widespread in all climate zones where seagrass are growing. The sulf ide toxicity and physiological stress can lead to deleterious eff ects of seagrass like growth performance reduction and dieback (Cambridge et al., 2012; Apostolaki et al.,2018). The research reviewed the sensitivity thresholds for sulf ide of diff erent plant species showed that the sulf ide toxicity threshold levels ofZosterawas 200-1 800 μmol/L (Lamers et al., 2013). Converted to the unit in our research, with the corresponding sediment moisture content, that range was about 1.6-14.4 μg/g.Because the sulf ide contents in eutrophic sediment of Swan Lake surpassed that range in general (Fig.4,Supplementary Table S2), the damage by this phytotoxin would be more severe.
Here, we addressed the reasons caused the diff erences of morphology, density, and distribution characteristics ofZ.japonicaroot system in sediments of distinct trophic status. The development of seagrass rhizome and root and its phenotypic plasticity are closely aff ected by nutrients available (Kiswara et al.,2009; Hovey et al., 2012). The relative rapid expanding rate, which ref lect as its longer node ofZ.japonicarhizome in oligotrophic sediment should be interpreted by the lower nitrogenous nutrients availability (Table 4). The explanation of N def iciency impact was consistent with previous study onZ.noltiiwhich owned longer internodes at low intertidal with less organic matter, lower N content (Cabaço et al.,2009). It is reasonable to infer thatZ.japonicain oligotrophic sediment relatively speeded up its root system development through high rhizome expanding rate in order to acquire more nutrients. Moreover, the relative deeper distribution ofZ.japonicarhizome in oligotrophic sediment (Table 3; Fig.3) should be attributed to N def iciency impact. The deeper rhizome distributing range of seagrass facilitate it to absorb nutrients from wider space (Delgard et al., 2016), that also indicated its urgency to relieve N def iciency.Meanwhile, the relative shorter rhizome node and shallower rhizome distribution in eutrophic sediment could be interpreted by the oxygen transport convenience. Seagrass transport oxygen to belowground part to support aerobic root respiration and protect against sulf ide intrusion (Connell et al.,1999; Martin et al., 2019). Even though the root length did not diff er signif icantly, the shorter rhizome node and shallower rhizome distribution in eutrophic sediment shortened the distance from seagrass leaves to root tips, and facilitatedZ.japonicato respirate and defend sulf ide in sediment with more sulf ide accumulation.
The shoot density diff erence ofZ.japonica, which was the primary reason caused diff erent root system densities, was resulted from the more serious sulf ide inhibition in eutrophic sediment (Table 4; Fig.4),rather than the eff ect of N def iciency in oligotrophic sediment. According to the investigation forZ.japonicameadow near the east bank of Swan Lake(Zhang et al., 2015), the leaf N content surpassed 1.8% and C/N value was less than 18.3, which indicated thatZ.japonica in this site hadn’t endured N def iciency. However, theZ.japonicadensity surpassed 3 356 shoots/m2, and was signif icantly larger than the 2 346 shoots/m2in our research. Hence,the impact of N def iciency was not the reason of higher shoot density in oligotrophic sediment.Although there was no sediment sulf ide content data of Zhang’s investigation (Zhang et al., 2015), our past survey in Swan Lake had recorded the sediment AVS contents inZ.japonicameadow near the east bank(Song, 2018), which was just about one third of the AVS level ofZ.japonicameadow sediment near the south bank in this study. Comparing the shoot densities and AVS contents in Huiquan Bay and the east and south banks of Swan lake, the sulf ide inhibition was demonstrated to be the main reason resulting in the less shoots in eutrophic sediment. The f inding on seagrass shoots reduction by sulf ide was consistent with other researches that demonstrated seagrass meadow deterioration with increased loading of organic matter and sulf ide accumulation (Borum et al., 2005; Calleja et al., 2007).
4.3 Advantages and disadvantages of the application of X-ray CT technology
The study also represents the f irst use of X-ray CT scanning to produce 3D images and quantitative data of seagrass root systems, and demonstrates this approach as feasible for future research. The 3D imagine could provide visual impression ofZ.japonicarhizomes and roots, and would be benef icial to grasp their morphological characteristics intuitively. The application of X-ray CT technology enhanced the effi ciency of measuring seagrass roots and rhizomes, because the relevant scanning,recognition, measuring, and calculating work are carried out automatically. Meanwhile, the whole procedure was non-destructive for core sample, thus it revealed the distributing information of seagrass roots and rhizomes as real as natural state, and avoid possible manual harm to them in traditional measurement. Furthermore, the digitalization of seagrass below-ground tissues makes more accurate and delicate measuring to be feasible, for instance, the root and rhizome densities ofZ.japonicain this research could be presented in hundreds of layers.
However, there are several disadvantages of X-ray CT technology emerged during our work, which remain to be considered or resolved in such researches of future. At f irst, the detection limitation of CT scanning was about 2-3 times the side length of voxel(Kaestner et al., 2006), that means the small roots whose diameter less than about 200 μm could not be recognized. However, the traditional approach by human eyes can recognize these small roots with ease.Meanwhile, though the imaging was powerful, the recognition function of software adopted could not automatically distinguish and acquire the detailed morphological characteristics like root length,rhizome node length, and roots amount of each node.Moreover, such information was supplemented by manual measurement work carried out synchronously in the same locations. Moreover, the size of core sample is restricted by the volume capacity of X-ray CT scanner. In spite of these disadvantages, the advantages of X-ray CT technology over traditional investigation methods be of benef it in exploring morphological, physiological, and ecological characteristics of seagrass below-ground tissues, and it still owns potential to be improved.
5 CONCLUSION
We used X-ray CT scanning to observe a diff erence in root and rhizome acclimation ofZ.japonicaliving in oligotrophic and eutrophic sediments. Morphology measures, and distribution of rhizomes and roots demonstrated thatZ.japonicain oligotrophic sediment, developed the root system with longer rhizome node, deeper rhizome distribution, larger allocation to below-ground tissues in order to acquire more nutrients and relieve the N def iciency, and the shorter rhizome node and shallower rhizome distribution ofZ.japonicain eutrophic sediment facilitate its oxygen transport. The lower root and rhizome densities ofZ.japonicain eutrophic sediment were mainly caused by fewer shoots and shorter longevity, which was resulted from the more serious sulf ide inhibition. Moreover, to the best of our knowledge, this is the f irst study to use X-ray CT methods in this manner, and demonstrates their feasibility for future seagrass research.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available upon request by contact with the f irst or corresponding author.
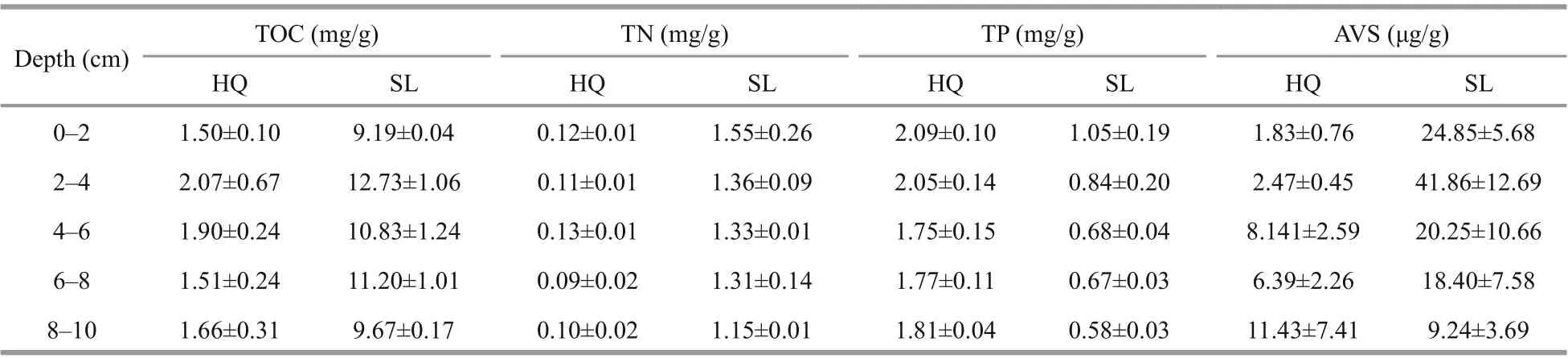
Supplementary Table S1 Chemical index values in sediments of Zostera japonica meadows from Huiquan Bay (HQ) and Swan Lake (SL)
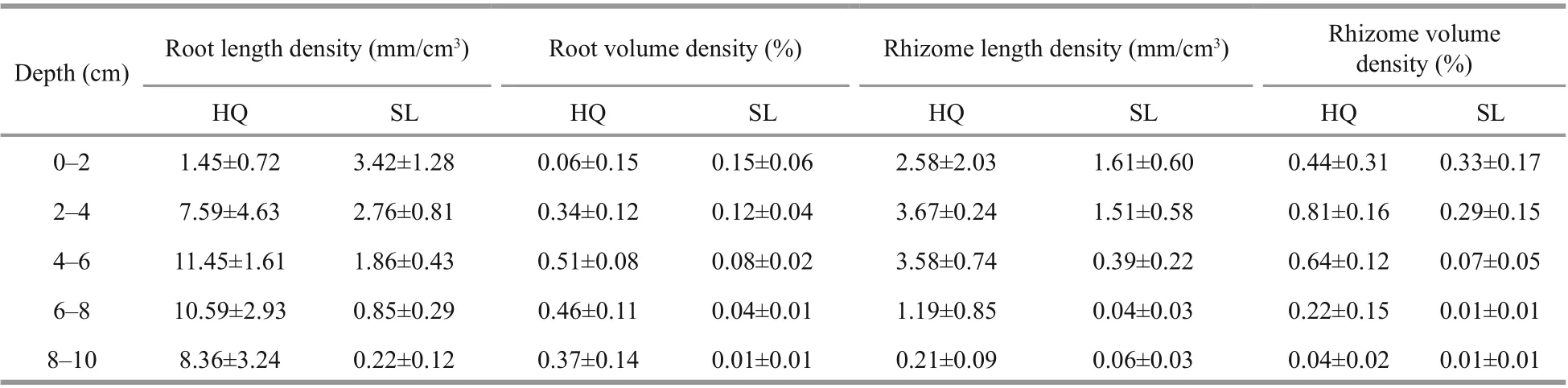
Supplementary Table S2 Root and rhizome densities values of Zostera japonica in Huiquan Bay (HQ) and Swan Lake (SL)
7 ACKNOWLEDGMENT
We are indebted to the help from Yumeng JIANG and Han YANG in Zhejiang Gongshang University,staff in the Environmental assessment laboratory of Key Laboratory of Engineering Oceanography,Second Institute of Oceanography, MNR, especially for their director, Dr. Hengtao XU. We also are grateful for our colleagues for their assistance during this work.
 Journal of Oceanology and Limnology2021年6期
Journal of Oceanology and Limnology2021年6期
- Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
