Redox control of magnetosome biomineralization*
Yingjie LI
State Key Laboratory of Microbial Technology, Shandong University, Qingdao 266237, China
Abstract Magnetotactic bacteria can orientate in the Earth’s magnetic f ield to search for their preferred microoxic environments, which is achieved by their unique organelles, the magnetosomes. Magnetosomes contain nanometer-sized crystal particles of magnetic iron minerals, which are only synthesized in lowoxygen environments. Although the mechanism of aerobic repression for magnetosome biomineralization has not yet fully understood, a series of studies have verif ied that redox modulation is pivotal for magnetosome formation. In this review, these advances in redox modulation for magnetosome biosynthesis are highlighted, mainly including respiration pathway enzymes, specif ic magnetosome-associated redox proteins, and oxygen- or nitrate-sensing regulators. Furthermore, their relationship during magnetosome biomineralization is discussed to give insight into redox control and biomineralization and inspire potential solutions for the application of respiration pathways to improve the yields of magnetosome.
Key word: magnetotactic bacteria; magnetosome; biomineralization; respiration; redox control
1 INTRODUCTION
Magnetotactic bacteria (MTB) biomineralize crystals of the iron oxide magnetite (Fe3O4) or the iron sulf ide greigite (Fe3S4) inside membrane-enveloped organelles termed magnetosomes (Fig.1). These mineral particles have inherent magnetic properties,and therefore are exploited by MTB for orientating the earth’s geomagnetic f ield to search for their preferred low oxygen environment (Jogler and Schüler, 2009). A huge diversity of the shape and size of magnetite or greigite nanoparticles is biosynthesized by diff erent MTB (Faivre and Schüler, 2008; Schüler,2008). Due to their relative ease of growth in the laboratory and genetic tractability, two closely related alphaproteobacteria,MagnetospirillumgryphiswaldenseMSR-1 (MSR-1) andMagnetospirillummagneticumAMB-1 (AMB-1),which produce single chains of cuboctahedral magnetite particles, have been used as model organisms for the mechanistic understanding of biomineralization. Over the past decades, genetic studies by using these twoMagnetospirillumspecies make signif icant progress in the identif ication of genes involved in magnetosome formation, which are clustered in a more than 100-kb genomic region,magnetosome island (MAI; Ullrich et al., 2005; Murat et al., 2010; Lohße et al., 2011). Although the mechanism of magnetosome formation has not been completely elucidated, a stepwise process for the synthesis and assembly of magnetite nanoparticles has been proposed: (i) invagination of magnetosome vesicles from cytoplasmic membrane, (ii) iron uptake,and (iii) magnetosome biomineralization and chain formation. Diff erent proteins arrested at various stages of magnetosome formation by individual deletion of each gene are summarized in some excellent reviews (Uebe and Schüler, 2016;McCausland and Komeili, 2020). In addition, it is suggested that the synthesis of mixed-valence iron oxide magnetite could occur by coprecipitation of ferric and ferrous iron in supersaturating concentrations, which thereby requires a proper ratio of ferrous and ferric iron (Mann et al., 1990; Faivre et al., 2004, 2007). This may be the reason why magnetite crystals are only biomineralized under microaerobic and anaerobic conditions, whereas atmospheric oxygen concentrations entirely inhibit the biosynthesis of magnetosomes. In this review, the author highlights the developments in understanding of the relationship between redox processes and biomineralization, and describes the mechanisms of redox associated proteins aff ecting magnetosome formation.
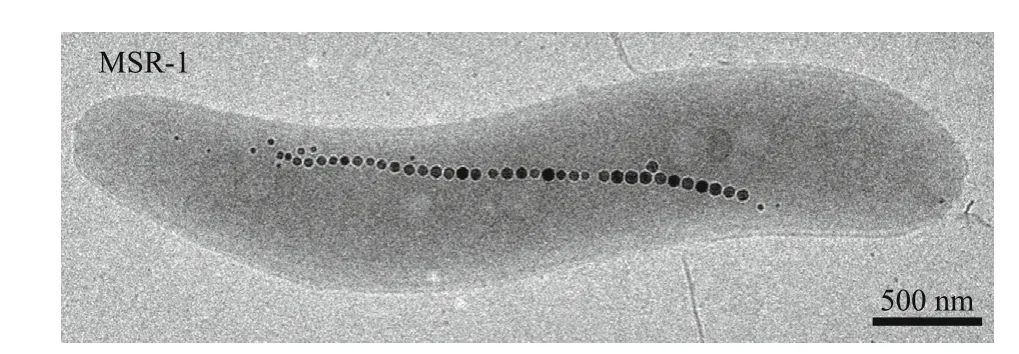
Fig.1 Magnetospirillum gryphiswaldense MSR-1 and magnetosomes
2 EFFECTS OF ENVIRONMENTAL OXYGEN ON MAGNETOSOME FORMATION
Since magnetosomes were early found to comprise of magnetite, oxygen molecules of Fe3O4was f irstly proposed to come from air. Therefore, early hypotheses focused on whether a competition for oxygen occurs between respiration and biomineralization (Blakemore et al., 1985). However,later isotope experiments demonstrate that oxygen molecules bound in biologically synthesized Fe3O4are not derived from O2but water (Mandernack et al.,1999). In spite of this, instead of being directly incorporated into Fe3O4, O2appears to be a crucial environmental factor for magnetite biomineralization.This speculation has been proposed based on the observations that magnetite crystals are only produced in low-oxygen environment while aerobic conditions completely inhibit their formation. Moreover, inMagnetospirillummagnetotacticum(MS-1), it has been found that changes of O2concentration clearly aff ect the synthesis of some proteins. For example, an increase of O2content resulted in enhanced activity of a manganese-type superoxide dismutase compared to an iron-type superoxide dismutase (Short and Blakemore, 1989). Also in the same strain, Sakaguchi et al. (1993) found that the presence of O2repressed the synthesis of a 140-kDa membrane protein. An O2-shift growth experiment on MSR-1 also showed some links between oxygen concentrations and magnetosome formation: after shifting aerobic MSR-1 strain to microaerobic conditions, cells remained non-magnetic for about 2 h. When MSR-1 cells were shifted from aerobic to anaerobic conditions, cells started to display a magnetic response only after about 1 h, which is faster than those shifted to microaerobic conditions (Supplementary Fig.S1). Therefore, it was initially speculated that the observed lag in magnetite biomineralization might be directly caused by perturbed redox conditions for iron oxidation, or alternatively, by deregulated expression of proteins involved in magnetosome formation.However, further studies on MSR-1 verif ied that the expression of key magnetosome proteins encoded in the genomic magnetosome island is not likely regulated by the concentrations of oxygen, a conclusion drawn based on the following observations:(i) the transcription of genes encoding magnetosome membrane proteins (Mam and Mms) is not regulated by oxygen (Schübbe et al., 2006; Wang et al., 2016);(ii) all tested magnetosome proteins, including MamA, MamC, MamK, and MamM are observed within the cell under aerobic conditions, and when quantif ied by immunodetection, these proteins show similar expression levels under diff erent oxygen conditions (Supplementary Fig.S2); (iii) nonmagnetic MSR-1 cells cultured under high O2conditions still form empty vesicles, while no electron-dense nanoparticles are formed (Raschdorf et al., 2016).Therefore, the question arose as to whether the aerobic repression for magnetite biomineralization is indirectly caused by changed cellular redox homeostasis, which thereby alters the redox state of the mineral.
3 DENITRIFICATION AND MAGNETITE BIOMINERALIZATION
In many anaerobic and microaerophilic bacteria such as MTB, one of the major redox pathways is the denitrif ication pathway, which stepwise reduces nitrate to nitrogen gas. So far, all cultured magnetospirilla have been shown to be capable of denitrif ication with nitrate as an alternative terminal electron acceptor, but not with Fe(III), sulfate, or fumarate (Bazylinski and Blakemore, 1983;Bazylinski and Williams, 2006; Lower and Bazylinski,2013). A potential link between denitrif ication and magnetite biomineralization has been realized for decades. For instance, in MS-1 more cells were found to contain magnetosomes in the presence of nitrate than its absence (Bazylinski and Blakemore, 1983).Also in AMB-1 nitrate supports magnetite biomineralization at low oxygen concentrations(Matsunaga et al., 1991; Matsunaga and Tsujimura,1993; Yang et al., 2001). However, no genetic evidence was available to elucidate the relationship between denitrif ication and magnetosome formation.Later, studies upon genetic analyses of denitrif ication pathway in MSR-1 revealed a complete denitrif ication pathway including genes encoding nitrate (napoperon), nitrite (nirS), nitric oxide (noroperon), and nitrous oxide (nosZ) reduction (Li et al., 2012, 2013)(Fig.2a), which are explained in detail in the following.
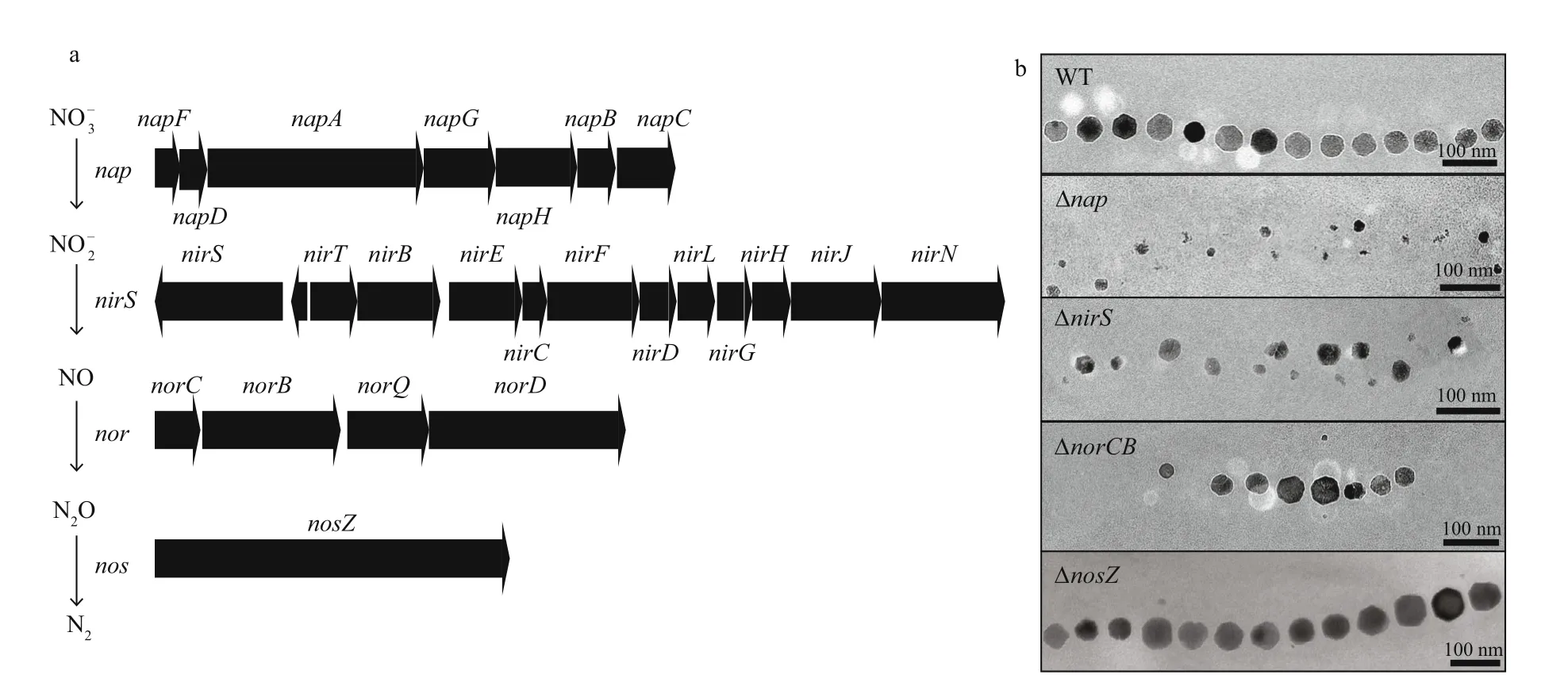
Fig.2 Denitrif ication pathways are involved in magnetite biomineralization
3.1 The nitrate reductase Nap poises an optimum redox conditions for biomineralization
In magnetospirilla, nitrate is reduced to nitrite by periplasmic nitrate reductase Nap, which serves as the major energy-generating step. It is essential for anaerobic growth, while the subsequent steps of denitrif ication do not support anoxic growth (Li et al.,2012). In addition, inactivation of Nap delayed aerobic growth and severely impacted magnetite biomineralization during denitrif ication and microaerobic oxygen reduction. The Δnapmutant strain only produced irregular and smaller magnetosome particles and formed a loose chain (Li et al., 2012) (Fig.2b). Therefore, a role independent of nitrate reductase is speculated in magnetite biomineralization, that is to maintenance of the intracellular redox balance, thereby poising an optimum redox potential for magnetite synthesis (Li et al., 2012).
3.2 The nitrite reductase NirS oxidizes ferrous iron for anaerobic magnetosome formation and requires NirN for proper d1 heme assembly
NirS, a cytochromecd1nitrate reductase is not absolutely required for anaerobic growth and the deletion ofnirSdid not completely abolish growth without oxygen (Li et al., 2013). In 1995, NirS was for the f irst time suggested to be involved in magnetosome formation since NirS purif ied from MS-1 was able to oxidize ferrous iron in the absence of oxygen (Yamazaki et al., 1995). This hypothesis is demonstrated in MSR-1, and the deletion ofnirSgene caused a defect of biomineralization: fewer, irregular,and smaller nanoparticles (Li et al., 2013) (Fig.2b).During nitrite reduction, NirS displays an activity of ferrous iron oxidation directly for magnetite biosynthesis (Li et al., 2013). In addition, similar phenotypes (poor anaerobic growth and impaired magnetite synthesis) were observed in AMB-1 upon concomitant interruption ofnorBandnirS(amb1395)(Wang et al., 2011). Diff erent from onenirSin MSR-1, twonirSgenesamb4165andamb1395are present in the genome of AMB-1, and the deletion ofamb1395completely repressed the reduction of nitrite to nitric oxide (Wang et al., 2011). However, the expression of bothamb1395andamb4165from AMB-1 under the control of the MSR-1nirSpromoter can completely rescue the growth and magnetosome formation of MSR-1 ΔnirSstrain, indicating that the observed lacking function ofamb4165in AMB-1 might be caused by transcriptional inactivity under tested conditions (Li et al., 2013).
NirN, a protein with previously unknown function shares a conspicuous sequence similarity to NirS.This raised the question whether NirN can also catalyze the reduction of nitrite to nitric oxide.However, despite the surf icially similar phenotypes in growth and magnetosome formation, closer inspection revealed that ΔnirNstrain is still capable of nitrite reduction upon prolonged incubation, and NirN alone is not able to support nitrite reduction in the absence of the NirS (Li et al., 2013). It has been further shown that proper assembly of thed1heme inholo-NirS depends on the functional interaction with the NirN,thereby providing the f irst evidence for a physiological function of NirN in vivo (Li et al., 2013). Consistent with this observation, inPseudomonasaeruginosathe cofactor content of NirS in ΔnirNmutant strain was found to be diff erent from that in the wild type(WT) strain by UV-visible absorption spectroscopy of periplasmic fractions (Nicke et al., 2013). Later, NirN inP.aeruginosahas been demonstrated to act as a novel electron-bifurcating dehydrogenase catalyzing the last step of hemed1synthesis, and the deletion ofnirNresulted in the accumulation of dihydro-hemed1,which f inally led to a diff erent form ofholo- NirS and decreased enzymatic activity (Adamczack et al.,2014; Klünemann et al., 2019).
3.3 The nitric oxide reductase Nor is important for biomineralization by yet-unknown functions
The NO reductase Nor also participates in magnetosome formation by yet unknown functions.In both MSR-1 and AMB-1, genetic inactivation of Nor resulted in the formation of fewer magnetosome particles during denitrif ication pathway (Wang et al.,2011; Li et al., 2012) (Fig.2b). In addition, deletion ofnorCBalso led to thicker, shorter cell morphology,which may be caused by accumulated NO stress (Li et al., 2012). Diff erently, inP.aeruginosaPAO1 NO stress was shown to be the major cause for cell elongation and subsequent anaerobic biof ilm formation (Hamada et al., 2014).
3.4 Nitrous oxide reductase Nos
Although in MSR-1 the deletion ofnosZdoes not aff ect magnetosome biosynthesis, a putative periplasmic Fe(II) oxidase identif ied inMagnetovibrioblakemoreistrain MV-1 using N2O as the terminal electron acceptor, was proposed as N2O reductase NosZ (Bazylinski and Williams, 2006). Thus, N2O reductase NosZ might be also involved in magnetite biomineralization.
Overall, denitrif ication enzymes, especially nitrate reductase Nap, nitrite reductase Nir, and nitric oxide reductase Nor, have important but non-essential functions in magnetite biomineralization, and phenotypes of these deletions’ TEM micrographs are summarized in Fig.2b. However, their role in magnetosome formation has not yet been fully elucidated. For example, it has remained unknown which gene(s) of thenapoperon is involved in redox homeostasis for biomineralization. Siponen et al.(2012) have suggested that NapC (a Nap protein essential for nitrate reduction, Li and Schüler,unpublished data) might be required to transfer electrons from the quinone pool to magnetochrome containing proteins. Moreover, it is worth mentioning that all sequenced MTB belonging toAlphaproteobacteriacontain homologues ofnapandnorclusters, although some of them are not capable of denitrif ication, further implying a key role of Nap and Nor in magnetosome formation (e.g. redox maintenance), which is independent of denitrif ication pathway. In other clades of MTB, distinct anaerobic respiratory enzymes may be evolved in biomineralization and resemble function with denitrif ication enzymes.
4 AEROBIC RESPIRATION AND MAGNETOSOME FORMATION
In addition to being an important environmental factor to aff ect magnetosome biosynthesis, O2mainly functions as a preferred electron acceptor for respiration and energy generation used for aerobic respiration by all magnetospirilla under microaerobic and aerobic conditions. By visible absorption spectroscopy, cytochromes for respiration were identif ied in MS-1, includinga-,a1-,b-,c-,cd1-, ando-type hemes (O’Brien et al., 1987). More than 85%of the detected cytochromes belong to thec-type,which are mostly soluble, while thea- andb-type cytochromes are mainly found in membrane fractions.Sincea1hemes (‘low aeration’ cytochrome oxidase)andohemes (‘high aeration’ cytochrome oxidase)were simultaneously identif ied in MS-1, O’Brien et al. (1987) proposed that the aerobic respiration chain is branched. Later, a novel ‘cytochromea1-like’hemoprotein was purif ied from MS-1, which displayed weak cytochromecoxidase activity in vitro(Tamegai et al., 1993). The observation that the hemoprotein is present in higher amounts in magnetic compared to nonmagnetic cells suggests that it might be involved in magnetosome formation (Tamegai et al., 1993). In the same organism, Tamegai and Fukumori (1994) identif ied a novelcbb-type cytochromecoxidase, which is assumed to function as the terminal oxidase for O2respiration under microaerobic conditions.
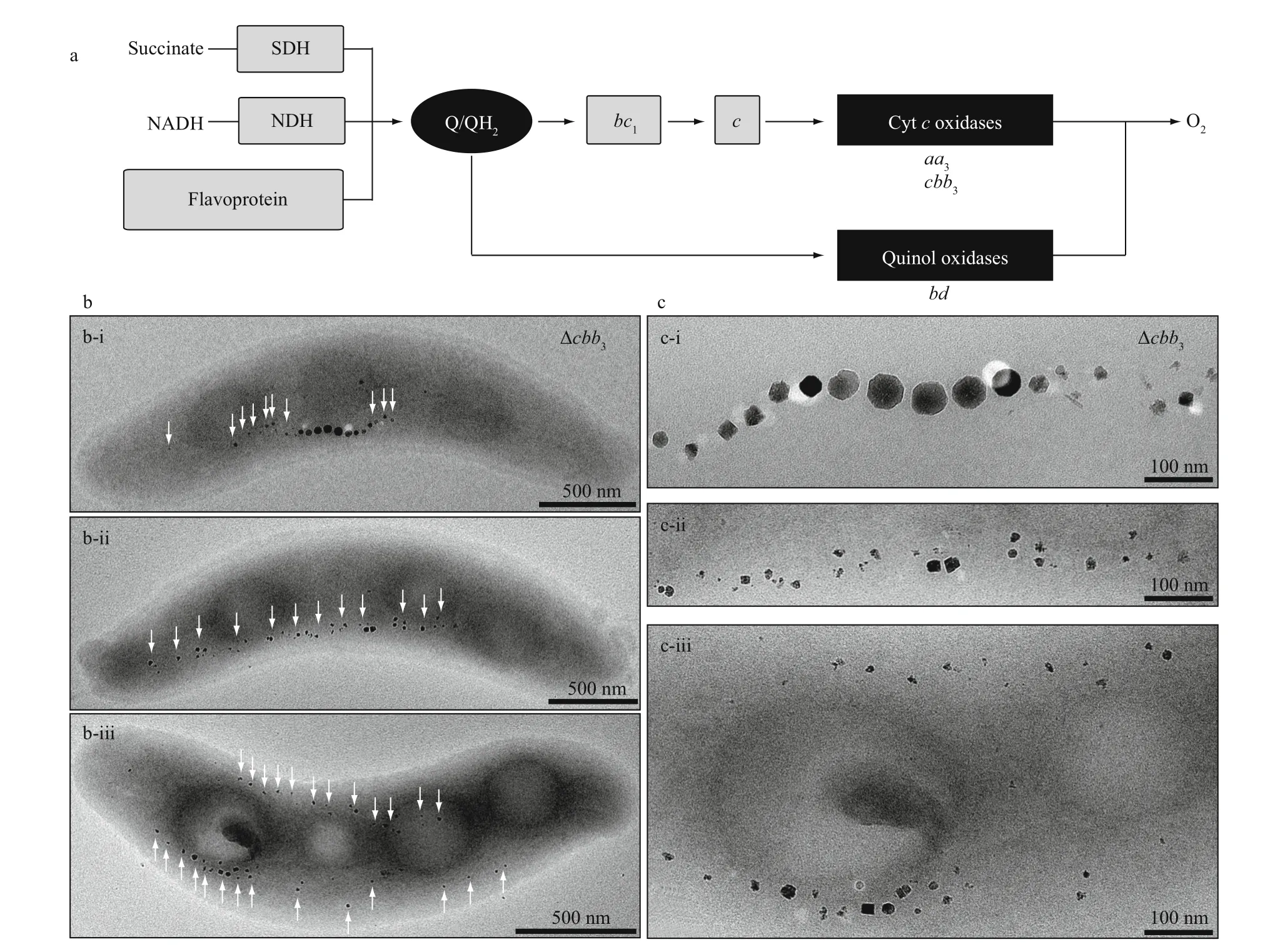
Fig.3 The cbb 3deficientstrain of MSR-1 shows various magnetosome phenotypes under microaerobic conditions in the presenceofnitrate
Studies on oxygen terminal oxidases provide mechanistic insight into the function of aerobic respiration on magnetite biomineralization. Although in the genome of MSR-1 three operons encoding putative terminalcbb3-type,aa3-type, andbd-type oxidases are present (Fig.3a), onlycbb3andbdwere found to be essential for aerobic respiration, whereasaa3oxidase had no physiological signif icance under tested conditions (Li et al., 2014a). Thebdoxidase is only required for O2-dependent growth in the absence ofcbb3, and not involved in magnetosome formation.Thecbb3oxidase appears to be the most important enzymes for not only O2-dependent growth, but also denitrif ication and biomineralization. For example,cbb3mutant strains are incapable of simultaneous nitrate and O2reduction, but reduce O2f irstly followed by nitrate reduction (Li et al., 2014a). With respect to biomineralization, loss ofcbb3leads to subtle eff ects on biomineralization in the absence of nitrate under microaerobic conditions. However, pleiotropic eff ects on magnetosome formation and organization were observed in the presence of nitrate, mainly including(i) WT-like particles in the center of magnetosome chain, accompanied by smaller and irregularly shaped particles at each end, (ii) small and irregular particles arranged in loose chains, and (iii) irregular magnetosomes present two loose chains bent and adapted to the inner and outer curvatures (Fig.3b-c).The most conspicuous phenotype was thatcbb3def icient strains form two loose magnetosome chains at each side of the cell, resembling those in ΔmamYmutant cells (Toro-Nahuelpan et al., 2019). Therefore,a potential link may be present betweencbb3oxidase and MamY. Furthermore, reduced redox states incbb3def icient strains indicate that observed defects in magnetosome biosynthesis and organization probably results from the disturbed redox balance required for magnetosome biomineralization (Li et al., 2014a). In agreement with this, dynamic light scattering shows that MamY oligomerization in vitro is controlled by pH changed and fully reversible (Toro-Nahuelpan et al., 2019). Therefore,cbb3oxidase probably provides an initial redox environment to facilitate the function of magnetosome proteins (e.g. MamY) for biomineralization and magnetosome organization.
5 MAGNETOSOME-ASSOCIATED PROTEINS INVOLVED IN REDOX CONTROL OF BIOMINERALIZATION
Once soluble iron ions are obtained by MTB, they must be precipitated chemically to become insoluble magnetite crystal deposits, which requires a balanced ratio of ferrous and ferric iron (Mann et al., 1990;Faivre et al., 2004, 2007). Faivre et al. (2004) found that the formation of abiotic magnetite requires a rather high pH and high concentration of iron. In addition to general respiration pathways, some magnetosome-associated proteins are also suggested to be involved in redox control for magnetite biomineralization (Raschdorf et al., 2013; Müller et al., 2014). Some of these proteins, such as MamZ and FtsZm, display diff erent eff ects on iron biomineralization between in the presence of nitrate and its absence (Raschdorf et al., 2013; Müller et al.,2014). MamZ even show similar but independent roles in redox control of magnetite synthesis with the mentioned Nap (Raschdorf et al., 2013). In contrast,FtsZm is involved in magnetosome formation only under nitrate deprivation (Müller et al., 2014).Recently, Wang et al. (2019a) found that FtsZm is capable of polymerization and subsequently recruits MamY, MamX, and MamZ.
In addition, MTB also contain a set of special redox proteins including MamE, MamP, MamT, and MamX,which are encoded by genes within magnetosome island (Siponen et al., 2012; Raschdorf et al., 2013).These proteins share a unique conf iguration of two close CXXCH heme-binding motifs (Grünberg et al.,2004), termed the magnetochrome domain, and spectral and redox characteristics of purif ied MamE and MamP further conf irms that the identical magnetochrome domain is a cytochromec-like domain (Siponen et al., 2012). Indeed, deletions of these genes resulted in the formation of irregular magnetosome particles (Murat et al., 2010; Raschdorf et al., 2013; Yang et al., 2013; Lohße et al., 2014). In addition, an allele ofmamEwith a point mutation in the CXXCH motif only partially complemented magnetite formation in themamEdef icient strain,further indicating a role for heme-binding domain in magnetite biomineralization (Quinlan et al., 2011).Loss of MamX putative magnetochrome domains also caused an identical biomineralization defect,demonstrating again, this CXXCH motif is associated with crystal formation (Raschdorf et al., 2013).Because some of these proteins including MamP and MamE also contain protein-interaction PDZ domain,Siponen et al. (2012) suggested that these proteins may form a protein complex, which serves as an electron transport chain to further regulate electron f low for magnetosome biomineralization.Furthermore, the crystal structure of MamP from marine magnetotactic ovoidal bacterium MO-1 provided the f irst insight into the role of this new type of cytochromes in iron biomineralization (Siponen et al., 2013). Studies of MamP and MamT in AMB-1 further verif ied that double CXXCH heme-binding motifs of MamP and MamT are essential for magnetite formation, supporting a physiological function for redox process (Jones et al., 2015).
6 REGULATION OF REDOX CONTROL FOR MAGNETOSOME FORMATION
As mentioned above, more and more observations imply that MTB need to control the redox state of the minerals by using multiply electron transport chains or pathways. Since MTB seem to employ general respiratory enzymes for magnetite biomineralization,it might be a common feature of bacterial biomineralization, which has been proposed by Rahn-Lee and Komeili (2013). If so, the modulation or redox control of magnetite biosynthesis may be accomplished by respiration pathways. InEscherichiacoliand other bacteria, regulatory switch between aerobic and microaerobic metabolism is primarily governed by an Fnr (fumarate and nitrate reduction)regulator (Unden et al., 1995; Bueno et al., 2012). In MSR-1, inactivation of the Fnr homologue MgFnr not only decreased N2production due to reduced N2O reductase activity, but also impaired magnetite biosynthesis under microaerobic conditions in the presence of nitrate (Li et al., 2014b). When MgFnr is overexpressed in the WT, cells would synthesize smaller magnetite nanoparticles during denitrifying growth, suggesting that its proper expression is critical for WT-like magnetite formation (Li et al.,2014b). Analyses of transcriptionalgusAreporter fusions revealed that MgFnr is involved in governing the expression of denitrif ication genes and thereby plays an indirect role in keeping proper redox conditions required for magnetosome formation (Li et al., 2014b). Recently, another Fnr homologue Mg2046 is also shown to be involved in biomineralization.The Δmg2046strain displayed similar growth with WT cells, but synthesized fewer and smaller magnetosomes (Wang et al., 2019b). Further transcriptional analysis revealed that Mg2046 severs as a redox regulator to modulate genes coding for iron uptake, oxygen respiration, and denitrif ication. In the same organism, a global carbon and energy metabolism regulator Crp (3′-5′-cyclic adenosine monophosphate receptor protein) was found to play an important role in magnetosome biosynthesis (Wen et al., 2016). Disruption ofcrpdramatically reduced intracellular iron content and resulted in fewer magnetosome particles produced in the mutant strains. Transcription expression prof ile analyses showed that lack ofcrpnot only aff ected the expression of both genes involving in carbon and energy metabolism, but also downregulated the transcription of all tested MAI genes, includingmamJ,mamC,feoB1,mms6, andftsZm(Wen et al.,2016). In 2017, a novel OxyR homologue OxyR-Like was identif ied to be involved in the regulation of carbon metabolism, and its deletion led to magnetosome consisting of not only magnetite, but also α-Fe2O3and ε-Fe2O3(Zhang et al., 2017). In addition to genes encoding tricarboxylic acid (TCA)cycle, MAI genes (mamJ,mamC,mms6, andftsZm)are also controlled by OxyR-Like, and disruption of OxyR-Like caused a reduced expression of these genes (Zhang et al., 2017).
However, since their relative subtle eff ects on oxygen-dependent magnetosome formation, none of these identif ied regulators can account for the observed complete inhibition to produce magnetite particles under aerobic conditions. In this case,magnetite biomineralization is probably governed by other unknown O2sensors through controlling intracellular redox conditions. The global transcriptome analysis between aerobic- and microaerobic-culturing MSR-1 may provide some candidate sensors (e.g. Anr-Dnr, DnaA, and PvdS)responsible for redox control, which exhibit diff erent expression under diff erent oxygen conditions (Wang et al., 2016). Therefore, a more expanded investigation for regulators involved in redox control may gain insights into the mechanism of oxygen-repressed magnetosome formation.
7 OUTLOOK
7.1 What is the role of denitrif ication and oxygen respiration in magnetosome formation?
There is no doubt that general respiratory pathways,such as denitrif ication and oxygen respiration play a vital role in redox control for magnetosome formation.Based on current data, I propose that respiratory enzymes, such as denitrif ication enzymes Nap and O2terminal oxidasecbb3, act as the primary contributors controlling intracellular redox conditions. In addition,magnetochrome proteins (MamE, MamP, and MamT)f ine-tune microenvironmental redox balance for magnetosome synthesis. In turn, the suitable redox environment promotes other redox-associated magnetosome proteins, such as MamY and FtsZm, to polymerize and recruit other players for magnetosome synthesis and chain formation (Fig.4b). From the schematic overview, it becomes apparent that a core cofactor, heme, is present in most proteins involved in redox modulation (Fig.4, yellow rectangles). On the other hand, heme as a versatile and indispensable cofactor in vivo, can catalyze a wide range of redox reaction in the company of diff erent proteins (Mayf ield et al., 2011). Although transcription and translation of magnetochrome proteins are not modulated by oxygen, their cofactor heme is able to mediate electron transfer in response to diff erent redox conditions.Therefore, it is possible that heme is the major player controlling redox process for magnetosome formation,which may be the reason why the global iron regulator Fur plays only a rather minor role in magnetite synthesis in MSR-1 (Uebe et al., 2010; Qi et al.,2012). Alternatively, there are threeirrhomologues(irrA,irrB, andirrC) present in the genome of MSR-1, which mainly perceive and respond to the synthesis of heme (Chandrangsu et al., 2017). These Irr regulators are probably involved in the control of redox process for biomineralization. The deletion ofirrBresulted in not only impaired growth and magnetosome synthesis, but also decreased expression of genes coding for iron transport and storage, heme biosynthesis, and Fe-S cluster assembly (Wang et al.,2015). However, the links among the three Irr regulators, as well as between heme and biomineralization, are yet little known. A comprehensive analysis of the relationship between Irrs orthologs and heme might provide new insight into the role of heme in redox control and magnetosome formation.
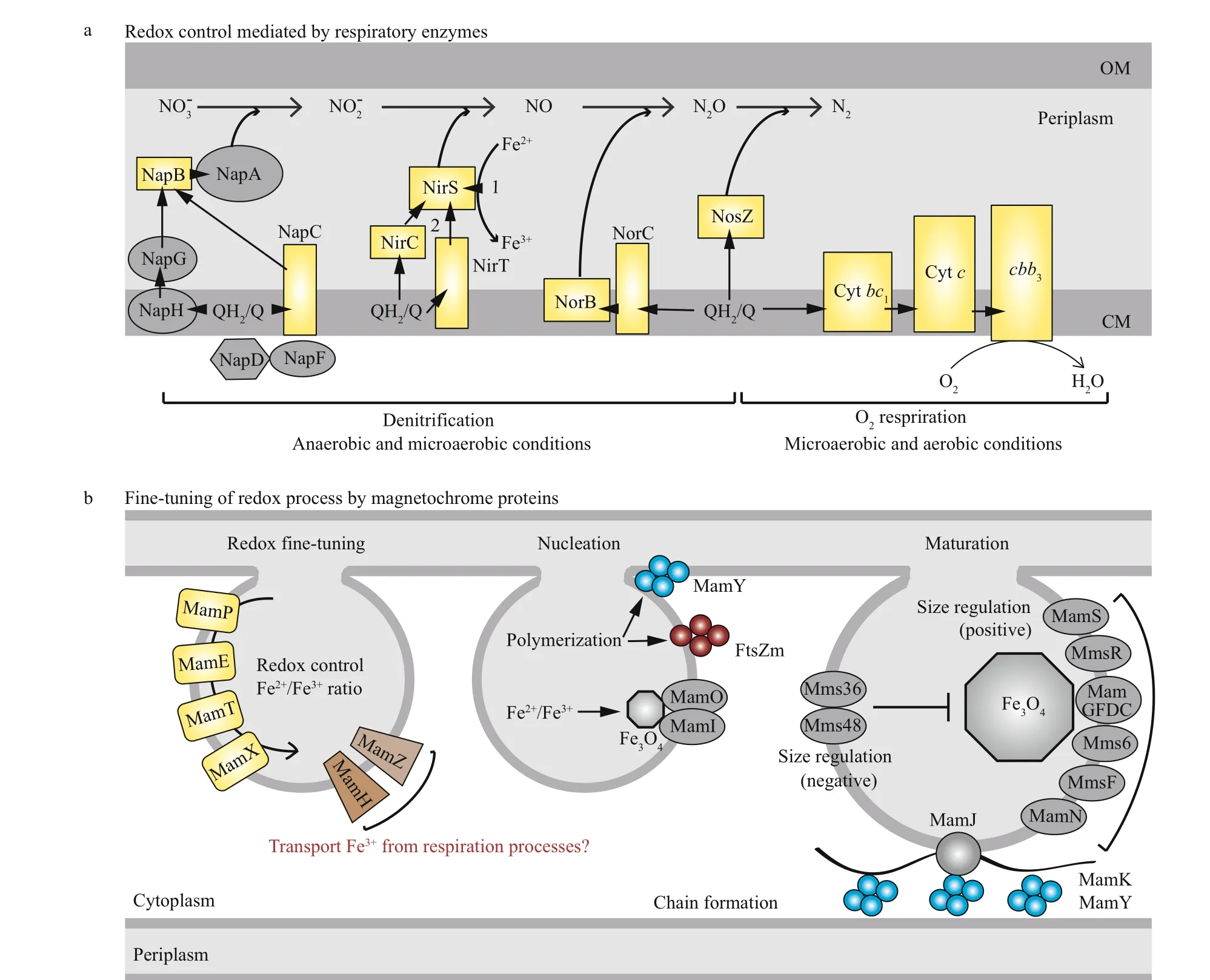
Fig.4 Proposed link between redox processes and biomineralization
7.2 Is it possible to engineer denitrif ication pathway or oxygen respiration to improve magnetosome yields?
Due to their proved biocompatibility,magnetosomes were found to be outstanding magnetic nanoparticles in various biotechnological and biomedical applications. However, most magnetotactic bacteria are recalcitrant to grow in the laboratory, and furthermore, even ones capable of cultivating, as mentioned above are only to synthesize magnetosomes under microoxic conditions with poor yields of magnetosomes. Therefore, a major challenge for potential applications is the requirement of the enormous amount of magnetosomes by mass cultivation. In 2014, Schüler and colleagues (Kolinko et al., 2014) for the f irst time successfully developed a “magnetized non-magnetotactic bacterium”Rhodospirillumrubrumby heteroexpressing genes associated with magnetosome formation. A subsequent study revealed that overexpression of magnetosome genes in MSR-1 by genomic amplif ication of gene clusters via sequential chromosomal insertion dramatically improves magnetosome yields, a potential strategy for the design and mass production of size adjusted nanoparticles with tuned magnetic properties (Lohße et al., 2016). Here there are also some clues as to the genetic factors during respiration pathways that play an important role in biomineralization. For example,because the conversion of nitrate to nitrite catalyzed by periplasmic reductase Nap is the major energygenerating step during denitrif ication, the use of stronger promoter in front ofnapcluster or more energy-producing membrane nitrate reductase Nar might possibly lead to increased growth, and consequently to supply more energy required for magnetosome biosynthesis. For example, the incomplete denitrif ierEnsifermeliloti1021 cannot grow anoxically due to low expression level ofnapdespite of containing a complete set of denitrif ication genes, while the overexpression ofnapcluster rescues the capability of anaerobic growth in the presence of nitrate (Torres et al., 2018). Therefore, it may be possible to improve cultivation and magnetosome yield by genetic engineering of redox pathways in the future.
8 DATA AVAILABILITY STATEMENT
All data generated and analyzed during the current study are available from the corresponding author upon request.
 Journal of Oceanology and Limnology2021年6期
Journal of Oceanology and Limnology2021年6期
- Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
