Synthesis,Structures,Luminescence and Magnetic Properties of Co(Ⅱ) and Ni(Ⅱ)Coordination Compounds Based on Dibenzoyl-Tartaric Acid
ZHANG XueSUN Yuan-YuanFENG Si-Si*,YUAN Cai-Xia*,
(1Institute of Molecular Science,Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education,Shanxi University,Taiyuan 030006,China)
(2Xi′an Manareco New Material Co.,Ltd.,Xi′an 710000,China)
Abstract:Two coordination compounds[Ni(DBTA)(DMF)(H2O)4](1)and[Co(DBTA)(DMF)(H2O)4](2)(D-H2DBTA=(+)-2,3-dibenzoyl-D-tartaric acid,DMF=N,N-dimethylformamide)have been synthesized at room temperature,and characterized by elemental analyses(EA),FT-IR,X-ray single-crystal and powder diffraction analyses.The structural analysis shows that complexes 1 and 2 are isomorphic.They belong to the monoclinic system with P21space group and display a zero-dimensional structure constructed from coordination bonds,which is further interlinked to form a three-dimensional network via hydrogen-bonding interactions.Fluorescence measurements indicate that 1 and 2 can exhibit strong fluorescence using 280 nm as the excitation wavelength.Though 1 and 2 are isomorphic,they displayed different magnetic properties.Complex 1 dominates weak antiferromagnetic couplings between Ni2+cations,while complex 2 exhibits the magnetic anisotropy of the Co2+center and antiferromagnetic interactions between the Co2+cations.CCDC:2072164,1;2065592,2.
Keywords:coordination compounds;dibenzoyl-tartaric acid;crystal structure;luminescence;magnetism
0 Introduction
Over the last few years,the rational design and development of novel metal coordination compounds have attracted extensive interest not only because of their intriguing variety of structures but also due to their potential applications in many fields[1-2],including catalysis[3-5],separation and gas storage[6-8],magnetism[9],luminescence[10-13],chirality[14],etc.However,it is still a great challenge to construct target coordination compounds with pre-designed structures and desirable properties because many factors need to be considered in the structural design,such as metal centers,organic ligands,reagent ratios,solvents,pH values and temperatures[15-19].
Luminescent complexes have been importantly explored for various applications,for instance,lightemitting,display devices,environmental probes,molecular sensors and biomedicine[20-21].Meanwhile,singlemolecule magnets(SMMs)have attracted considerable attention in magnetic chemistry due to their potential applications in high-density data storage,high-speed quantum computing,spin qubits,nanoscale spintronic devices,etc[22-25].Moreover,chirality,as a fundamental property of nature,has become the hot spot and frontier of contemporary study,especially in chemical and biological research fields.Therefore,it is significant and meaningful to synthesize multifunctional complex materials with chiral,optical and magnetic properties.
Organic linkers act as building pillars in the construction of coordination compounds.Thus,the design and rational selection of the organic ligands are important in building the purposed complexes[26-27].In this work,wefocusontheligand(+)-2,3-dibenzoyl-D-tartaric acid(D-H2DBTA),one of the chiral derivates of tartaric acid.The ligand combines the rigidity of the benzene ring with the flexibility of tartaric acid,and possesses multiple coordination points formed by the two carboxyl groups as well as two ester groups.Therefore,the complexes based on such ligand may exhibit diverse structures.
Hence,two complexes formulated as[Ni(DBTA)(DMF)(H2O)4](1)and[Co(DBTA)(DMF)(H2O)4](2)(DMF=N,N-dimethylformamide)have been synthesized successfully from D-H2DBTA and structurally characterized by elemental analyses(EA),FT-IR,X-ray single-crystal diffraction analyses and X-ray powder diffraction analyses(PXRD).Structural analysis reveals that 1 and 2 are isostructural.They belong to the monoclinic system with P21space group and feature a zerodimensional(0D)structure,which was further interlinked to a 3D network via hydrogen-bonding interactions.The solid-state luminescence properties and magnetic properties of complexes 1 and 2 have also been systematically investigated.
1 Experimental
1.1 Materials and measurements
D-H2DBTA wasboughtfrom TCI(Shanghai)Development Co.,Ltd.and used directly without further purification.Other reagents and solvents were purchased from commercial sources and were used without further purification.The samples for EA were dried under vacuum and performed with the CHN-O-Rapid instrument.FT-IR spectra were obtained on a Bruker TENSOR27 spectrometer in a range of 4 000-400 cm-1on KBr pellets.PXRD patterns were collected on the Bruker D8 Advance X-ray diffractometer employing Mo Kα radiation(λ=0.071 069 nm)at a scan speed of 5(°)·min-1in a 2θ range of 5°-50°.The operating voltage and current were 40 kV and 25 mA,respectively.Thermogravimetric analyses(TGA)were performed on the Dupont thermal analyzer under a nitrogen atmosphere with a heating rate of 10℃·min-1.Fluorescence spectra were characterized at room temperature using a Fluoromax-4 Spectrofluorometer with a xenon arc lamp as the light source.Magnetic susceptibility was measured with a SQUID magnetometer(Quantum MPMS)with an applied field of 1 000 Oe in a temperature range of 2-300 K.All magnetic data were corrected for diamagnetism by using Pascal′s constants.
1.2 Preparations of complexes 1 and 2
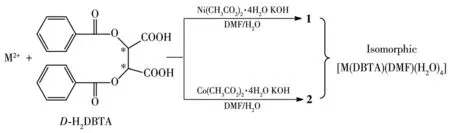
Scheme 1 Synthesis routes of complexes 1 and 2
D-H2DBTA(1.5 mmol,0.537 3 g)was dissolved in the mixed solvent of 7.5 mL DMF and 15 mL water in a 50 mL flask.Ni(CH3CO2)2·4H2O(0.75 mmol,0.186 5 g)was dissolved in 5 mL water,and was gradually added to the ligand solution.Then 3 mL KOH(0.5 mol·L-1)was added to the mixture.After stirring for eight hours,the filtrate was maintained unperturbed for two weeks.Pale green block crystals of 1 were obtained in a yield of 20%(based onD-H2DBTA).EA for C21H27NO13Ni(%):C 44.84(Calcd.44.98);H 4.73(Calcd.4.82);N 2.42(Calcd.2.50).IR(KBr,cm-1):3 500s,3288s,1723s,1661s,1494w,1452m,1418m,1388m,1330m,1315m,1275s,1181m,1129s,1112s,1071m,1 029w,941w,802w,762w,714s,689m,564m,473w.
D-H2DBTA(1.3 mmol,0.465 5 g)was dissolved in the mixed solvent of 7.5 mL DMF and 15 mL water in a 50 mL flask.Co(CH3CO2)2·4H2O(0.65 mmol,0.161 5 g)was dissolved in 5 mL water,and was gradually added to the solution of ligand.Then 2.6 mL KOH(0.5 mol·L-1)was added to the mixture.After stirring for eight hours,the filtrate was maintained unperturbed for two weeks.Pale pink block crystals of 2 were obtained in a yield of 21%(based on D-H2DBTA).EA for C21H27NO13Co(%):C 44.82(Calcd.44.97);H 4.72(Calcd.4.82);N 2.41(Calcd.2.50).IR(KBr,cm-1):3 500s,3 291s,1 723s,1 662s,1 494w,1 452m,1 418m,1388m,1330m,1315m,1275s,1181m,1129s,1112s,1 071m,1 029w,942w,849w,795w,715s,686m,564m,473w.
1.3 X-ray crystallography
Single-crystal X-ray diffraction data for 1 and 2 were collected on a Bruker SMART APEXⅡdiffractometer with a CCD area detector and MoKαradiation(λ=0.071 069 nm)at 298(2)K.Absorption corrections were applied by using the SADABS program.The structures were solved by the direct method and refined by the full-matrix least-squares method onF2using the SHELXS-2014.After all the non-hydrogen atoms were refined anisotropically,H atoms attached to C atoms were placed geometrically and refined using a riding model approximation,with C—H bond lengths of 0.093-0.098 nm.H atoms attached to O atoms were located from difference Fourier maps,and their bond lengths were restrained in a range of 0.075-0.089 nm;then,they were refined using a riding model,withUiso(H)=1.5Ueq(O).A summary of the crystallographic data for complexes 1 and 2 is listed in Table 1.Selected bond lengths and angles for 1 and 2 are shown in Table 2.
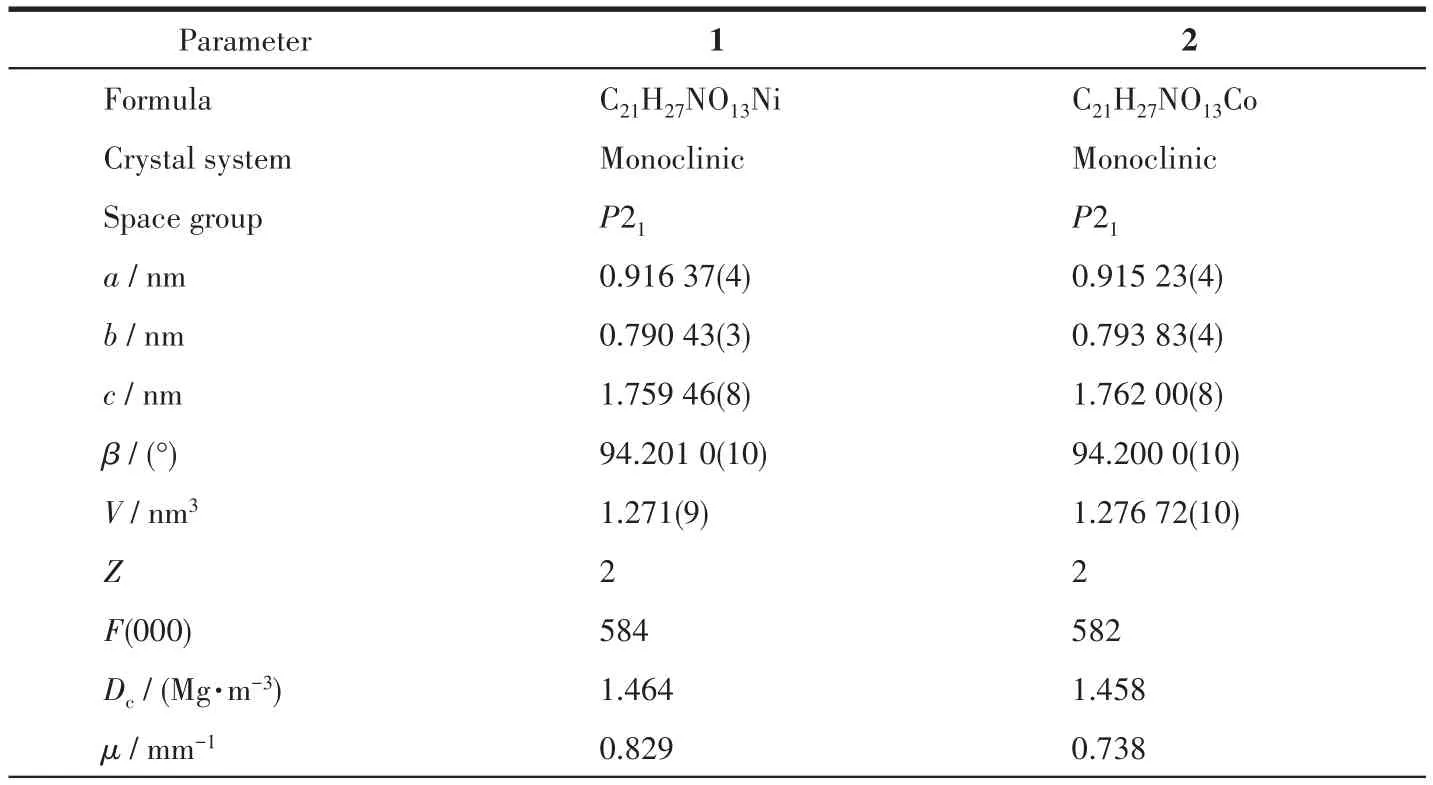
Table 1 Crystal data and structure refinement for complexes 1 and 2
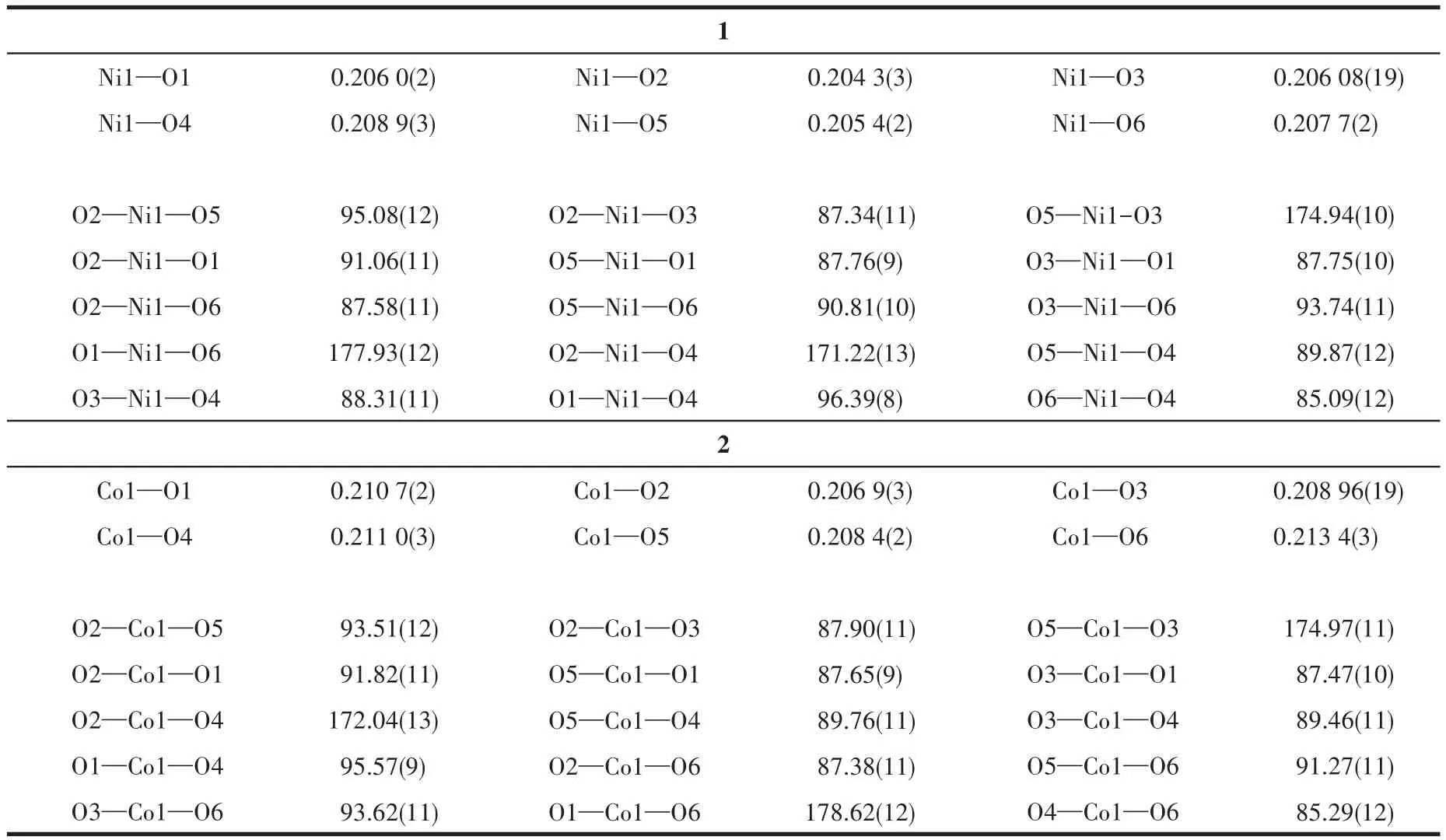
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
CCDC:2072164,1;2065592,2.
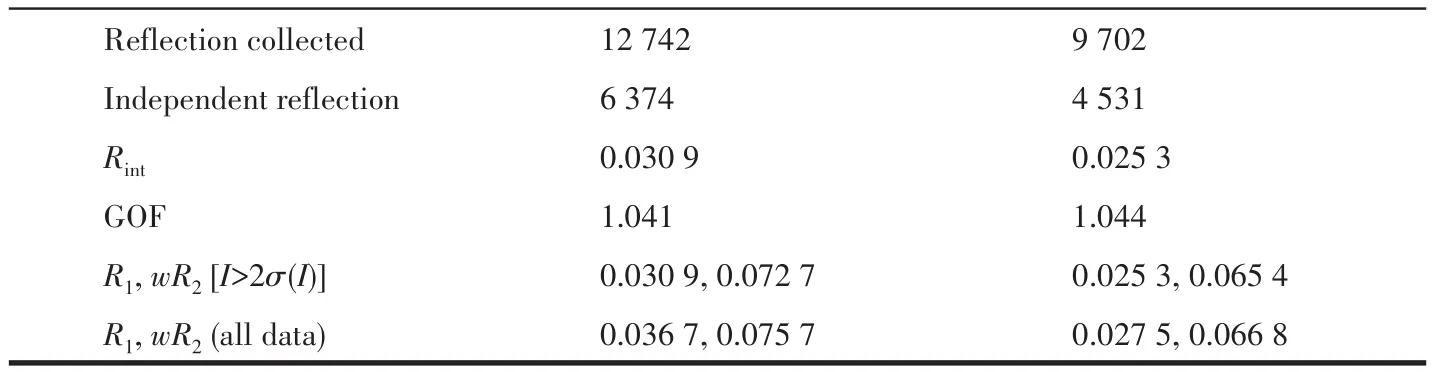
Continued Table 1
2 Results and discussion
2.1 IR spectra
IR spectra ofD-H2DBTA and complexes 1 and 2 were gained at room temperature(Fig.1),and the main characteristic absorption peaks present the typical stretching vibrations of COO-and O—H groups[28].The broad band near 3 500 cm-1is assigned to the O—H stretching vibration of coordinated water molecules.Compared with the ligand,the characteristic absorption peaks of the complex showed an obvious shift.The asymmetric and symmetric stretching vibrations of carboxylate groups appeared at about 1 494 and 1 452 cm-1for the complexes,which were shifted to lower wavenumbers in comparison with the free ligand.The strong bands at 1 129 and 1 723 cm-1for the complexes are attributed to the ester C—O and acyl C=O stretching vibrations,respectively,indicating a certain shift compared with the free ligand.It is proved that carboxyl oxygen atoms in the ligands participate in the coordination to the metal ion.These structural features are in accord with the results of the X-ray diffraction analysis.
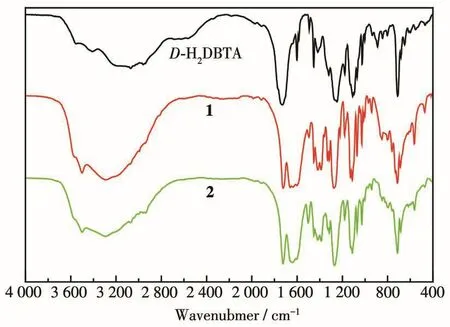
Fig.1 IR spectra of D-H2DBTA and complexes 1 and 2 at room temperature
2.2 Crystal structure description
The X-ray single crystal diffraction shows that complexes 1 and 2 are isomorphic,so we take 1 as the representative to describe their crystal structures.Complex 1 belongs to the monoclinic crystal system with the P21space group,and the asymmetric unit includes one Ni2+cation,one DBTA2-ligand,one coordinated DMF molecule and four coordinated water molecules(Fig.2).Each Ni2+cation is coordinated with six oxygen atoms from one DBTA2-,one DMF molecule and four water molecules,showing the octahedral coordination geometry.The bond length range of Ni—O is 0.204 3(3)-0.208 9(3)nm.The DBTA2-ligands are involved in hydrogen bonds with coordinated water molecules.As depicted in Fig.3a,the mononuclear[Ni(DBTA)(DMF)(H2O)4]units are connected by intermolecular hydrogen bonds between the coordinated water molecules and the oxygen atoms of DBTA2-(O4—H4…O10ii,symmetry code:iix,y-1,z;Table 3)into a 1D chain structure propagating along the b axis.The adjacent 1D chains are further linked by the remaining O—H…O hydrogen bonds to generate 2D planes parallel to ab(O5—H5B…O10iv,O6—H6A…O9iv,symmetry code:iv-x,y-1/2,-z+1)and bc(O3—H3D…O1i,O6—H6…O11i,symmetry code:i-x+1,y-1/2,-z+1)planes as shown in Fig.3b and 3c,respectively.So,it finally forms a 3D network structure(Fig.3d).The shortest inter-molecular distance between Ni2+cations in 1 is 0.526 1(1)nm,while the distance between Co2+cations in 2 is 0.524 8(2)nm.

Table 3 Hydrogen bond parameters of 1 and 2
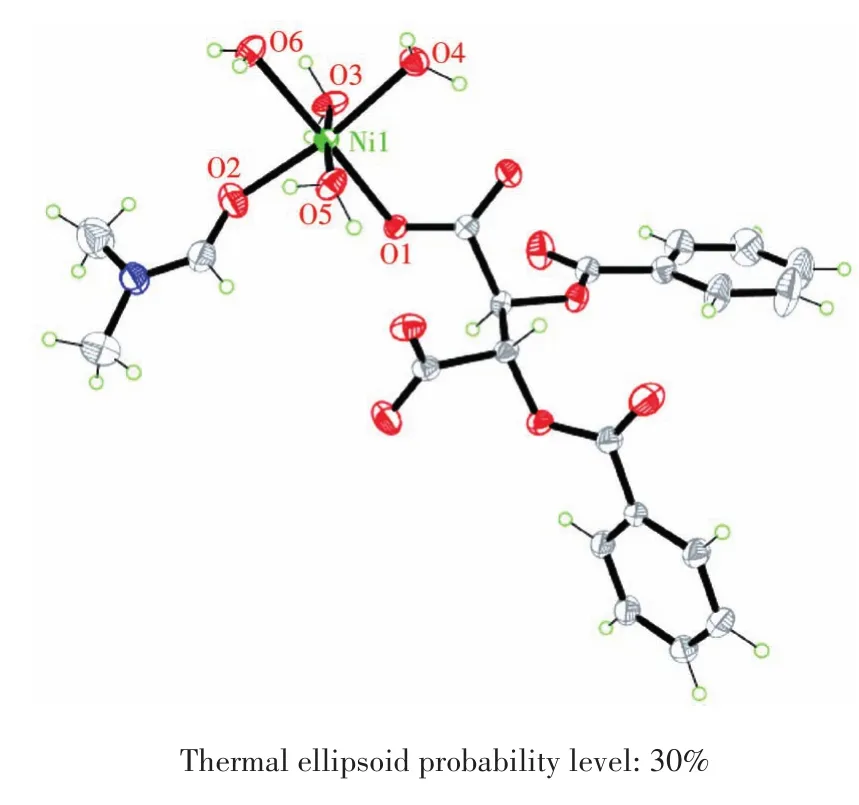
Fig.2 Coordination structure of complex 1

Fig.3 (a)1D chain of complex 1 via hydrogen bonds;(b)2D layer parallel to ab plane of complex 1 via hydrogen bonds;(c)2D layer parallel to bc plane of complex 1 via hydrogen bonds;(d)3D framework of complex 1 formed by hydrogen bonds
2.3 PXRD analyses and thermal stability
To verify the phase purity of the complexes,PXRD analyses were performed.The experimental PXRD patterns were in good agreement with the simulated data based on the results from the X-ray singlecrystal diffractions,demonstrating the high phase purity of the complexes(Fig.4).The thermal stabilities of complexes 1 and 2 were characterized by TGA from room temperature to 800℃at the heating rate of 10℃·min-1under an N2atmosphere(Fig.5).Due to the isomorphic structures of complexes 1 and 2,1 is discussed in detail as a representative.For 1,the weight loss in a range of 85-203℃was 25.14%,which corresponds to the release of four coordinated water molecules and a DMF molecule(Calcd.25.90%).Then with the temperature further heating,the framework decomposed gradually without displaying any plateau.

Fig.4 PXRD patterns(simulated and experimental)of complexes 1(a)and 2(b)at room temperature
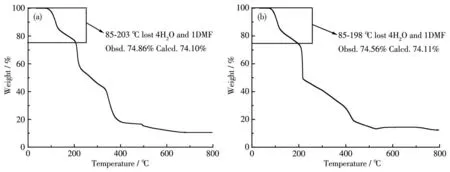
Fig.5 TG curves of complexes 1(a)and 2(b)
2.4 Luminescence properties
The solid-state photoluminescent properties of DH2DBTA and complexes 1 and 2 were investigated at room temperature.When the excitation wavelength was 280 nm,D-H2DBTA exhibited emission peaks at 318 and 622 nm,which can be considered as the π -π*transition or n-π*transition of the ligand[29-30].Under the same conditions,complexes 1 and 2 displayed emission peaks at 306 and 614 nm,respectively.Compared with the emission peaks of the ligand,the emission peaks of the complexes showed a slight blue shift,which may be attributed to ligand-to-metal charge transfer(LMCT)[31].Moreover,the strength of the emission of the complexes was obviously higher than that of the ligand,suggesting the possible decrease of nonradiative transition due to the better stability of the complex resulting from the hydrogen bonds(Fig.6)[32].

Fig.6 Luminescence spectra of D-H2DBTA ligand and complexes 1 and 2 at 298 K in the solid-state
2.5 Magnetic properties
Variable-temperature magnetic susceptibilities of complexes 1 and 2 were measured in a temperature range of 2-300 K with an applied magnetic field of 1 000 Oe.The χmT value of 1 was 1.31 cm3·mol-1·K at 300 K,which was close to the spin-only value for the magnetically isolated Ni2+cation(S=1 and assuming g=2.3)[33].Upon cooling,the χmT value decreased slowly and reached 1.26 cm3·mol-1·K at 14 K,then the χmT value abruptly decreased to a minimum(1.05 cm3·mol-1·K)at 2 K,indicating weak antiferromagnetic exchange between the Ni2+centers.The χm-1value showed a linear relationship with the temperature in a range of 2-300 K,which obeyed the Curie-Weiss law,χ=C/(T- θ),with C=1.31 cm3·mol-1·K and θ=-0.84 K(Fig.7a).The small negative Weiss constant indicates the weak antiferromagnetic interactions between the Ni2+cations,which is consistent with the Ni…Ni distance of 0.526 1(1)nm in complex 1[34].The field dependence of magnetization(M)for 1 was determined at 2 K in a range of 0-70 kOe(Fig.8a),which displayed a gradual increase of the magnetization at low fields,and following with almost saturation at 70 kOe.For complex 2,the χmT value was 2.95 cm3·mol-1·K at room temperature.It was larger than the spin-only value of an isolated S=3/2 cobalt cation(1.875 cm3·mol-1·K,g=2.0),which may be due to the orbital contribution[35].Upon cooling to 125 K,the χmT value decreased to 2.79 cm3·mol-1·K,and then dropped dramatically,and finally reached 1.77 cm3·mol-1·K at 2 K.The variation of χmT values can be interpreted as the intrinsic magnetic anisotropy of the Co2+center[36].The temperature dependence χm-1also followed the Curie-Weiss law,with C=3.12 cm3·mol-1·K,θ=-10.42 K.The nega-tive value of θ indicates the strong antiferromagnetic couplingsbetween Co2+cationsthrough hydrogen bonds and the spin-orbit coupling of the Co2+center(Fig.7b)[37].The M-H plots for 2(Fig.8b)did not reach the theoreticalsaturation value even at 70 kOe,indicating the presence of antiferromagnetic interactions among adjacent metal cations.
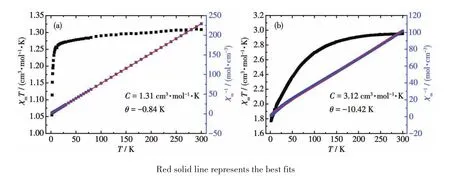
Fig.7 Temperature dependence of χmT and χm-1for 1(a)and 2(b)collected in an applied field of 1 000 Oe

Fig.8 Plots of field dependence of magnetization(M)of 1(a)and 2(b)at 2 K
Interestingly,although complexes 1 and 2 are isomorphic structures,their antiferromagnetic interactions were quite different,which is caused by different electron configurations of Ni2+and Co2+cations.In the hexagonal coordination octahedral field,Co2+cation has a relatively small energy level difference between the excited state and the ground state after the splitting of the d orbitals,resulting in the large spin-orbit coupling,thus having high magnetic anisotropy,which easily leads to strong antiferromagnetic interaction[38].Similar phenomena of different magnetic properties of isomorphic materials have also been reported for other complexes[39].Moreover,at a zero external field,no obvious χm″signal for the alternating current(ac)susceptibilities can be observed at frequencies from 10 to 1 000 Hz in a temperature range of 2-20 K for the two complexes;therefore,there may be no slow relaxation of the magnetization for these complexes.
3 Conclusions
In conclusion,we successfully synthesized two coordination complexes 1 and 2 based on the reaction between the tartaric acid derivative D-H2DBTA and transition metals by solvent volatilization.Their structures were determined by EA,FT-IR,X-ray singlecrystal and powder diffractions methods.The structure analysis showed that complexes 1 and 2 are isomorphic.They belonged to the monoclinic system with P21space group and displayed the 0D structure,which was further interlinked to form the 3D network via hydrogen-bonding interactions.Fluorescence analysis showed that complexes 1 and 2 exhibited strong fluorescence due to the structural stability of the complexes derived from the hydrogen bonds.The results of the magnetic properties analyses showed that complex 1 displayed weak antiferromagnetic coupling between the Ni2+cation,while complex 2 exhibited spin-orbit contribu-tions and strong antiferromagnetic interactions.The different magnetic properties are caused by the diverse electron configurations of Ni2+and Co2+cations.In summary,complexes 1 and 2 are potential difunctional materials combining optical and magnetic properties.

