Folic acid protects against fluoride-induced oxidative stress and testicular damage in rats
Ray Dibyendu, Chatterjee Tiasa, Monalisha Das, Panda Pradip, Mukherjee Sandip
1Department of Physiology, Serampore College, Serampore, Hooghly - 712201, West Bengal, India
2Department of Statistics, Serampore College, Serampore, Hooghly - 712201, West Bengal, India
ABSTRACT
Objective: To investigate the effects of folic acid on testicular oxidative damage in sodium fluoride-induced male Wistar rats.
Methods: A total of 24 male Wistar rats were divided into 4 groups: the control, sodium fluoride (fed with 100 mg/L sodium fluoride through drinking water orally for 21 days), folic acid(36 μg/kg body weight/day, orally), and sodium fluoride plus folic acid (received similar dose orally) groups. At the end of 21 days, epididymal sperm parameters, biochemical analysis of testicular tissue, and serum hormonal levels were performed along with histopathological studies.
Results: Sodium fluoride intoxication resulted in marked reduction in gonado somatic index, serum luteinizing hormone, and testosterone level along with 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase activities. In addition, reduction in sperm density, as well as loss of sperm motility and sperm viability, were also observed.Besides, increased levels of testicular malondialdehyde, nitrite,interleukin-6 and tumor necrosis factor-α as well as decreased levels of superoxide dismutase and catalase activities and reduced glutathione content were found to be associated with this toxicity. Folic acid co-treatment, on the other hand, could prevent all the sodium fluoride-induced testicular pathophysiology and oxidative stress related parameters. Histological examinations of testicular sections from the experimental rats supported these results.
Conclusions: Combining all, this study suggests that being an antioxidant, folic acid plays a beneficial role against fluorideinduced adverse effects on the male reproductive system.
KEYWORDS: Sodium fluoride; Folic acid; Testicular damage; Sperm; Oxidative stress; Antioxidants✉To whom correspondance may be addressed. E-mail: rayd30@gmail.com
1. Introduction
Due to the widespread fluoride contamination in drinking water, fluoride toxicity has become a global problem[1].According to the World Health Organization (WHO), the critical level of fluoride in drinking water is 1.5 ppm. However, in India,the majority of states often have a higher level of fluoride in their drinking water than the WHO recommendations[1]. Longterm fluoride drinking has been shown to have harmful effects on nearly all major organs of the body, raising serious public health concerns.
Significance
Sodium fluoride disrupts the oxidant-antioxidant status and causes free radicals-damage in the testis. Folic acid is an antioxidant/anti-inflammatory B vitamin whose beneficial effect has been established in many tissues. This study showed that folic acid attenuates fluoride-induced testicular damage, and improves sperm profile and testosterone level by modulating oxidative stress, interleukin-6 and tumor necrosis factor production. These results are also consistent with our histological findings. In conclusion, folic acid protects fluorideinduced testicular damage in rats.
Furthermore, emerging data suggest that fluoride can impair male reproductive function and lower fertility by reducing the quality and quantity of sperm in experimental animals[2,3]and humans[4]. Despite the extensive investigations, the exact mechanism by which fluoride exerts its reproductive toxicity effects remains unexplored. Results of in vivo and in vitro studies suggest that oxidative stress is a common underlying mechanism of fluoride toxicity[5]. Furthermore, oxidative stress is a common cause of male reproductive dysfunction, emphasizing the importance of fluoride as an oxidative stress inducer. Indeed,increasing evidence has established that fluoride is a testicular toxicant that can easily cross the blood-testis barrier and adversely impair the endocrine and reproductive functions of testes[6] by generating excess free radicals and inducing oxidative stress in the testicular microenvironment[7,8]. Both the testes and the sperm are well enriched with polyunsaturated fatty acids, which increase their vulnerability to attack by reactive oxygen species (ROS) and consequently oxidative damage may occur. At the level of the testes,fluoride-induced oxidative stress has been linked with degeneration of Leydig and Sertoli cells, and inhibition of spermatogenesis[9-11],while at the level of spermatozoa, it is associated with a decrease in sperm count and motility[2,12]. Apart from these effects, fluoride acts as an endocrine disrupter[13] that changes the hormonal milieu of the testis by directly inhibiting 3β-hydroxysteroid dehydrogenase(3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD), the key enzymes associated with testosterone synthesis[14].
Fluoride contamination, thus, has become a life-threatening health hazard, challenging the researchers to come out with effective therapeutic substitutes. The use of dietary antioxidants, especially antioxidant vitamins could be a promising therapeutic approach in mitigating the fluoride-induced testicular pathophysiology. In this respect, folic acid or naturally occurring folate is considered a potential nutritional factor owing to its antioxidant, antiinflammatory, and anti-apoptotic properties[15-17]. Folic acid, a vitamin from the B group is required to maintain DNA synthesis and spermatogenesis[18]. Deficiency of folic acid may cause declination of sperm count, suggesting that normal folate status is essential for male reproductive function[19]. Folic acid supplementation has been shown to reduce male reproductive damage in previous investigation[20]. Furthermore, folic acid administration has been reported to restore testicular dysfunction by eliminating ROS[21].Interestingly, folic acid, alone or in combination with other vitamins has been reported to have promising protective benefits against oxidative stress-mediated diverse tissue damage produced by various xenobiotic substances such as nicotine, bisphenol A, and insecticides[20,22,23], mainly through its potent anti-inflammatory and antioxidant activities.
To the best of our knowledge, rare studies have been reported on the role of this vitamin in ameliorating fluoride-induced reproductive toxicity. Therefore, the present study was undertaken to find out the protective efficacy of folic acid against fluoride-induced overproduction of ROS and testicular damage in a rat model.
2. Materials and methods
2.1. Chemicals and reagents
Sodium fluoride (NaF) and folic acid were purchased from Sigma-Aldrich. Sulfanilamide, phosphoric acid, naphthyl ethylene diamine dihydrochloride, thiobarbituric acid (TBA), trichloroacetic acid(TCA), nitroblue tetrazolium (NBT), xanthine oxidase,5,5 dithiobsis-2-nitrobenzoic acid (DTNB), potassium dihydrogen phosphate, HOGSH and NaF were purchased from Sisco Research Laboratories, Mumbai, India. Bovine serum albumin (BSA) and folic acids were obtained from Sigma-Aldrich (St. Louis, USA).All other reagents and chemicals were purchased commercially and were of analytical grade.
2.2. Experimental animals
In the present study, sexually mature male Wistar-albino rats(aged 10 weeks, weighing 110-125 g) were selected and housed in the plastic cage under standard environmental conditions of the temperature of (23±2) ℃, 12-h light/dark cycle, and 10% relative humidity. The animals were provided with standard pellet diets and purified water ad libitum. They were acclimatized to the new environment for about 7 days before the experiment.
2.3. Experimental design
After one week of acclimation, rats were randomly divided into four groups (n=6 in each group) and they were treated as follows:Group A served as the control group and received purified drinking water) without test chemicals. Group B was treated with a dose of sodium fluoride (100 mg/L) orally through drinking water. Group C was treated orally with only folic acid (36 μg/kg body weight/day).Group D received orally both sodium fluoride (100 mg/L) plus folic acid (36 μg/kg body weight/day). The duration of the treatment was 21 consecutive days. The dose and route of sodium fluoride were selected based on the previous study[12], whereas the dose of folic acid was chosen according to our earlier report[22]. The experimental groups B, C and D were pair-fed with the animals of group A to counteract the influence of any altered food intake.
2.4. Blood collection and serum preparation
At the end of the treatment period, the experimental animals were then euthanized after being anaesthetized via an intraperitoneal injection of ketamine. Blood was then carefully collected by cardiac puncture. Samples were then ejected into non heparinised glass tubes and allowed to clot at 25 ℃. Serum was separated by centrifugation of blood at 1 200 ×g for 15 min, and preserved at-20 ℃ until analysis.
2.5. Testicular tissue collection and measurement of gonadosomatic index (GSI)
Before cervical dislocation, the body weight of all animals in each group was recorded by digital balance. After sacrifice, each testis of experimental rats was carefully removed, dried on blotting paper,and weighed (in grams) and finally the ratio of the testis weight to the body weight of each experimental animal was measured to calculate the percentage of GSI by using the following formula:

All the right testes of experimental rats were set aside for histological studies, while all the left testes were used for biochemical studies after washing with phosphate-buffered saline (PBS).
2.6. Evaluation of sperm parameters
The cauda epididymal duct was exposed and incised. The epididymal duct was then uncoiled and minced. The semen that oozed out was quickly sucked into a capillary tube and transferred to an eppendorf tube. It was diluted 200 times in 10 mM PBS. After mixing, the sperm suspension was used for the analysis of sperm density, motility and viability[12].
2.6.1. Sperm density
In the study, 10 μL of the diluted sperm suspension was placed on a Neubauer haemocytometer and observed at ×400 magnification by an optical microscope (Carl Zeiss, Germany). The results were determined by counting at least ten microscopic fields. Total number of sperm density was expressed in millions per millilitre as per dilution.
2.6.2. Sperm motility
Only sperm suspensions showing vigorous motility was used for sperm motility. A 10 μL of the sperm suspension was put on a clean glass slide, covered with a 22 mm × 22 mm cover slip. The results were recorded by counting at least ten microscopic fields. Total sperm motility was expressed as the % of motile sperms.
2.6.3. Sperm viability
Sperm viability was measured by using Eosin-Nigrosin staining.A fraction of sperm suspension (10 μL) was mixed with a 10 μL Eosin-Nigrosin stain, and a smear was prepared on a clean glass slide. At least 200 sperm were evaluated by an optical microscope.Finally, the dead and live sperms were observed as dark-red and pale-pink, respectively.
2.7. Assay of serum testosterone and luteinizing hormone (LH)concentration
Testosterone and LH concentrations in serum were analyzed by using the enzyme-linked immunosorbent assay (ELISA) kit (obtained from DRG Inc., Germany) following the standard methods stated previously[12].
2.8. Estimation oxidant/antioxidant status in testis
2.8.1. Preparation of testicular homogenate
For oxidative stress measurement, a specimen from the left testis was homogenized (10% w/v testicular tissue) by glass Teflon homogenizer and then centrifuged at 12 000 ×g for 30 min at 4 ℃ to obtain supernatant. For the estimation of malondialdehyde (MDA),and superoxide dismutase (SOD), testicular tissue extract was prepared in ice-cold Tris-HCl buffer (pH 7.4), while for catalase(CAT) and reduced glutathione (GSH) estimations, the tissue homogenate was prepared in ice-cold PBS. The clear supernatant containing protein was used for the experiments.
2.8.2. Measurement of lipid peroxidation (LPO)
The LPO in terms of MDA formation was measured by the method of Wills[24] with slight modification. Briefly, 2 mL testicular homogenate was diluted with 1 mL TCA (20%) and 1 mL of TBA(0.8%) and then boiled at 95 ℃ for about 60 min. The contents were cooled and 2 mL n-butanol and pyridine mixture (15:1) was added. Following centrifugation, the absorbance of supernatant was measured spectrophotometrically at 532 nm. The LPO levels were expressed as nanomoles of MDA per milligram of protein using the molar extinction coefficient (1.56×10cm/mmol).
2.8.3. Nitrite concentration assay
Nitrite accumulation is generally used as an indicator of nitric oxide (NO) production. The compound NO has a short half-life and is quickly converted to the stable end products nitrate and nitrite. In this study, nitrate was converted to nitrite by cadmium which was followed by colour development with Griess reagent (sulphanilamide and N-napthyl ethylenediamine). The total nitrite accumulation was measured by using the Griess reaction according to our previous studies[25]. The absorbance was measured at 540 nm with a spectrophotometer.
2.8.4. SOD activity assay
Testicular SOD activity was measured by using the method of Sun et al[26]. In this method, the SOD activity was determined based on the inhibition of NBT reduction by SOD. In brief, 2.5 mL of 0.05 mol NaCObuffer (pH 10) was mixed with 0.1 mL of 3 mmol/L ethylenediamine tetraacetic acid., 3 mmol/L of xanthine, 1.5 mg/mL BSA, 0.75 mmol/L NBT and the testicular samples. The reaction mixture was then incubated for 30 min after adding 0.1 mL of 56 mU/mL xanthine oxidase. It was then centrifuged at 350 ×g for 10 min and stopped by adding 6 mmol/L CuCl. The absorbance of blue formazan formed was measured at 560 nm. The relative absorbance was then converted into unit of SOD activity/mg protein, where one unit SOD activity was equivalent to the quantity of SOD that caused a 50% decrease in the background rate of NBT reduction.
2.8.5. Testicular CAT activity assay
CAT activity was measured according to the method described by Aebi[27] by following the decomposition of HOat 240 nm. In brief,50 mM hydrogen peroxide (HO) was added to a mixture of 50 mM phosphate buffer solution (pH 7.0) and 50 mM supernatant. After mixing, the change in absorbance was computed for 2 min with 10 s interval at 240 nm in a UV spectrophotometer. One unit of CAT activity is defined as 1 μM of HOthat is decomposed in 1 min.
2.8.6. GSH assay
GSH levels were measured according to the method of Ellman[28]by using DTNB. A 720 μL supernatant was centrifuged with 5%TCA to remove the protein content. To 0.1 mL of this homogenate,2 mL phosphate buffer (pH 7.4), 0.5 mL of DTNB and 0.4 mL of double distilled water were added. The mixture was vortexed and the absorbance of reduced chromogen was measured spectrophotometrically at 412 nm. The GSH content was then determined from a standard curve and expressed as mM/mg of protein.
2.9. Assay of testicular 3β -HSD and 17β -HSD activities
Testicular 3β -HSD and 17β -HSD were two rate-limiting enzymes in testosterone biosynthesis. These two crucial enzymes were measured according to methods described earlier[22]. One unit of the enzyme activity is to a change in the absorbency of 0.00 1U/min at 340 nm.
2.10. Determination of interleukin (IL)-6 and tumor necrosis factor (TNF)-α
Testicular homogenates were used to estimate TNF-α and IL-6 by the commercially available ELISA kit obtained from Ray Biotech(USA). All samples were assayed in duplicate. The values were expressed as pg/mL.
2.11. Histopathological examinations of the testis
For histological evaluation, testes were fixed with Bouin’s fluid at room temperature for 24 h and embedded in paraffin wax. Thin sections of about 4-5 μm were prepared from the mid-portion of each testis with a rotary microtome and then stained with the Harris hematoxylin-eosin (H-E) stain. The microscopic investigation of these sections was investigated by a bright-field microscope (Carl Zeiss, Germany) and images were obtained at 200× magnification.
2.12. Statistical analysis
All values were expressed as mean±standard deviation (mean±SD).One-way analysis of variance test was first performed to test for any differences between the mean values of experimental groups.To test the intergroup significant difference, the Dunn’s post-hoc multi comparison tests were performed. P<0.05 was considered statistically significant.
2.13. Ethics statement
The experimental procedure was approved by the Institutional Animal Ethics Committee of Serampore College [Registration Number-1946/PO/Re/18/CPCSEA], Serampore, West Bengal, India,with approval No: 02/p/s/sc/IAEC/2017. All animal experiments were performed according to the international guidelines on ethical use of animals.
3. Results
3.1. Effect of folic acid on GSI of the testis
Table 1 depicts the changes in GSI ratio. Administration of fluoride caused a significant decrease of GSI compared to the control group(P<0.001). In the case of folic acid co-administered animals, the decrease in GSI was prevented and increased significantly (P<0.01)compared to only the fluoride-treated group.
3.2. Effect of folic acid on sperm parameters
The effects of different treatments on epididymal sperm density,viability and motility are shown in Table 1. Treatment of sodium fluoride caused significant reduction in the above mentioned sperm parameters compared with the control group (all P<0.001)(Table 1). Co-administration of folic acid to the fluoride-exposed group resulted in significant improvements in sperm count, sperm viability, and motility when compared with fluoride-treated rats(P<0.001). Furthermore, folic acid alone improved sperm density(P<0.001), sperm viability (P<0.001), except sperm motility(P>0.05) when compared with the untreated control group at the end of the experiment.

Table 1. Effect of folic acid on the gonado-somatic index, sperm density, viability, and motility in rats.
3.3. Effect of folic acid on serum level of testosterone and LH
Fluoride-treated rats showed a significant reduction in serum concentration of testosterone and serum LH, compared to the control group (both P<0.001) (Table 2). Folic acid administration also elevated serum testosterone level compared to the control group,although there was no significant difference in serum LH level between the folic acid-treated group and the untreated control group(P>0.05). Concomitant administration of folic acid to the fluoridetreated group showed significant restoration of both serum levels of LH and testosterone compared to the sodium fluoride group(both P<0.001) (Table 2).

Table 2. Effect of folic acid on the serum level of luteinizing hormone and testosterone.
3.4. Effect of folic acid on testicular MDA and nitrite content
The concentration of lipid peroxidative product MDA significantly increased in the fluoride group in comparison with the control group (P<0.01). There was a significant increase in testicular nitrite concentration after fluoride exposure (P<0.01). Folic acid co-treatment with fluoride significantly improved these parameters compared to the fluoride-treated group (P<0.001), toward the level of the control group. However, no significant alteration in the testicular MDA and nitrite levels were noticed in the only folic acidtreated groups in comparison to the control group (P>0.05) (Table 3).
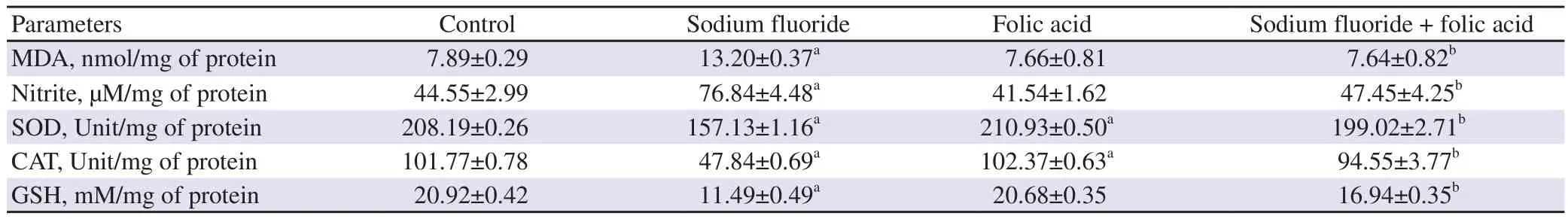
Table 3. Effect of folic acid administration on oxidant-antioxidant parameters of rat’s testes.
3.5. Effect on antioxidant parameters
A drastic inhibitory response on the testicular antioxidant status was observed, followed by sodium fluoride exposure (Table 3). The testicular activities of SOD, CAT and GSH content were significantly decreased in sodium fluoride-treated animals in comparison with the controls (P<0.01). In the case of folic acid, the above changes in the co-supplemented animals (folic acid + sodium fluoride) were significantly enhanced (P<0.001) compared to the only fluoridetreated group, and testicular antioxidant status was restored.
3.6. Effect on testicular 3β -HSD and 17β -HSD
In the sodium fluoride-treated group, the testicular steroidogenic enzyme activities of both 3β -HSD and 17β -HSD were significantly attenuated (3β-HSD: P<0.001, 17β-HSD: P<0.001) compared to the control group (Figure 1 A, B). But, fluoride-mediated alterations of both 3β -HSD and 17β -HSD activities were found to be significantly improved by supplementation of folic acid (3β-HSD: P<0.001, 17β-HSD: P<0.001) (Figure 1 A, B).

Figure 1. Effect of folic acid against sodium fluoride-induced changes in 3β-HSD (A) and 1 7β-HSD (B) activities of the testes. The values are expressed as mean±SD (n=6 in each group). Data are analyzed by one-way analysis of variance followed by Dunn’s post-hoc multiple comparison test. a: Compared to the control group, P<0.001; b: Compared to the sodium fluoride group, P<0.001. HSD: hydroxysteroid dehydrogenase.
3.7. Effect on testicular IL-6 and TNF-α
Testicular IL-6 was significantly higher in the fluoride treated group compared to the control group; folic acid co-administration,however, significantly ameliorated this alteration in the sodium fluoride-cotreated group (P<0.001) (Figure 2A). Similarly, testicular tissue TNF-α was substantially elevated in sodium fluoride-treated rats compared to the controls (P<0.001). Folic acid co-treatment,thus, significantly lowered the fluoride-induced rise in TNF-α in testicular tissue extract (P<0.001) (Figure 2B).

Figure 2. Effect of folic acid against sodium fluoride-induced changes in the levels of cytokines (A: IL-6; B: TNF-α). The values are expressed as mean±SD (n=6 in each group). Data are analyzed by one-way analysis of variance followed by Dunn’s post-hoc multiple comparison test. a: Compared to the control group, P<0.001; b: Compared to the sodium fluoride group, P<0.001. IL-6: interleukin-6; TNF-α : tumor necrosis factor--α.
3.8. Histopathological assessment results
As demonstrated in Figure 3, the control rats showed typical seminiferous tubular structures with normal spermatogenic cell organisation (Figure 3A). Sodium fluoride treatment for 21 days resulted in evident histological alteration in the testis of rats, including atrophy of seminiferous tubules, lack of luminal spermatozoa, degeneration of germinal epithelium and Leydig cells(Figure 3B). The rats treated with folic acid alone showed normal spermatogenic cell organisation (Figure 3C). All of these adverse histopathological alterations were considerably attenuated by the concurrent treatment with folic acid after sodium fluoride exposure(Figure 3D).

Figure 3. Effect of folic acid on testicular architecture in rats. Sections of testicular tissues of rats are stained with hematoxylin-eosin (magnification 200×). A: The control group shows normal cellular morphology of seminiferous tubules (ST) with luminal spermatozoa and presence of interstitial Leydig cells(LC). B: The sodium fluoride group shows a marked degeneration and collapse of the seminiferous tubules such as disruption of germinal epithelium (thick yellow arrow), vacuolation (v) of seminiferous tubules as well as oedematous stroma containing no or small group of Leydig cells (o). C: The folic acid group shows normal morphology of seminiferous tubules (ST) in rats like that of the control group. D: The sodium fluoride plus folic acid group shows noticeable restoration of seminiferous tubular structure (ST) with the presence of normal germinal epithelium and moderate presence of Leydig cells (LC) in interstitial space. PS: primary spermatids; IS: interstitial space without Leydig cells.
4. Discussion
Where fluorosis is endemic, millions of individuals are adversely affected by the exposure to fluoride. Previous scientific and investigational studies have demonstrated that fluoride-induced oxidative stress plays a decisive role in male reproductive dysfunction[29]. Interestingly, increased attention has been focused on dietary factors because the toxicity of environmental chemicals can be modified by the consumption of dietary factors[30]. In the present study, folic acid dietary intervention ameliorates sodium fluorideinduced testicular dysfunction via combating oxidative stress and anti-inflammatory signs due to its antioxidant and anti-inflammatory properties.
The GSI is an excellent measure of reproductive activity and is frequently employed as a major criterion in the assessment reproductive toxicity. In the current investigation, GSI was shown to be lower after fluoride exposure. This finding is in consistent with the previous findings that fluoride treatment decreased the testicular weight and hence GSI in rats[25,31]. Decreased GSI is known to indicate an adverse impact on reproduction in experimental animals[32]. Normal testicular weight mainly dependents on the LH and testosterone level[33]. Therefore, decreased GSI may be due to inhibited synthesis of various reproductive hormones which are required for maturation and development of reproductive organs[34].In the present study, folic acid co-administration in fluoride-treated rats showed a significant restoration of GSI. In addition, loss of organ weight may also be due to loss of appetite and impaired metabolism. Folic acid co-administration substantially enhanced body weight and testicular-somatic index, which may be due to folic acid’s influences on appetite recovery[35].
Sperm quality and the number is an important parameter used in the evaluation of fertilizing ability of males. Any alteration of sperm quality may reduce fertility. Several experiments on animals showed that fluoride exposure reduced male fertility by altering the structural and functional properties of spermatozoa[36]. In the present study,the spermiotoxic effects of fluoride in male rats were manifested by the decreased sperm count, motility, and viability. Fluoride can easily enter into the blood circulation of the testis by crossing the blood-testis barrier and may have a direct cytotoxic effect on spermatozoa. Besides, the decrease in the epididymal sperm number and quality in fluoride-treated rats might be due to the low concentration of testosterone as the sperm formation and the sperm maturation in the epididymis are controlled by testosterone[13]. The above findings in this experiment are in harmony with the previous studies in animals where fluoride-induced male reproductive toxicity has been associated with a low level of testosterone and reduction in sperm numbers and alteration of sperm qualities[37].However, co-administration of folic acid successfully counteracted the spermatoxic effects of fluoride and improved testosterone level along with sperm number and quality as evidenced from the current study, which was in harmony with the previously reported benefits of folic acid on sperm parameters[21,23,38]. It has been found that folic acid supplementation has positive effects on spermatogenesis,DNA synthesis, and repair mechanism[18]. We speculate that folic acid preserved the sperm count and quality possibly by promoting the spermatogenesis process.
Testosterone is the primary male reproductive hormone that is essentially required for the normal growth and development of sex organs and also for the maintenance of spermatogenesis. This steroid hormone is synthesized in Leydig cells, which is under the control of two rate-limiting steroidogenic enzymes: 3β - HSD and 17β-HSD.In our study, a significant reduction in serum levels of testosterone was noticed in the fluoride-treated group. To expose the possible reason for the reduction in testosterone in the fluoride-treated group,the activity of two rate-limiting steroidogenic enzymes: 3β-HSD and 17β-HSD were further examined in the current investigation and marked decreases in the activity of testicular 3β-HSD and 17β-HSD were observed[6]. In addition to it, a diminished serum level of LH controlling the steroidogenic activity of these two enzymes in Leydig cells was observed. Therefore, it can be concluded that the reduction in serum level of testosterone might also be caused by low level of LH. Likewise, Zhou et al[4] reported that the level of LH and testosterone were diminished after fluoride exposure. A reduction in LH level in fluoride intoxicated rats also confirmed the decreased testicular somatic index (as mentioned earlier), since testicular growth is dependent on plasma LH[39].However, the actual cause of the decrease in plasma LH in the present experiments was not dissected out. In our experiment, cotreatment of folic acid improved the serum level of LH, testosterone,and 3β -HSD and 17β-HSD activity. It may be due to folic acid scavenges ROS and free radicals. Similar observations have reported that folic acid and vitamin Btreatment on nicotine intoxicated rats restored reproductive hormones and reproductive status[12].
Although the specific mechanism of fluoride-induced testicular toxicity is unknown, there is evidence that fluoride-induced oxidative stress is a major pathological mechanism responsible for testicular damage and the development of male infertility. In this study, the administration of fluoride resulted in excessive ROS generation and oxidative stress as reflected by testicular MDA level, a marker of LP and NO levels. These findings are in agreement with the induction of LPO and oxidative stress upon fluoride exposure[12]. However,folic acid treatment significantly decreased the content of MDA and NO in fluoride-exposed rats. It has been well established that folic acid behaves as a powerful antioxidant and free radical scavenger,and is reported to inhibit LPO[15,40]. When folic acid reacts with oxidizing free radicals, theOH present on its purine type ring plays an important role in counteracting the oxidation effects.
Balancing ROS and antioxidants is vital for normal testicular functions and sperm fertilization ability. Under normal conditions,endogenous free radical scavengers such as SOD, CAT, and GSH may effectively combat the cellular damage induced by ROS.Therefore, we measured testicular SOD and CAT activities and GSH content as antioxidant markers to assess oxidative stress more comprehensively. The superoxide anions (O) are decomposed by the enzyme SOD into HOwhich may be further converted into inactive forms by Fenton’s reaction or by other enzymes such as CAT. Our results showed that fluoride administration caused a significant declination of enzymatic activities of SOD and CAT in testicular tissue which may further cause overproduction of ROS and LPO. Folic acid treatment significantly attenuated fluorideinduced oxidative toxicity in testicular tissue of the fluoride group by improving the activities of these antioxidant enzymes. The results, therefore, demonstrate that folic acid can potentially improve testicular SOD and CAT activities in the fluoride-intoxicated animal model, consequently improving reproductive function.
GSH, a thiol-containing non-enzymatic tripeptide, performs multiple functions in cells such as maintenance of cellular antioxidant status, antioxidant enzyme functions, and detoxification of HOand organic peroxides. Further, GSH and glutathione S-transferase are important regulators of germ cells proliferation and differentiation and they protect the germ cells against the toxic effects of free radicals[41]. Fluoride ions bind with GSH and inactivate it, and any decline in testicular GSH levels in the fluoride-treated group indicates that free radical scavengers fail to counteract the ROS generation. It is well established that in fluoride intoxication, the decrease in testicular enzymatic activities (SOD and CAT) and non-enzymatic level (GSH) is accompanied by increased ROS level, which causes oxidative stress and its associated testicular damage[7,40].
The inhibition of testicular steroidogenic enzyme activities after fluoride treatment in the present study might be the result of the overproduction of ROS in testicular tissue as microsomal steroidogenic enzyme activities in the testis are attenuated in presence of ROS[14]. The reduced activity of testicular 3β-HSD and 17β-HSD is supported by the decrease in plasma testosterone level seen in this study. The inhibitory effect of fluoride-induced oxidative stress on testicular steroidogenesis is consistent with other studies[14]. The reduction in testicular somatic index and alteration in epididymal sperm physiology also support the conception of inhibition of testicular steroidogenesis.
The alteration epididymal sperm profile in fluoride-treated rats is possibly mediated by oxidative stress. Spermatozoa was particularly vulnerable to ROS-induced peroxidative damage as their plasma membrane is enriched with a large amount of polyunsaturated fatty acids and also has a low concentration of antioxidant enzymes.Moreover, oxidative stress leads to DNA damage, adenosine triphospahte depletion, and decreases the function of the membrane and fluidity, consequently, severe loss of sperm motility. Cell death occurs following damage of cell membrane and DNA, which can be the probable cause of reduced cell viability and the sperm count in the present study. Recent studies revealed that long-term fluoride exposure promotes the production of pro-inflammatory cytokines IL-6 and TNF-α[42]. These cytokines are known to involve in inflammation. Furthermore, an increased level of IL-6 is known to inhibit testosterone production by Leydig cells. This literature coincides with our result, which reveals a rise in IL-6 and TNF-α owing to fluoride administration. However, co-administration of folic acid successfully attenuated the production of these cytokines in fluoride-treated rats. Thus, in line with the previous studies, the present experiment demonstrated an anti-inflammatory mechanism of folic acid in the prevention of fluoride-induced testicular toxicity in rats[16].
The protective effect of folic acid was further confirmed by histopathological examination. The results of histological examination of testes in fluoride-treated animals supported oxidative damage to testicular tissue, as evidenced by deformation and atrophy of seminiferous tubules, a decrease in luminal spermatozoa,and degeneration of Leydig and Sertoli cells, vacuolization and oedema. These findings are also corroborated with previous findings associated with the effects of fluoride on testicular structure[7,8,12]. Histological section of the testes of rats given folic acid simultaneously with fluoride showed a noticeable improvement of testicular histoarchitecture, which also asserts the preventive role of folic acid against oxidative damage. Similar findings were observed by Shalaby et al[23] who used folic acid against methomyl insecticide in rats.
However, the study has some limitations. In this study, we examined only a single dose of folic acid based on the previous studies.Therefore, we need further studies for different comparative doses to find the accurate dose. Furthermore, total oxidant-antioxidant and cell apoptosis should be investigated to elucidate the mechanism of action of folic acid.
In conclusion, supplementation folic acid could effectively restore quality and quantity of spermatozoa, serum testosterone level, and testicular damage by preventing excess production of free radicals,alteration in antioxidant status and cytokines level in fluoride treated rats. The findings proposed that being an easily available and lowcost nutritional factor, folic acid may be a promising candidate to prevent some reproductive health consequences associated with fluoride exposure for many populations at risk worldwide.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
This work was performed in collaboration between all authors. All authors discussed the results and contributed to the final manuscript.The main ideas behind the experiments were conceived by Dibyendu Ray with many helpful suggestions from Sandip Mukherjee.Tiasa Chatterjee wrote the first draft of the manuscript. Material preparation, experiment, and data collection were performed by Tiasa Chatterjee and Monalisha Roy. Author Pradip Panda performed the statistical analysis. Dibyendu Ray supervised and wrote the revised manuscript. Before the article was submitted, it was read and approved by the authors.
 Asian Pacific Journal of Reproduction2021年6期
Asian Pacific Journal of Reproduction2021年6期
- Asian Pacific Journal of Reproduction的其它文章
- Quality of life of infertile couples in the Gaza Strip, Palestine
- Neutrophil-lymphocyte ratio in pregnancy-associated maternal complications:A review
- Protective effects of honey compound syrup on busulfan-induced azoospermia in male rats
- Association between eNOS gene promoter polymorphism (-786T>C) and idiopathic recurrent pregnancy loss in Iranian women
- Effects of L-arginine on preeclampsia risks and maternal and neonatal outcomes:A systematic review and meta-analysis
