Human umbilical cord-derived mesenchymal stem cells treatment for refractory uveitis: a case series
Jing Yang, Xin-Jun Ren, Xi-Teng Chen, Yuan-Feng Jiang, Zhi-Bo Han, Zhong-Chao Han,3,Xiao-Rong Li, Xiao-Min Zhang
1Tianjin Key Laboratory of Retinal Functions and Diseases,Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
2National Engineering Research Center of Cell Products,Tianjin 300457, China
3Beijing Engineering Laboratory of Perinatal Stem Cells,Beijing Institute of Health and Stem Cells, Beijing 300457,China
Abstract
INTRODUCTION
Uveitis is a broad term for intraocular inflammation of various origins. It is one of the leading causes of blindness, and mainly affects adult working people[1-2]. Uveitis can be secondary to an infectious etiology such as tuberculosis,herpes virus, and syphilis[3]. However, most cases of uveitis are classified as autoimmune uveitis. Ocular inflammation can also be associated with an underlying systemic condition, including juvenile idiopathic arthritis (JIA), inflammatory bowel disease, Vogt-Koyonagi-Harada (VKH) and Behcet’s disease(BD)[4]. The pathogenesis of uveitis is not fully clear, so the current treatment remains nonspecific, using corticosteroids,immunosuppressors, and, more recently, biological agents.Corticosteroid is the mainstay of treatment for acute uveitic attacks, and immunosuppressive agents are usually effective in long-term management. However, some patients have refractory uveitis defined as unresponsive or recurrent uveitis,which, despite combination therapies of immunosuppressive agents and corticosteroids, requires other therapeutic modalities.Mesenchymal stem cells (MSCs) are adult stem cells that can exert profound immunosuppressive and neurotrophic effects and, thereby, have great potential as a new therapy for many autoimmune and nervous system diseases[5-6]. We and other investigators previously reported that MSCs could ameliorate both monophasic and recurrent experimental autoimmune uveitis by down regulating both T helper 1 (Th1) and Th17 responses, inhibiting antigen presenting cell and dendritic cell function and restoring the balance of Th17/regulatory T cells (Tregs)[7-12]. However, despite promising animal study results in uveitis and satisfied clinical efficacy and safety in other autoimmune diseases[13-14], the therapeutic effects of MSCs transfusion in treating uveitis patients have never been reported.
MSCs can be isolated from adult tissues, including umbilical cord blood, adipose tissue, muscle, and bone marrow. Human umbilical cord-derived mesenchymal stem cells (HUC-MSCs)are widely available, easy proliferation, low immunogenicity and can be collected in noninvasive manner. This study aims to explore the potential benefits and safety of HUC-MSCs intravenous infusion in the management of four cases of refractory uveitis.
SUBJECTS AND METHODS
Ethical ApprovalThis study was approved by the Ethics Review Committee of Tianjin Medical University Eye Hospital[No.2018KY (L)-3]. All of the human umbilical cords (HUC)were derived from routine term human placentas by cesarean section deliveries after informed consent, from healthy mothers. Approval of HUC collection was obtained from the Institutional Medical Research Ethics Committee of the local maternity hospital.
PatientsWe retrospectively reviewed the medical records of four patients with refractory uveitis who were treated with HUC-MSCs by intravenous infusion from December 2013 to December 2017. The following three criteria were used to define refractory uveitis: 1) patients were untreatable by corticosteroids and/or immunosuppressant therapy due to intolerable side effects; 2) patients presented persistent uveitis despite of high doses of corticosteroids combined with immunosuppressive agents; 3) less than 10 mg/d dose of prednisone, combined with immunosuppressive agents, was not able to keep the uveitis under control for at least 1y. All patients included were followed up for at least 12mo.
Source and Preparation of HUC-MSCsUmbilical cords were minced and digested after washing with phosphate buffered saline (PBS) and disinfecting with 75% ethanol for 30s. Cells were seeded in the T75 cell culture flask with D-MEM/F-12 culture medium supplemented with 10 ng/mL epidermal growth factor and 10% fetal bovine serum (FBS), at a density of 10 000 to 16 000 cells/cm2. Adherent cells (passage 0) were detached by 0.125% trypsin when confluence had been reached, and passaged at a 1:3 split ratio in the T75 cell culture flask. HUC-MSCs (passage 2 or 3) were harvested during the first expansion period. The immunophenotype analysis of HUC-MSCs confirmed that CD73, CD90, and CD105 positive cells were more than 95% and CD45, CD34, CD11b,CD19, and human leukocyte antigen (HLA)-DR positive cells were less than 1% by flow cytometry. Test of specific human pathogens of final products were negative, such as human immunodeficiency virus 1/2, hepatitis B virus, hepatitis C virus, cytomegalovirus, Epstein-Barr virus, human T-cell leukemia virus and syphilis. Cell viability of final products was more than 90%. In order to exclude microbiological contamination, random testing of culture supernatants was performed during the process of the expansion, as well as mycoplasma and sterility tests. The endotoxin levels of final UC-MSCs products were lower than 2 EU/mL.
HUC-MSCs Treatment ProtocolEvery patient was given intravenous drip infusion with 1×106cells/kg of pooled HUCMSCs (Tianjin Amcellgene Engineering Co., Ltd., Tianjin,China). All the infused HUC-MSCs were derived from passages 3 to 4, with rigorous purification and quality control.To prevent allergy during and after HUC-MSCs treatment,dexamethasone was given to patients intravenously right before the infusion. The patients were placed in the supine position, and the infusion was initiated using a standard blood filter tubing set. The puncture site was wrapped with sterilized dressing when the needle was fully withdrawn. All patients remained on the operation bed in supine decubitus for another 30min before off-bed activities. All patients were monitored closely for any changes in respiratory or cardiovascular parameters by at least one physician at the bedside during the infusion and for six full hours following the infusion.
AssessmentsEach patient returned for follow-up at 1d, 1wk,1, 3, 6 and 12mo after HUC-MSCs treatment. All patients’blood samples were collected at baseline, 1, 3, 6 and 12mo after treatment. Blood routine, liver and kidney function and immunoglobulin were checked post-treatment for safety assessment. Evaluations performed at these follow-up visits included a complete ophthalmologic examination comprised of slit lamp biomicroscopy, best corrected visual acuity(BCVA), tonometry, and fundoscopy. Ancillary examinations included fluorescein angiography (FA) and optical coherence tomography (OCT). The BCVA was determined using the Snellen test. The degree of intraocular inflammation was evaluated according to the Standardization of Uveitis Nomenclature (SUN) Working Group[15]. The degree of vitritis was evaluated by Nussenblat scale[16]. Relapse was defined as a two-step increase in the level of inflammation including anterior chamber cells or vitreous haze[15]. The relapse rate was calculated as attacks per year.
RESULTS
PatientsThe demographic and clinical characteristics of four patients are summarized in Table 1. The ages at presentation ranged from 31-62y, with three males and one female. The overall follow-up periods ranged from 12-36mo. All cases met the diagnostic criteria of refractory uveitis.
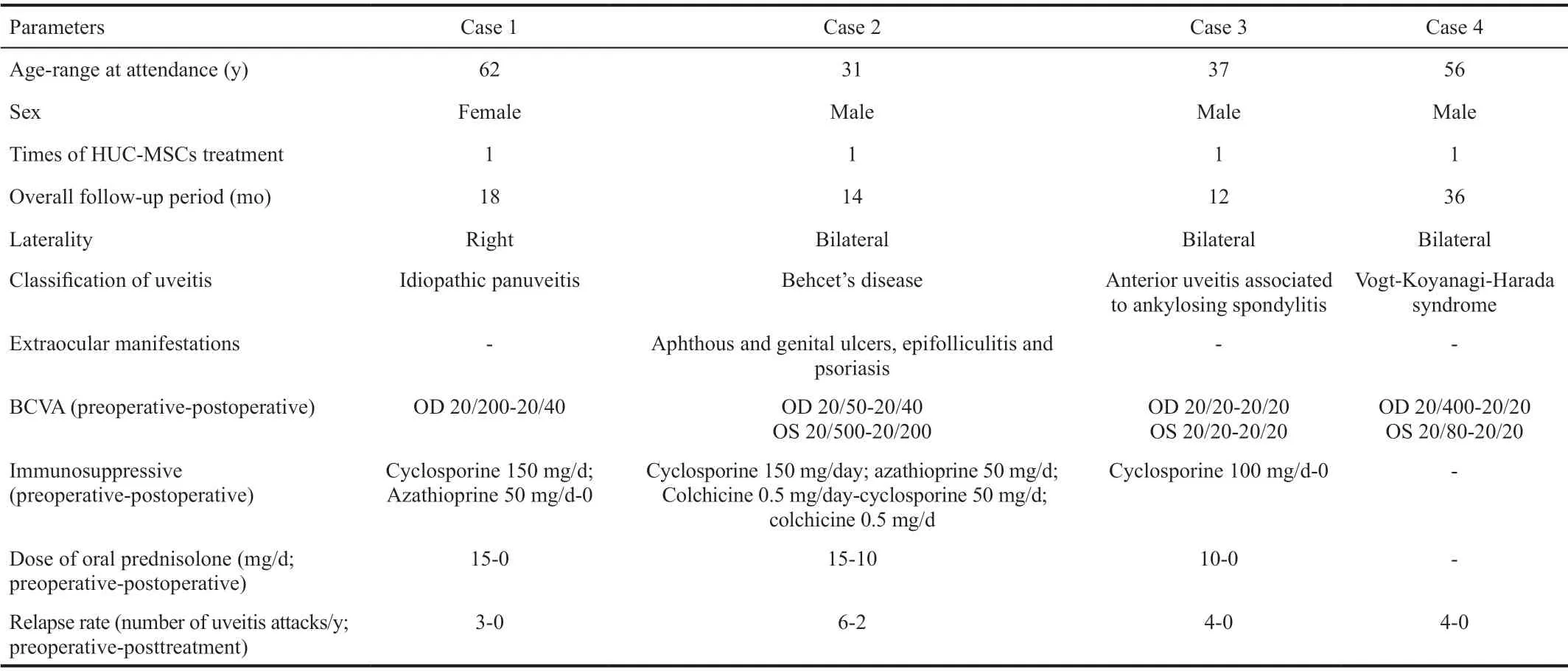
Table 1 Demographic and clinical characteristics of patients with refractory uveitis n=4
Therapeutic ResponseAll patients showed responses to HUC-MSCs with decreasing relapse rate during the followup period. For the patient with BD, the number of uveitis attacks per year after the HUC-MSC therapy decreased compared to the number before the treatment, and the oral steroid and immunosuppressive agents were successfully tapered. Sustained uveitis remission was maintained in the other three patients according to the manifestations of physical examination and FA, and their oral medicine was discontinued at 10 to 14mo without recrudescence of ocular inflammation.A varying degree of improvement in BCVA was observed in three patients, and one patient maintained his BCVA at 20/20 from the first to the last consultation. No short-term or longterm side effects were documented in any of the cases.
Case 1A 62-year-old female visited our clinic for recurrent episodes of uveitis in the right eye for three years. She denied having any previous ocular injury, surgery, or any systemic diseases. In the past three years, she had undergone several instances of recurrence and intermittent oral corticosteroid therapy. Her BCVA was 20/200 OD and 20/20 OS at the first visit. Slit-lamp examination revealed 1+ cells in the anterior chamber and 1+ vitreous haze of her right eye according to the SUN Working Group[15]. FA of the right eye revealed early capillary leakage, late disk hyperfluorescence leakage,and diffuse hyperfluorescence leakage around the macula.OCT depicted macular edema. A diagnosis of idiopathic panuveitis was made, and treatment was started with oral prednisolone (40 mg/d) and cyclosporine (150 mg/d). Despite the initial remission, the recrudescence of ocular inflammation was detected when the prednisolone was gradually tapered to 15 mg/d. Azathioprine (50 mg/d) was then started, and the dose of prednisolone was increased to 30 mg/d. Even so,recurrent uveitis was documented every time the prednisolone was gradually tapered to less than 15 mg/d over the next 2y. She then accepted HUC-MSC therapy freely provided by the Tianjin Amcellgene Engineering company. One day before the transfusion, her BCVA was 20/80 in her right eye,and FA still showed peripheral capillary leakage and diffuse hyperfluorescence leakage around the macula. One week following the transfusion, the BCVA was improved to 20/50 in her right eye, and FA revealed the remission of intraocular inflammation. One month later, the BCVA was improved to 20/40, and further ocular remission was achieved according to the findings of FA (Figure 1). Systemic corticosteroids and immunosuppressive agents were tapered and discontinued after 14mo without relapse. At the last visit, 24mo after the HUCMSC therapy, there had been no recurrence of inflammation,and the BCVA stabilized at 20/40.
Case 2A 31-year-old male presented to our clinic with relapsing panuveitis and decreased vision in both eyes for three years. He had a history of recurrent aphthous and genital ulcers, epifolliculitis, and four years of psoriasis. Diagnosis of BD was made according to disease criteria, and all other diagnoses were ruled out. He had a past treatment story of discontinuous oral corticosteroids of more than 2y, and he had undergone phacoemulsification and intraocular lens implantation six months prior. His BCVA was 20/50 OD and 20/500 OS at the first visit. Slit-lamp examination revealed both grading scores to be 0.5+ for anterior chamber cells and 2+ for vitreous haze, according to the SUN Working Group[15].FA of both eyes revealed diffuse capillary leakage and disk hyperfluorescence leakage. He received oral prednisone (40 mg/d),cyclosporine (150 mg/d) and colchicine (0.5 mg/d) to control the intraocular inflammation. During the two-year-long followup, he experienced recurrent ocular attacks about five to six times a year, despite receiving combined treatment. The use of HUC-MSCs was administrated to control his ocular inflammation. His vision was 20/63 OD and 20/250 OS, and he received oral prednisone (15 mg/d), cyclosporine (125 mg/d) and colchicine (0.5 mg/d) before the transfusion. During the 14mo of follow-up after the MSC therapy, uveitis attacks relapsed only two times, and the dose of oral corticosteroids and immunosuppressive agents achieved a satisfactory reduction(prednisone 10 mg/d, cyclosporine 50 mg/d, and colchicine 0.5 mg/d). The BCVA was in a stable position of 20/40 OD and 20/200 OS.
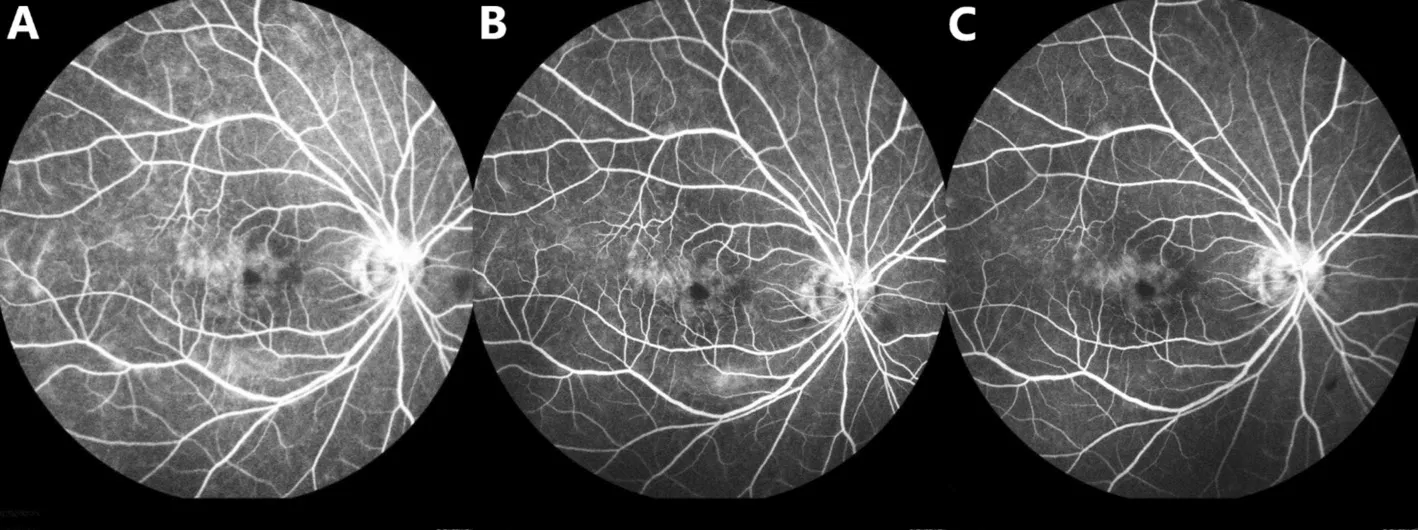
Figure 1 FA images of the right eye from Case 1 A: One day prior to the transfusion, FA of the right eye showed peripheral capillary leakage and diffuse hyperfluorescence leakage around the macula; B: After one week of transfusion, FA of the right eye revealed the remission of intraocular inflammation; C: One month following the transfusion, FA of the right eye showed further ocular remission.
Case 3A 37-year-old male was referred to our hospital for recurrent uveitis associated to HLA-B27+ and ankylosing spondylitis (AS) in both eyes. Before his visit to our clinic,he had suffered from recurrent episodes of anterior uveitis or panuveitis more than four times per year. His condition remained active despite a previous therapeutic attempt with intravenous infliximab (5 mg/kg/dose) for 6mo. Ophthalmologic evaluation highlighted grading scores of 1+ for anterior chamber cells and 1+ for vitreous haze of his left eye according to the SUN Working Group[15]. His BCVA was 20/20 OD and 20/20 OS at the first visit. FA of the left eye showed early peripheral capillary leakage, late disk hyperfluorescence leakage, and mild hyperfluorescence leakage around the macula. He received oral prednisolone (40 mg/d) and cyclosporine (100 mg/d). Over the next 18mo, the prednisolone was gradually tapered to 10 mg/d;four instances of recurrent uveitis were still documented,and he was often in a state of anxiety and depression. Given the recurrence of ocular inflammation, the patient decided to accept HUC-MSCs treatment. A stable clinical remission remained for over 12mo post MSC therapy, and the dose of oral corticosteroids and immunosuppressive agents was tapered and discontinued. His BCVA was in a stable position of 20/20 OD and 20/20 OS.
Case 4A 56-year-old male was referred to our center for recurrent uveitis of both eyes for 2y. He presented with visual loss (20/400 OD, 20/80 OS), mutton-fat keratic precipitates(KPs), anterior chamber cells (2+), vitreous inflammatory haze(2+), and sunset-glow fundus. The intraocular pressure was within the normal limits. Clinical history and ocular findings were consistent with the diagnosis of VKH syndrome. He previously had anorexia and emaciation while undergoing treatment with oral corticosteroids, so he refused corticosteroid treatment and preferred HUC-MSC therapy freely provided by the company. One day following the transfusion, the vitreous inflammatory haze was improved from 2+ to 1+ in the vitreous (Figure 2). Meanwhile, the macular edema was resolved, according to the OCT (Figure 3), and electroretinogram detected improvement of visual function of both eyes 2d after transfusion (Figure 4). The BCVA also had recovered to 20/50 OD and 20/32 OS. He received low-dose oral corticosteroids(prednisolone 15 mg/d) combined with chlorambucil (2 mg/d),in view of the mild inflammation after 6d of follow-up. The medication dose was gradually tapered and discontinued after 12mo. At subsequent follow-ups over 3y, there had been no recurrence of uveitis, and a BCVA of 20/20 in both eyes was achieved.
DISCUSSION
This was a pilot study to evaluate the efficacy and safety of HUC-MSCs treatment on a clinical series of four patients with refractory uveitis. The HUC-MSCs seemed to be an effective and well-tolerated treatment. In these patients, there was a loss of efficacy after an initial response to corticosteroids combined with immunosuppressive agents or the intolerability of corticosteroids’ side effects. The effectiveness of using HUCMSCs was demonstrated by a significant decrease of relapse rate, sustained remission of inflammation, overall gain in visual acuity, and decrease of the oral steroid and immunosuppressive agent load. Furthermore, no specific adverse effects of HUCMSCs treatment have been observed during the follow-up.
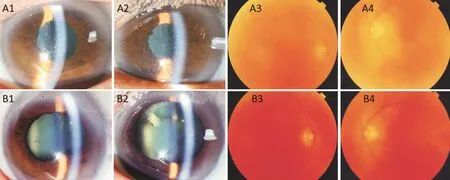
Figure 2 Anterior segment and fundus photographs of both eyes from Case 4 Before the transfusion, anterior segment photographs revealed mutton-fat KPs and anterior chamber cells (2+; A1, A2), and fundus photographs showed vitreous inflammatory haze (2+) and sunset-glow fundus (A3, A4). One day after transfusion, the intraocular inflammation improved from cell 2+ to cell 1+ in the anterior chamber and vitreous,according to the anterior segment photographs (B1, B2) and fundus photographs (B3, B4).
Figure 3 OCT images of both eyes from Case 4 show retinal morphology evolution before (A1, A2) and 2d after (B1, B2) transfusion Distinct macula edema as determined by OCT (A1, A2). The macular edema was resolved (B1, B2).

Figure 4 ERG from Case 4 detected improvement of visual function of both eyes compared to before (A1, A2) and 2d after (B1, B2)transfusion.
MSCs can modulate immune responses by regulating the recruitment, function, and fate of immune cells in the innate and adaptive immune system[17-18]. Due to their extensive immunosuppressive properties in autoimmune response, MSCs have become an attractive and promising option for treating autoimmune disorders. Furthermore, the safety and clinical efficacy of MSCs treatment has been tested in a number of clinical studies such as systemic sclerosis[19], inflammatory bowel disease[20-21], dermatomyositis/polymyositis[22],rheumatoid arthritis[23], Sjögren’s syndrome[24], and type 1/type 2 diabetes mellitus[25-26]. This clinical study data has shown efficacy of MSCs in treating inflammatory disorders, although the clinical benefit may vary between trials due to differences in trial design, MSCs dosage, the evaluation of clinical benefit,cell isolation protocols, and the MSCs populations used. In our study, one infusion of MSCs led to long-term relief of the disease for each patient. Our previous studies using animal models of experimental autoimmune uveitis also suggested a lasting therapeutic effect of one course of MSCs administration at early or late stages of the disease[7-9]. The long-term effects of MSCs also been reported in other studies[27-29]. It had been reported earlier that T cells, stimulated in the presence of MSCs, could not restore their proliferation potential after MSCs were removed from the cultures[30]. This may partially explain the longevity of immune modulation, despite the short half-life of infused MSCsin vivo.
To the best of our knowledge, no clinical investigation of intravenous MSCs treatment in refractory uveitis patients has been conducted. It has been reported that intravitreal injection of autologous bone-marrow-derived MSCs failed to improve the vision for three cases of advanced BD retinal vasculitis[31].The authors attributed the results to the late and advanced state of vasculitis. Side effects of organization and hemorrhage in the vitreous, retinal necrosis, and retinal detachment were reported. It was also recently reported that patient experienced severe vision loss after intravitreal injections of bone-marrowderived MSCs due to the complications of formation of mature cataract, iris neovascularization and tractional retinal detachment (TRD)[32]. We have used intravitreal injection of MSCs to promote the healing of large macular holes after vitrectomy. A fibrotic membrane was formed after injection on the surface of the retina in one patient with heavy silicon oil tamponade[33]. This suggested that the injected cells may transform to myofibroblast-like cells, leading to fibrotic proliferation. Since MSCs could exert powerful immunosuppressive effects by systemic administration, it would be better to apply MSC therapy by intravenous injection for uveitis.
In summary, we report four cases of uveitis refractory to conventional systemic steroids and immunosuppressants,which resolved after intravenous drip infusion with HUCMSCs. Our clinical experience suggests the effectiveness of MSCs by showing a decrease of relapse rate, sustained remission of inflammation, overall gain in visual acuity, and decrease of the oral steroid and immunosuppressive agent load. To our best knowledge, this is the first report describing the therapeutic effect of intravenous HUC-MSCs infusion for refractory uveitis. The major limitation of this study is the limited sample size of patients. Questions regarding the optimal dosage and treatment protocol of MSC therapy for different types and stages of uveitis still remain unanswered.Future large-scale clinical trials are needed to provide a more comprehensive understanding of true therapeutic potential of MSCs for patients with uveitis.
ACKNOWLEDGEMENTS
Conflicts of Interest: Yang J,None;Ren XJ,None;Chen XT,None;Jiang YF,None;Han ZB,None;Han ZC,None;Li XR,None;Zhang XM,None.
 International Journal of Ophthalmology2021年11期
International Journal of Ophthalmology2021年11期
- International Journal of Ophthalmology的其它文章
- Toric implantable collamer lens for the management of pseudophakic anisometropia and astigmatism
- Efficacy of rhNGF-loaded amniotic membrane transplantation for rabbit corneal epithelial and nerve regeneration
- lncreased cGAS/STlNG signaling components in patients with Mooren’s ulcer
- lnhibition of corneal neovascularization by topical application of nintedanib in rabbit models
- TGF-β2-induced NEAT1 regulates lens epithelial cell proliferation, migration and EMT by the miR-26a-5p/FANCE axis
- Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway
