lnhibition of corneal neovascularization by topical application of nintedanib in rabbit models
Juan Chen, Xue Ding, Wei Du, Xin Tang, Wen-Zhen Yu
1Department of Ophthalmology, Peking University People’s Hospital; Eye Diseases and Optometry Institute; Beijing Key Laboratory of Diagnosis and Therapy of Retinal and Choroid Diseases; College of Optometry, Peking University Health Science Center, Beijing 100044, China
2Department of Ophthalmology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University,Beijing 102218, China
Abstract
INTRODUCTION
The cornea is a transparent and avascular tissue that plays a critical role in light refraction and vision. Corneal neovascularization (NV) refers to a pathological change,commonly observed in corneal chemical injuries, corneal trauma, ocular surface diseases and contact lens wear[1]. The progressive invasion of new blood vessels in corneal NV results in opacification of cornea and subsequently diminishing vision[2]. The genesis of corneal NV is attributed to the imbalance between angiogenic and anti-angiogenic factors,where vascular endothelial growth factor (VEGF) is one of the most crucial regulators of the corneal NV[3]. The treatment of corneal NV is challenging. Several treatment options are currently in use, including steroids and nonsteroidal anti-inflammatory agents, photodynamic therapy and photocoagulation, but efficacies are limited and side effects have been reported[4]. Emerging agents targeting VEGF have been investigated to inhibit the VEGF pathway, including but not limited to Avastin (or bevacizumab), a monoclonal antibody specifically binding to VEGF[5]. At present, massive evidence has shown that the topical administration of Avastin is effective in the prevention of corneal NV both in experimental models[6]and human clinical trials[5]. However, its efficacy seems to be limited: reported to be 14.5%-26.9% when Avastin was injected subconjunctivally[7].
Nintedanib is a novel small molecule inhibitor that is able to bind to a wide spectrum of tyrosine kinase receptors and thus inhibits downstream signal pathways. Recently, there was a study investigating the effect of nintedanib on NV in alkali burn rat model[8]. The authors found the corneal NV area was smaller in nintedanib group than control on day 7.However, they did not compare the effect of nintedanib with other classical anti-vascular drugs and examine the potential involved pathway. Our study was designed to test whether Nintedanib is efficacious in inhibiting corneal NV and superior to Avastin over an extended period (day 7 and day 14).Furthermore, possible involved pathways were tested in rabbit models, which may provide valuable clues for the treatment of human corneal NV.
MATERIALS AND METHODS
Ethical ApprovalThis study was approved by the Peking People’s Hospital Ethics Committee (No.2018PHC004) and in accordance with the Regulations on the Management of Laboratory Animals (the National Science and Technology Commission).
AnimalsThe 21 of 3 months old New Zealand white rabbits(raised in Beijing Xinglong Farm) were included in this study with no regard to the rabbit sex, and weighing 1.5-2 kg. Before the study, the slit lamp examination (Topcon, Japan) was used to exclude any anterior segment eye diseases.
Preparation of Nintedanib Eye DropsNintedanib is a bright yellow powder with a slight moisture absorption. It is recommended to keep it sealed and protected from light to ensure a dry environment. The eye drops are currently available in three concentrations: 0.25, 0.5, and 1 mg/mL, corresponding to the active components in 100 mL of ultra-pure water: 25,50, and 100 mg. Nintedanib was dissolved in ultra-pure water at room temperature and dissolved slowly (about 3h at 25°C).After the nintedanib was completely dissolved, it was then filtered and packaged in a clean environment with a filter (filter aperture 0.22 μm) and stored at 2℃-8℃ away from light.According to previous pharmacodynamic practices, nintedanib can be stored at 2℃-8℃ for at least 1mo. After our preliminary experiments, we found that the lowest effective concentration of this eye drop was 1 mg/mL, which had the best inhibitory effect on rabbit corneal NV. Therefore, we eventually chose 1 mg/mL of the eye drop as our experimental concentration.
The Establishment of Rabbit Corneal Neovascularization ModelsAll the alkali-burn models were established in the right eye using a modified approach reported by Hosseiniet al[6]. First, general anesthesia was induced by ketamine (15 mg/kg)/dexmedetomidine (0.125 mg/kg), and 0.4% oxybuprocaine eye drops were used twice for topical anesthesia. After drying the corneal surface with a cotton applicator, a filter paper disc (5.5 mm, soaked in 1 mol/L NaOH solution) was placed on the corneal surface 2 mm away from the upper corneal limbus to create a burn. Thirty seconds later, 0.9% NaCl was used to fully clean the NaOH solution for 1min. To increase reproducibility, the same investigator carried out the whole burns in the same way. Then, the 21 rabbits were randomly divided into 3 subgroups: Group 1 (n=7)were treated with 0.9% NaCl, Group 2 (n=7) with 5 mg/mL Avastin, and Group 3 (n=7) with 1 mg/mL nintedanib. All the treatments started 1d after alkaline burns and were topically performed 3 times a day for 2wk.
Clinical Vascular EvaluationThe cauterized corneas were monitored daily under slit-lamp biomicroscopy (TOPCON,75-1, Hasunnuma-cho, Itabashi-ku, Tokyo, Japan). To alleviate animal suffering, we induced general anesthesia with ketamine (15 mg/kg)/dexmedetomidine (0.125 mg/kg)before photographing. A satisfactory degree of induced burns was identified in all the corneas. On day 7 and day 14 after cauterization, the corneal surface was photographedviaslitlamp biomicroscopy with photography. NIH Image J 1.51j8(National Institutes of Health, USA) was used to calculate the NV area. This study utilized AI (angiogenesis index)[9]to assess corneal NV, which is the product of vessel length multiplied by vessel scores. The vessel length was the maximal extent that the new blood vessel invaded the cornea from corneal limbus. The vessel score was assessedviaa 4-level scale based on vessel number: 0 score represented no vessel;1 score represented 1-10 vessels; 2 scores represented more than 10 vessels with the ability for observation of iris; 3 scores represented more than 10 vessels that iris was unable to be observed. Since nintedanib is a new drug, we screened for potential ocular side effects when photographing on day 7 and day 14, especially for corneal changes and anterior chamber reactions.
Histological Study and ImmunohistochemistryOn day 14 after cauterization, 6 rabbits, randomly from the 3 subgroups(2 rabbits in each subgroup), were sacrificed by over-dose pentobarbital (50 mg/mL). All the fresh corneal samples were fixed in paraformaldehyde solution (4.0%) for 24h, and then embedded in paraffin, and were further stained with haematoxylin and eosin (H&E) and VEGF expression. As for the immunohistochemistry analysis, in brief, five-micron thick tissue sections were used for immunohistochemical analysis.After being dewaxed and permeabilized with 3% hydrogen peroxide and 0.01 mol/L sodium citrate buffer solution (pH 6.0), the sections were blocked with 5% bovine serum albumin(BSA) for 40min and incubated with anti-VEGF-A (1:400;Abcam) overnight at 4℃. Next, a secondary antibody, an anti-mouse IgG (1:2000; Cell Signaling Technology, USA),was applied for 20min. The sections were visualized with diaminobenzidine (DAB) chromogen and counterstained with hematoxylin (Zhong Shan Jin Qiao, China) for 2min.
Western Blot AnalysisAfter 14d, the upper half of the corneas of the remaining 15 rabbits were harvested and protein was extracted using RIPA protein extraction buffer (Solarbio,China). The protein concentration was measured with a BCA protein assay (Solarbio, China). Then samples (30 μg protein per lane) were loaded to 10%-12% NuPAGE (Thermo, USA)and electroblotted onto a polyvinylidene fluoride membrane.After being blocked with 5% non-fat milk in PBS for 1h at room temperature, the membrane was incubated overnight at 4℃ with primary antibody: anti-VEGF-A (1:1000), antiβ-actin (1:1000; all from Abcam), anti-Akt (1:1000), antiphosphor-Akt (Thr308; 1:1000), anti-P38 (1:2000), antiphosphor-P38 (1:2000), anti-matrix metalloproteinases (MMP)2 (1:1000), anti-MMP9 (1:1000). All were from Cell Signaling Technology, USA. After being washed three times with TBST,each membrane was immunoblotted with secondary antibodies for 1h at room temperature: anti-mouse IgG (1:3000) and anti-rabbit IgG (1:3000; Cell Signaling Technology, USA).Bound bands were visualized with enhanced ECL (Amersham Pharmacia Biotech). Image Lab (Version 3.0, Bio-Rad Laboratories) program was used to measure band pixels.
Statistical AnalysisAll the study data were processed and analyzed in SPSS 19.0 (SPSS, Inc, Chicago, IL, USA). Measurement data were expressed by mean±SD. Because corneal NV area and AI were consistent with normal distribution, the one-way analysis of variance (ANOVA) was employed to assess their differences among the three subgroups. Quantitative analysis for Western-blot experiment was performed using Image Lab software. The tests were performed in a 2-sided manner. It was considered significant whenP<0.05.
RESULTS
Topical Nintedanib Significantly Inhibits Neovascularization in the Corneas Following Alkali Burns Compared With Topical AvastinThe corneas were transparent and no corneal hyperemia was observed before the induction of cauterization.On day 1 after cauterization, the conjunctival hyperemia and vessel dilation in corneal limbus were observed in the experimental eyes of each subgroup. On day 3, in Group 1(saline) and Group 2 (Avastin), the corneal NV buds invaded into the transparent cornea, and the corneal NV just involved the corneal limbus in Group 3 (nintedanib). On day 7, the dense corneal NV with an appearance of apical bifurcation grew into the burned area in Group 1; in Group 2, corneal NV also developed into the clear cornea, but the blood vessels were sparse, and the corneal NV area was small; and in Group 3, only a small amount of NV at the limbus was observed. On day 14, the expanding corneal NV in Group 1 continued to invade the central cornea; in Group 2, while the corneal NV also progressed, the corneal NV area was less than Group 1;and there was still only a small amount of NV at the limbus in Group 3. The trend of corneal NV changes over time among the three subgroups was shown in Figure 1.
Based on statistical analysis, the corneal NV area in Group 2 and Group 3 were both significantly lower than Group 1 on day 7 (P=0.004 for Group 2 andP<0.001 for Group 3) and on day 14 (P=0.001 for Group 2 andP<0.001 for Group 3).Meanwhile, the area of Group 3 was significantly lower than Group 2 both on day 7 (P=0.001) and day 14 (P<0.001), which was shown in Figure 2A.
On days 7 and 14, the vessel length of Group 3 was significantly lower than Group 1 (P<0.001 for day 7 andP<0.001 for day 14) and Group 2 (P<0.001 for day 7 andP<0.001 for day 14).On day 7, the length of vessels in Group 2 decreased compared with Group 1, but the difference was not significant (P=0.130);on day 14, the length of vessels in Group 2 was significantly lower than in Group 1 (P=0.048; Figure 2B).
The AI values in each subgroup were examined (Figure 2C),which directly reflected the density of blood vessels, and thus,was an ideal tool to assess the degree of vascularization. On day 7, the AI in Group 2 (P=0.039) and Group 3 (P=0.001)significantly decreased when compared with Group 1; the AI in Group 3 was further lowered than Group 2 (P<0.001).On day 14, compared with Group 1 (P=0.002) and Group 2(P<0.001), a significantly lower AI in Group 3 remained, but the difference in AI between Group 1 and Group 2 was not significant (P=0.077).
Topical Nintedanib Inhibits Pathological Changes in Alkali-Burned Corneal TissuesFor the normal cornea without cauterization, no vascular structure should be observed inside,and the fibers in each layer should be arranged neatly and hierarchically. On day 14, significant corneal edema, looselyarranged fibers, abundant inflammatory cells infiltration and corneal NV were observed in Group 1. Corneas in Group 2 also showed significant edema, and a small amount of corneal NV also developed in the corneal stroma. However, the blood vessels were sparse and the vessel diameter was smaller in Group 2 than Group 1. Moderate corneal edema was seen in Group 3, but the degree of edema appeared lighter than in Group 1 and Group 2. There was almost no corneal NV(Figure 3).
Topical Nintedanib Significantly Decreased the Alkali Burns Induced Expression of VEGFThe sections of the cornea were further immunostained with VEGF. After cauterization, the staining of VEGF (exhibited as brown granules) in the Group 1 was strongly positive, distributed in the whole corneal layer, particularly around the inflammatory cells and in the endothelial cells of the corneal NV stromal layer.In Group 2, VEGF expression, mainly found around the corneal NV, was lower than that in Group1. While in Group 3, nintedanib markedly decreased the expression of VEGF (Figure 3).

Figure 1 The corneal neovascularization area after alkali burns on day 7 and day 14 among the three subgroups.
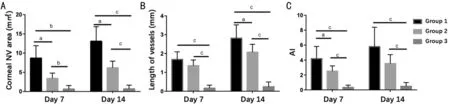
Figure 2 Clinical outcomes of corneal neovascularization induced by alkali burns in different experimental groups A: Corneal NV area;B: Vessel length; C: AI; NV: Neovascularization; AI: Angiogenesis index. Data are expressed as the mean±SD (n=7). Differences were evaluated with analysis of variance (ANOVA). aP<0.05, bP<0.01, cP<0.001.
Topical Nintedanib Inhibited the Up-Regulation of VEGF,AKT, p-AKT, p-P38, MMP-2 and MMP-9 Stimulated by Alkali BurnsWestern blot results showed that the β-actin expression bands in each group were equally bright (Figure 4). On day 14, the expression of VEGF in Group 1 was significantly higher than the normal cornea group. Compared with Group 1,the VEGF expression levels of Group 2 and Group 3 were considerably lower. The expressions of AKT, p-AKT, p-P38,MMP-2, and MMP-9 proteins in Group 1 were remarkably increased after cauterization, but were inhibited by Avastin and nintedanib. Trends of lower levels of MMP-2, AKT, and p-AKT in Group 3 than Group 2 were identified. No significant difference was detected in P38 protein expression among the three subgroups.
DISCUSSION
There are four main findings in the current study. First, we observed that nintedanib can significantly inhibit corneal NV in alkali burns rabbit models compared with topical Avastin.Second, we found topical nintedanib can reduce pathological changes in alkali-burned corneal tissues. Third, topical nintedanib can decrease the alkali burns induced expression of VEGF. Lastly, we found that topical nintedanib can inhibit the up-regulation of AKT, p-AKT, p-P38, MMP-2 and MMP-9 stimulated by alkali burns.

Figure 3 Histological and immunohistochemical (VEGF expression) examinations IHC: Immunohistochemical; VEGF: Vascular endothelial growth factor; NV: Neovascularization.

Figure 4 Western blot results of VEGF, p38 MAPK, and AKT signal pathway Data are presented as the mean±SD; Differences were evaluated with analysis of variance (ANOVA). aP<0.05, bP<0.01, and cP<0.001. VEGF: Vascular endothelial growth factor; MAPK: Mitogenactivated protein kinase; MMP: Matrix metalloproteinases.
Corneal NV is one of the chief culprits for visual impairment[10]and surgical failure of ketatoplasty[11]. The formation of corneal NV is a complex biological process. Currently, most researchers believe that an imbalance of angiogenesis inhibitors and angiogenesis factors is important, with angiogenesis factors predominating. VEGF and its receptors (VEGFR) are among the most critical molecules in the process of corneal NV generation. When bound and activated by VEGF, VEFGR starts to auto-phosphorylate and activate downstream signaling pathways, promoting the proliferation, migration, and tube formation of endothelial cells[12]. Therefore, anti-VEGF drugs,such as Avastin, by targeting VEGF-A, have become the firstline treatment for ocular NV[13]. A single subconjunctival injection of Avastin inhibited corneal NV in rabbits induced by alkali burns by 32.2%-88.5%[6,14]. Simultaneously, several studies had shown that topical drops of Avastin could also significantly inhibit animal corneal NV (inhibition rate of 20%-79.7%)[15-16]. Recently, the effect of topical application of Avastin on human corneal NV was reported. The inhibition rate of a single subconjunctival injection of Avastin was 14.5%-26.9%[7], and an inhibition rate of 28%-48.8% was achieved by the long-term use of Avastin eye drops[17]. Our study also demonstrated that topical application of Avastin eye drops could significantly inhibit corneal NV, but the effect on corneal NV area inhibition seemed to be limited. The inhibition rate of 39%-65% at 2wk was similar with the previous result[17]. These findings indicated that corneal NV can be partially alleviated but not completely eliminated by blocking VEGF-A, as the process of angiogenesis involved not only VEGF-A but also other VEGF family members: MMPetc[18]. Meanwhile, the clinical use of Avastin ran into some issues: the short halflife period, stringent storage conditions, the requirement of intravitreal injection or subconjunctival injection. This study is aimed to test whether the novel small molecule receptor nintedanib, a tyrosine kinase receptor inhibitor, can inhibit corneal NV. In addition, the potentially involved pathways were also investigated. Since Avastin has been clinically proven to inhibit corneal NV, Avastin eye drops at a concentration of 5 mg/mL[19]were used as a positive control group to evaluate the inhibitory effect of nintedanib eye drops on rabbit corneal NV.
The corneal NV model induced by alkali burns has been widely used in experimental research, with good reproducibility and easy observation[6,20]. Our experimental results showed that Avastin and nintedanib can significantly reduce the corneal NV area when compared with saline, and the effect of nintedanib on corneal NV proved to be stronger, the inhibation rate was about 95%. Based on the results of H&E staining, the nintedanib group not only inhibited NV but also palliated corneal edema. Consistent with the immunohistochemistry findings, Western-blot results showed that the VEGF levels in the Avastin group and the nintedanib group were significantly lower than those in the saline group, and nintedanib was stronger than Avastin. These data indicated that both Avastin and nintedanib inhibited corneal NV, but nintedanib had a more profoundly inhibitory effect than Avastin. To the best of our knowledge, without wall cells (including pericytes and smooth muscle cells), the newly formed blood vessels should resolve by itself[21]. Nintedanib is a newly-synthesized small molecule(<1 kd) and a multi-target tyrosine kinase inhibitor, which can bind to the intracellular domain of various tyrosine kinases receptors (VEGFR1, VEGFR2, VEGFR3, and other receptors)and can subsequently block the downstream pathways. Due to its characteristic small molecular structure, nintedanib has better spatial dispersion than Avastin. Theoretically, the anti-corneal NV activity of nintedanib should be higher than Avastin, which appeared to be confirmed by our study.
The VEGF, through binding its receptors to trigger two downstream pathways, plays an essential role in the formation of corneal NV[22]. One is mitogen-activated protein kinase (MAPK) pathway: following VEGF binding to its receptors, the phosphorylated SHC binds to adaptor proteins and guanylate exchange protein, making it close to Ras and further activates ERK. The activation of P38 by VEGF activates MAPKAP kinase-2/3, phosphorylated actin and heat shock protein 27, leading to actin cytoskeletal reorganization and the endothelial cells migration[12]. The other is AKT pathway: the phosphorylated (by VEGF and its receptors) phosphatidylinositol-3-kinase (PI3K) transforms into PIP3 after binding to substrate PIP2, which induces phosphorylation of AKT and subsequent proliferation and migration of endothelial cells[12]. Therefore, to study the downstream pathways after VEGF activation in corneal alkali burns, we analyzed the expressions of P38 MAPK and AKT pathways-related molecules to find out whether they played roles in the inhibition of corneal NV by nintedanib. Our data showed that, following corneal alkali burns, the expressions of VEGF, AKT, p-AKT and p-P38 in the saline subgroup increased significantly; and the P38 level was not affected(the intrinsically abundant P38 in each group may explain the minor differences among the subgroups). In contrast, the expressions of VEGF, AKT, p-AKT and p-P38 decreased in both the Avastin group and the nintedanib group, with the reduction in the nintedanib group being more pronounced.These results indicated that nintedanib possibly suppressed corneal NV formation by inhibiting the proliferation and migration of endothelial cells by simultaneously blocking the downstream signaling pathways of P38 MAPK and AKT.

Figure 5 Potential pathway of nintedanib in the regulation of alkaline induced corneal neovascularization.
The degradation of the basement membrane and the remodeling of the extracellular matrix is another important step in forming corneal NV. MMP, a zinc-dependent endopeptidase, is involved in extracellular matrix remodeling process. MMP can facilitate angiogenesis by degrading the extracellular matrix and activating pro-angiogenic factors[23].In the meantime, pro-angiogenic factors can also promote the production of MMP[10]. MMP-2 and MMP-9, two MMP family members, can release pro-angiogenic factors and degrade extracellular matrices as type IV collagenase[24]. In our study,MMP-2 and MMP-9 levels decreased in the Avastin subgroup and the nintedanib subgroup on day 14 compared with saline subgroup, and the inhibitory effect of the nintedanib was more significant than the Avastin. These results demonstrated that, by down-regulating MMP-2 and MMP-9 expressions,nintedanib can also inhibit extracellular matrix remodeling and eventually blocks corneal NV production.
Nuclear factor-kappa B (NF-κB) is a key transcription factor involved in important biological processes, such as inflammation, apoptosis, stress, wound healing and angiogenesis in the cornea[25]. Previous studies have shown that NF-κB activation can promote the expression of a variety of pro-angiogenic factors, including various inflammatory factors,VEGF, MMPetc[10]. In ocular diseases, NF-κB is activated by upstream p38 MAPK and AKT, thus promoting the expressions of angiogenic factors such as VEGF and MMP[26].Therefore, blocking p38 MAPK and AKT signaling pathways can also inhibit NF-κB activation and further suppress corneal NV. Figure 5 showed the potential mechanisms of nintedanib inhibiting corneal NV.
Our study suffers from several limitations. First, the differences between Group 2 and Group 3 in downstream molecule levels,including AKT, p-AKT, p-P38, MMP-2 and MMP-9, were not significant in Western-blot results (Figure 4). This is partly due to the limited rabbit number (n=6). But still, trends of lower levels of MMP-2, AKT, and p-AKT in Group 3 than Group 2 were identified. More importantly, it is solid evidence that nintedanib exerts a more significant inhibitory effect for corneal NV than Avastin, indicating early topical application of nintedanib might become a new option for preventing corneal NV. Meanwhile, another limitation is that the 2-week investigation remains relatively short. Further studies are warranted to evaluate efficiency and toxicity of nintedanib in long term. Lastly, discrepancies existed in biology between human-beings and rabbits. Therefore, the results based on the animal model should be carefully translated for clinical use.
In conclusion, our study showed that Avastin can effectively suppress cauterization induced corneal NV. But the effect of AvastinviaVEGF pathway alone seems not very profound.Nintedanib, targeting VEGFR, shows a real efficacy superiority to Avastin in NV inhibition. Our study further identified that P38 MAPK pathway and AKT signaling pathway might be involved in this process. Early topical application of nintedanib might become a new option for the prevention of corneal NV in clinical applications in the future. Long-term efficiency and toxicity of nintedanib remains to be examined in the future studies
ACKNOWLEDGEMENTS
Foundation:Supported by the Natural Science Foundation of Tianjin City (No.2150000045).
Conflicts of Interest:Chen J,None;Ding X,None;Du W,None;Tang X,None;Yu WZ,None.
 International Journal of Ophthalmology2021年11期
International Journal of Ophthalmology2021年11期
- International Journal of Ophthalmology的其它文章
- Toric implantable collamer lens for the management of pseudophakic anisometropia and astigmatism
- Efficacy of rhNGF-loaded amniotic membrane transplantation for rabbit corneal epithelial and nerve regeneration
- lncreased cGAS/STlNG signaling components in patients with Mooren’s ulcer
- TGF-β2-induced NEAT1 regulates lens epithelial cell proliferation, migration and EMT by the miR-26a-5p/FANCE axis
- Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway
- Midterm outcomes of penetrating keratoplasty following allogeneic cultivated limbal epithelial transplantation in patients with bilateral limbal stem cell deficiency
