lncreased cGAS/STlNG signaling components in patients with Mooren’s ulcer
Ya-Ni Zhang, Yan-Ling Dong, Wen-Pei Hao, Xiao-Fei Bai, Xia Qi, Ting Liu,Xiao-Tong Sun, Chao Wei, Xiao-Lin Qi,5
1Xi’an Children’s Hospital, Xi’an 710004, Shaanxi Province,China
2Shandong Eye Institute, Shandong First Medical University& Shandong Academy of Medical Sciences, Qingdao 266071,Shandong Province, China
3Qingdao Eye Hospital of Shandong First Medical University,Qingdao 266071, Shandong Province, China
4State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao 266071, Shandong Province,China
5Eye Hospital of Shandong First Medical University, Jinan 250021, Shandong Province, China
Abstract
INTRODUCTION
Mooren’s ulcer (MU) is a rare refractory chronic disease,characterized with painful peripheral or central corneal ulceration, and the ultimate perforation of the eye[1-2]. Currently,there is no specific treatment for MU, immunosuppressive agents, glucocorticoids, and combined with surgical treatment were mainly used[3]. Several lines of evidence indicated that MU is an organ-specific autoimmune disease, especially the findings of autoantibody against corneal-associated antigen[4].Histopathological analysis showed the aggravated infiltrations of immune cells, including CD4+T cells, macrophages and B-lymphocytes[5-6]. Complement system was also reported to be associated with MU[7]. These findings suggested both cellular and humoral immunity contributed to the pathogenesis of MU[4,8-9]. However, due to the shortage of animal models,the etiopathogenesis of MU remains largely unknown.
Cyclic GMP-AMP synthase (cGAS) is one of the important cytosolic DNA sensors, which recognizes nuclear or mitochondrial DNA, and then catalyzes the production of cyclic GMP-AMP (cGMP-AMP or cGAMP)[10-12]. The cGASinduced cGAMP binds to stimulator of interferon genes(STING) and leads to the production of type I interferons(IFN-Is) and inflammatory cytokines through phosphorylation of interferons regulatory factor 3 (IRF3) and activation of NF-κB signaling[13-14]. As an important innate signaling, the cGAS/STING signaling pathway reportedly plays important roles in defense against pathogen infections and anti-tumor immunity[15]. The pathological roles of cGAS/STING signaling in inflammatory and autoimmune disorders were also well established, such as age-related macular degeneration,ischemic stroke, aging, systemic lupus erythematosus (SLE),and neurodegeneration[16-19].
However, the information on the associations of cGAS/STING signaling with MU pathogenesis remains limited. In this study, we examined the expression of cGAS/STING signaling components in MU samples and investigated the effect of cGAS/STING signaling on inflammatory response using human corneal epithelial cells (HCECs).
MATERIALS AND METHODS
Ethical ApprovalThis study was approved by the ethical committees of Shandong Eye Institute and strictly adhered to the guidelines of the Declaration of Helsinki. Written informed consents were obtained from the patients, and no patients received a stipend in the study.
Cell CultureHCECs were cultured in DMEM/F-12 medium containing 10% fetal bovine serum. The cGAMP (1 μg/mL)(InvivoGen, USA) was used to investigate the effect on cGAS/STING signaling on HCECs according to Daiet al’s methods[13].Briefly, the HCECs in six-well plates were permeabilized in permeabilization buffer (50 mmol/L HEPES, 100 mmol/L KCl,3 mmol/L MgCl2, 0.1 mmol/L DTT, 85 mmol/L sucrose, 0.2%BSA, 1 mmol ATP and 0.1 mmol GTP, pH 7) with 1 μg/mL digitonin (Sigma, USA) and cGAMP in the presence or absence of C-176 (20 μmol, MedChemExpress, USA). After incubation at 37℃ for 30min, the permeabilization buffer was replaced with DMEM/F-12 medium and cells were cultured for indicated time. The supernatants and cells were then collected for subsequent experiment, respectively.
Clinical SamplesClinical samples were obtained from Qingdao Eye Hospital of Shandong First Medical University,and used to investigate the potential participation of cGAS/STING signaling in MU development. The diagnosis was based on the clinical presentation, past medical history, and laboratory investigations. Patients with other systemic diseases that cause the same eye manifestations have been excluded.The samples were surgically removed from the diseased corneas of the patients with MU. Some clinical specimens in this study also came from wax blocks embedded in the Department of Pathology. The residual corneal-scleral rings of healthy donor corneal grafts were collected for controls.
Western BlotThe cells/tissues lysates were prepared in RIPA lysis buffer containing a mixture of phosphatase inhibitors(Millipore, Germany) and protease inhibitors (Solarbio,Beijing, China). The protein concentrations were quantified by BCA protein assay kit (Beyotime, Shanghai, China), and then separated by SDS-PAGE using a 10% SDS-PAGE gel and transferred to a PVDF membrane. The specific primary antibodies were as follows: anti-IRF3 (1:1000, 4302s, Cell Signaling Technology, USA), anti-phospho-IRF3 (1:1000,4947s, Cell Signaling Technology, USA), anti-cGAS (1:500,40192, arigo, Shanghai, China), anti-STING (1:1000, 13647s,Cell Signaling Technology, USA). Horseradish peroxidaseconjugated goat anti-rabbit (ZB-2301, Beijing Zhongshan Golden Bridge Biotechnology, China) second antibody was used to visualize the target proteins using immobilon western chemiluminescent HRP substrate (Millipore, USA).
Real-time Polymerase Chain ReactionThe total RNA of the cells was extracted by RNA extraction kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The reverse transcriptase reactions were performed using HiScript®III RT SuperMix for quantitative polymerase chain reaction (qPCR) (Vazyme, Nanjing,China). Real-time qPCR was performed using a 7500 real-time PCR system with a ChamQ universal SYBR qPCR master mix (Vazyme, Nanjing, China). Data were calculated by using a comparative cycle threshold (CT) method(2-ΔΔCt) and normalized using GAPDH primers as an internal control. The primers used in this study were as follows:GAPDH, 5’-CATGTTCGTCATGGGTGTGAA-3’,5’-GGCATGGACTGTGGTCATGAG-3’; IFN-β,5’-TACTGCCTCAAGGACAGGATGA-3’,5’-CCAGCCAGTGCTAGATGAATCTT-3’; CXCL-10,5’-CGCTGTACCTGCATCAGCAT-3’, 5’-TT CTGGATTCAGACATCTCTTCTCA-3’; IFIT-1,5’-CCGCTTAAATCCAGACAATGG-3’,5’-AACTTGGCTGCATATCGAAAGAC-3’; IL-6,5’-TGGCTGAAAAAGATGGATGCT-3’,5’-TCTGCACAGCTCTGGCTTGT-3’.
Enzyme-linked Immunosorbent AssayThe supernatants of HCECs were collected to detect IFN-β content at 6h after stimulation with cGAMP using a human IFN-β enzyme-linked immunosorbent assay (ELISA) kit (KE00187, Proteintech,USA) according to the manufacturer’s instructions.
Immunofluorescence StainingThe cGAMP-treated HCECs were collected and fixed with 4% paraformaldehyde for 20min at room temperature. Cells were then permeabilized with 0.1% Triton X-100 for 20min, and blocked with 5% BSA for 1h. Anti-phospho-IRF3 (1:200, 29047s, Cell Signaling Technology, USA) was used to label the activated IRF3. DAPI was used to reveal all of the cells in each section.
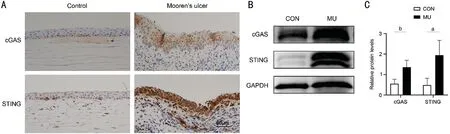
Figure 1 The increased protein levels of cGAS/STING signaling components in MU samples A: Representative images of IHC staining against cGAS and STING in the patients with MU (magnification 200×, scale bar 50 μm); B: Western blot analysis of cGAS and STING protein levels in the patients with MU; C: Quantitative analysis of cGAS and STING in MU samples versus healthy donors. aP<0.05, bP<0.01, n=3.
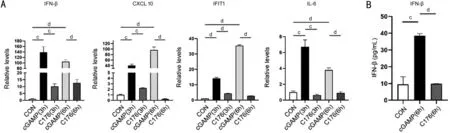
Figure 2 The up-regulation of IFN-β and ISGs in cGAMP-treated HCECs A: Quantitative analysis of mRNA level of IFN-β and ISGs in cGAMP treated HCECs in the presence or absence of C-176; B: ELISA analysis of IFN-β in the supernatants of HCECs treated with cGAMP in the presence or absence of C-176. cP<0.001, dP<0.0001, n=3.
ImmunohistochemicalFor immunohistochemical (IHC)staining, the paraffin sections were deparaffinized and rehydrated, and then immersed into sodium citrate antigen retrieval solution (pH 6.0) for antigen retrieval. Tissue sections were treated with 3% hydrogen peroxide (H2O2)at room temperature and then incubated overnight in 4℃with the following primary antibodies: anti-IRF3 (1:400,ab68481, abcam, UK), anti-cGAS (1:500, ab224144, abcam,UK) and anti-STING (1:200, ab227704, abcam, UK).Staining was developed using DAB followed by hematoxylin counterstaining. Finally, the slides were dehydrated in gradient ethanol and mounted with neutral resin. Nucleus stained with hematoxylin is blue. The positive cells developed by DAB reagent were brown yellow.
Statistical AnalysisAll experiments were performed at least in triplicate, and the data was expressed as the mean±standard deviation (SD). Two-tailed Student’st-tests were used for comparisons between two groups, One-way ANOVA was employed for 3 or more groups.P-value of less than 0.05 was considered statistical significance.
RESULTS
Increased Expression of cGAS/STING Signaling Components in MU SamplesThe protein levels of cGAS/STING signaling components in the MU samples were detected using IHC and Western blot. The IHC staining showed that the levels of cGAS and STING in MU samples were much higher than those in control group (Figure 1A).cGAS and STING were mainly located in ocular epithelial cells and infiltrated inflammatory cells. As shown in Figure 1B and 1C, the levels of cGAS and STING protein in MU patients were significantly up regulated compared with the controls,which was quite similar with the results from IHC staining.
Up-regulation of IFN-β and ISGs in cGAMP-treated HCECsAlthough the location of cGAS and STING were mainly in ocular epithelial cells and infiltrated inflammatory cells in MU patients through IHC staining, the exact roles of cGAS/STING signaling pathway on MU remains unknown.Therefore, HCECs were selected to investigate the roles of cGAS/STING signaling pathway in MU pathogenesis. As presented in Figure 2A, cGAMP treated HCECs showed a much higher transcriptional level of IFN-β and ISGs (including CXCL10, IFIT1, and IL-6) than in untreated controls at 3 and 6h. The concentration of IFN-β in the supernatants was also significantly elevated after stimulation with cGAMP at 6h(Figure 2B), which was consistent with the results from realtime PCR. On the contrary, when pre-treated with C-176 (an inhibitor of cGAS/STING signaling pathway[20]), the effect of cGAMP on HCECs was pronouncedly reversed, with less expression of IFN-β and ISGs. The results indicated that cGAS/STING signaling pathway can be activated in HCECs.

Figure 3 The elevated IRF3 phosphorylation in HCECs after cGAMP treatment A: Western blot analysis of phosphorylated IRF3 in HCECs stimulated with cGAMP at 0.5, 1 and 3h in the presence or absence of C-176; B: Quantitative analysis of phosphorylated IRF3 in cGAMP-treated HCECs versus control group; C: Representative images of IF against p-IRF3 in the HCECs treated with the same conditions(magnification 400×, scale bar 10 μm). aP<0.05, bP<0.01, n=3.
Elevated IRF3 Phosphorylation in HCECs after cGAMP TreatmentTo further characterize the activation of cGAS/STING signaling pathway in HCECs after cGAMP treatment,we detected the phosphorylation of IRF3, a key downstream transcription factor of cGAS/STING signaling pathway. As shown in Figure 3A and 3B, the much more phosphorylation of IRF3 in HCECs at 0.5, 1 and 3h after treatment with cGAMP was observed. The immunofluorescent (IF) staining also revealed increased phosphorylation and more nuclear translocation of IRF3 in HCECs after treatment with cGAMP(Figure 3C). While when pre-treated with C-176, the cGAMPstimulated the phosphorylation in HCECs was reduced.These findings indicated that the activation of cGAS/STING signaling pathway in HCECs probably depended on the IRF3 phosphorylation.
Augmented Expression of Phosphorylated IRF3 in MU SamplesAlthough we detected the increased expression of cGAS and STING in MU samples using Western blot and IHC, whether the cGAS/STING signaling pathway is activated remains uncertain. Therefore, we determined the levels of phosphorylated IRF3 in MU samples. As shown in figure 4A, the phosphorylated IRF3 was mainly located at ocular epithelial cells and infiltrated inflammatory cells in the MU patients. Moreover, we also observed the increased phosphorylation of IRF3 in MU samples when compared with the control group (Figure 4B and 4C).
DISCUSSION
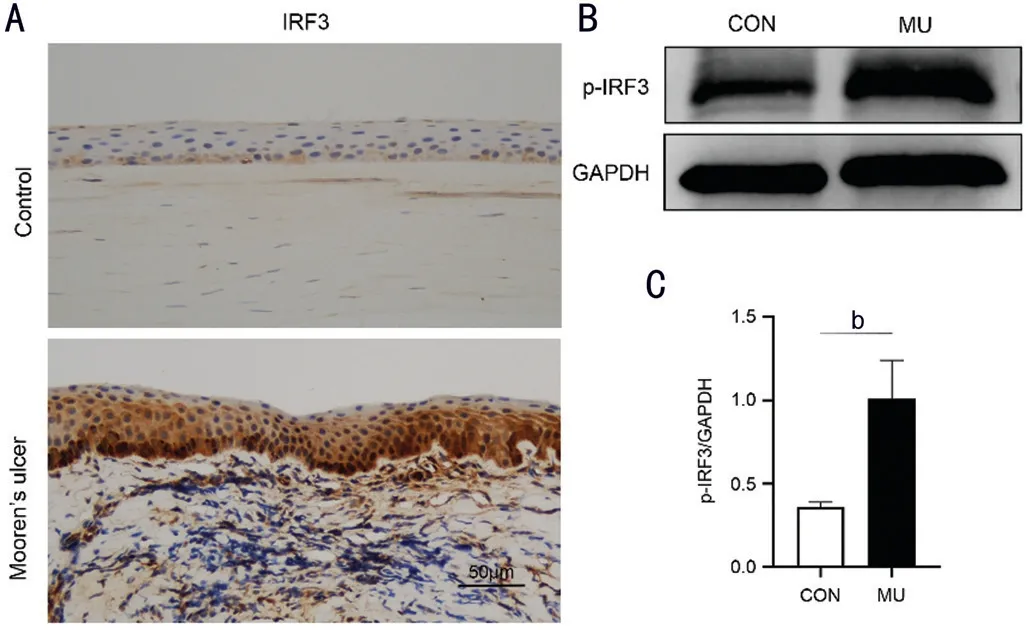
Figure 4 The augmented expression of phosphorylated IRF3 in MU samples A: Representative images of IHC staining against IRF3 in the patients with MU (magnification 200×, scale bar 50 μm); B: Western blot analysis of p-IRF3 in the patients with MU; C: Quantitative analysis of p-IRF3 in MU samples versus healthy donors. bP<0.01, n=3.
MU is an idiopathic chronic corneal ulcer, which begins in the periphery of the cornea and then progressively involves the circumferential and central cornea. A large evidence indicates that MU is an autoimmune disease, histopathologically characterized by the presence of autoantibody against cornealassociated antigen and more infiltrations of multiple types of immune cells, including CD3+T cells, CD68+macrophages,neutrophils, and B7-2+antigen-presenting cells[5,21]. However,the pathogenesis of MU remains unclear. In this study, we revealed a robust expression of cGAS/STING signaling components in MU samples compared with the healthy controls, suggesting the involvement of cGAS/STING signaling pathway in the pathogenesis of MU.
Accumulative evidence indicates that cytosolic DNA sensor pathways are essential for innate immune signaling that alerts the host immune system to mount a defense against microbial infections[22]. However, the dysregulations of these cytosolic DNA sensor pathways result in aberrant innate immune response, and ultimately cause autoimmune and inflammatory disorders[23]. As one of the major cytosolic DNA sensors, cGAS can recognize the cytosolic DNA and generate cGAMP which binds STING and ultimately promotes IFN-Is and NF-κB mediated generation of pro-inflammatory cytokines[12].The overactivation of cGAS/STING signaling was reported to contribute to the autoimmune and inflammatory diseases,such as SLE, asthma, Aicardi-Goutières Syndrome (AGS)and amyotrophic lateral sclerosis (ALS)[24-27]. As autoimmune disease, we speculate that cGAS/STING signaling could be involved in the development of MU. Therefore, we collected the MU subjects and evaluated the expression of cGAS/STING signaling components. This study showed a higher protein expression of cGAS and STING in patients with MU versus the control samples, as well as the increased phosphorylated IRF3 in MU samples. These findings suggested the increased expression of cGAS and STING in MU samples probably contributed to the downstream activation of IRF3 and ultimate MU development.
Through IHC, we found that major location of cGAS and STING in MU samples was infiltrated inflammatory cells and ocular epithelial cells. These observations further demonstrated the involvement of inflammatory cells and epithelial cells in the MU pathogenesis through cGAS/STING signaling.To investigate the effect of cGAS/STING signaling on MU,the HCECs were stimulated with cGAMP in the presence or absence of C-176 (an inhibitor of STING). We showed the increased levels of IFN-Is and ISGs in HCECs after cGAMP treatment, as well as increased phosphorylation of IRF3. When in the presence of C-176, the effect of cGAMP on HCECs was significantly reversed, as indicated by decreased expression of IFN-Is and ISGs, as well as less IRF3 phosphorylation.These findings indicated that the activation of cGAS/STING signaling pathway in HCECs probably depended on the IRF3 phosphorylation, which was essential to produce IFN-β and ISGs. Moreover, the IFN-Is and ISGs have been documented to contribute to the pathogenesis of the inflammatory and autoimmune diseases[28-29]. Therefore, these results, coupled with the findings of increased cGAS/STING signaling components in MU subjects emphasized the potential of cGAS/STING signaling on the onset and development of MU.Overall, our study demonstrated the up-regulation of cGAS/STING signaling components in MU samples, and further confirmed the activation of its downstream IRF3 and expression of its target genes. We speculate that the exogenous and self-DNA triggered the activation of cGAS/STING pathway and contributed to the MU development. Therefore,further research should focus on the mechanism by which the cGAS/STING signaling is activated in MU pathogenesis and be required to investigate the possibility of cGAS/STING signaling as therapeutic target for MU.
ACKNOWLEDGEMENTS
Foundations:Supported by National Natural Science Foundation of China (No.81900907); the Young and Middle-Aged Scientists Research Awards Fund of Shandong Province(No.ZR2017BH004).
Conflicts of Interest: Zhang YN,None;Dong YL,None;Hao WP,None;Bai XF,None;Qi X,None;Liu T,None;Sun XT,None;Wei C,None;Qi XL,None.
 International Journal of Ophthalmology2021年11期
International Journal of Ophthalmology2021年11期
- International Journal of Ophthalmology的其它文章
- Toric implantable collamer lens for the management of pseudophakic anisometropia and astigmatism
- Efficacy of rhNGF-loaded amniotic membrane transplantation for rabbit corneal epithelial and nerve regeneration
- lnhibition of corneal neovascularization by topical application of nintedanib in rabbit models
- TGF-β2-induced NEAT1 regulates lens epithelial cell proliferation, migration and EMT by the miR-26a-5p/FANCE axis
- Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway
- Midterm outcomes of penetrating keratoplasty following allogeneic cultivated limbal epithelial transplantation in patients with bilateral limbal stem cell deficiency
